Abstract
This study was conducted to determine if a low monotonic dose of zearalenone (ZEN) affects the immunohistochemical expression (IE) of oestrogen receptor alpha (ERα) and oestrogen receptor beta (ERβ) in the intestines of sexually immature gilts. Group C (control group; n = 18) gilts were given a placebo. Group E (experimental group; n = 18) gilts were dosed orally with 40 μg ZEN /kg body weight (BW), each day before morning feeding. Samples of intestinal tissue were collected post-mortem six times. The samples were stained to analyse the IE of ERα and Erβ in the scanned slides. The strongest response was observed in ERα in the duodenum (90.387—average % of cells with ERα expression) and in ERβ in the descending colon (84.329—average % of cells with ERβ expression); the opposite response was recorded in the caecum (2.484—average % of cells with ERα expression) and the ascending colon (2.448—average % of cells with ERα expression); on the first two dates of exposure, the digestive tract had to adapt to ZEN in feed. The results of this study, supported by a mechanistic interpretation of previous research findings, suggest that ZEN performs numerous functions in the digestive tract.
Key Contribution:
Qualitative changes were manifested by a shift in oestrogen receptor expression levels from absorption level 0 to 3; particularly in ERβ expression in the descending colon.
1. Introduction
Oestrogens and oestrogen-like substances found in the natural environment including the mycoestrogen ZEN, affect the developing reproductive and non-reproductive tissues [1,2]. Oestrogens are synthesised by the body, but they are also present in the environment, in the form of xenobiotics and naturally occurring compounds (undesirable substances) [3]. Most of these substances (not necessarily pollutants) are known as endocrine disruptors (EDs) [4], and they are usually found in soil, air, water, food and feed (i.e., the environment) [5,6]. Phytoestrogens (genistein, coumestrol) and the mycoestrogen ZEN (fungal metabolite) are naturally occurring EDs [7,8,9].
Zearalenone and α-zearalenol (α-ZEL) have an oestrogen-like structure. However, they are not steroids and do not originate from sterane structures [10]. EDs such as zearalenone are involved in several processes [11,12] that influence the endocrine system [13] and induce side effects [14]: (i) in prepubertal gilts, EDs compete with endogenous oestrogens for the binding sites of oestrogen receptors (ERs), which can alter mRNA expression levels and protein synthesis and reduce the efficacy of endogenous steroids [10,15,16,17]; (ii) EDs can bind to the inactive receptor (i.e., blocking it), thereby preventing the binding of natural hormones to that receptor (antagonistic effect) [11,17]; (iii) EDs reduce the levels of circulating natural hormones because they bind to blood transporting proteins, [2]; and (iv) EDs can also affect the body’s metabolism by influencing the rates of synthesis, decomposition, and release of natural hormones [10,18,19,20].
When ingested, ZEN can prevent or delay the clinical and subclinical spread of oestrogen-dependent tumours [2,21,22]. Sex hormones and exogenous oestrogen-like chemicals are frequently implicated in the aetiology of tumours in various tissues [8]. Many oestrogen-sensitive tumours are termed oestrogen receptor-positive tumours because ERs are mediators of oestrogens or oestrogen-like substances that cause cancer [14,21]. Zearalenone may be a selective oestrogen receptor modulator, but its binding affinity for ERs is 10,000 times lower than that of 17-oestradiol (E2) [2]. Zearalenone has agonistic or antagonistic effects on target tissues, depending on the type of ER [1,2]. The chemopreventive effect of ZEN can be attributed to its antagonistic influence on ERs [18]. There is evidence that ZEN can inhibit circulating oestrogen precursors and slow the development and progression of oestrogen-dependent tumours by binding to ERs, and ERs can probably also inhibit the activity of steroid hormones that convert circulating hormones to E2 [18,23].
Elements of the oestrogen response have been investigated in studies involving endogenous oestrogens and oestrogen-containing drugs [12,13,18]. When endogenous oestrogens exert genomic effects via ERs, oestrogen response elements bind with ERs or other response elements in the neighbouring genes that respond directly to oestrogens [3]. The resulting bonds influence the transcription of oestrogen-responsive genes. Mycoestrogens trigger similar responses by binding to ERs and initiating molecular cascades that alter gene expression [8]. Zearalenone is involved in molecular mechanisms, but its oestrogenic activity remains insufficiently investigated. Previous research has demonstrated that the presence of ZEN in feed or food affects the mRNA expression of ERs [8,24] and the activity of other genes encoding metabolic processes in enterocytes [25,26]. Subclinical symptoms of ZEN mycotoxicosis can cause changes in hormonal signalling when enterocytes in different intestinal segments are exposed to this mycotoxin [19]. The role of zearalenone in the digestive system should be evaluated to determine possible risks for gilts before puberty [2,27,28,29,30]. Therefore, this experiment aimed to find out whether a low monotonic dose of ZEN affects the immunohistochemical expression (IE) of ERα and ERβ in the gut of prepubertal gilts. The findings may contribute to a mechanistic understanding of changes in ERα and ERβ expression.
2. Results
2.1. Clinical Observations
Clinical manifestations of ZEN mycotoxicosis were not noted during the experiment. However, histopathological analyses, ultrastructural analyses, and analyses of the metabolic profile of samples taken from same gilts frequently revealed changes in certain tissues or cells. These findings have been posted in various articles [2,19,20,31,32,33,34,35].
2.2. Optical Density
The brown background staining of the slides (Figure 1 and Figure 2) was not specific to all intestinal segments, and it may have occurred in staining assays examining the ERα and ERβ expression in DAB-stained gastrointestinal tissues (most samples exhibited light-brown, non-specific staining).
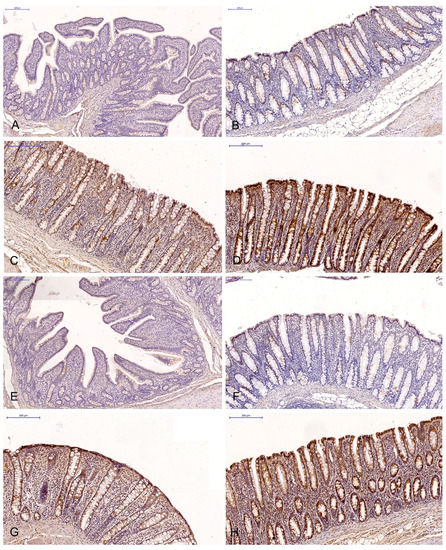
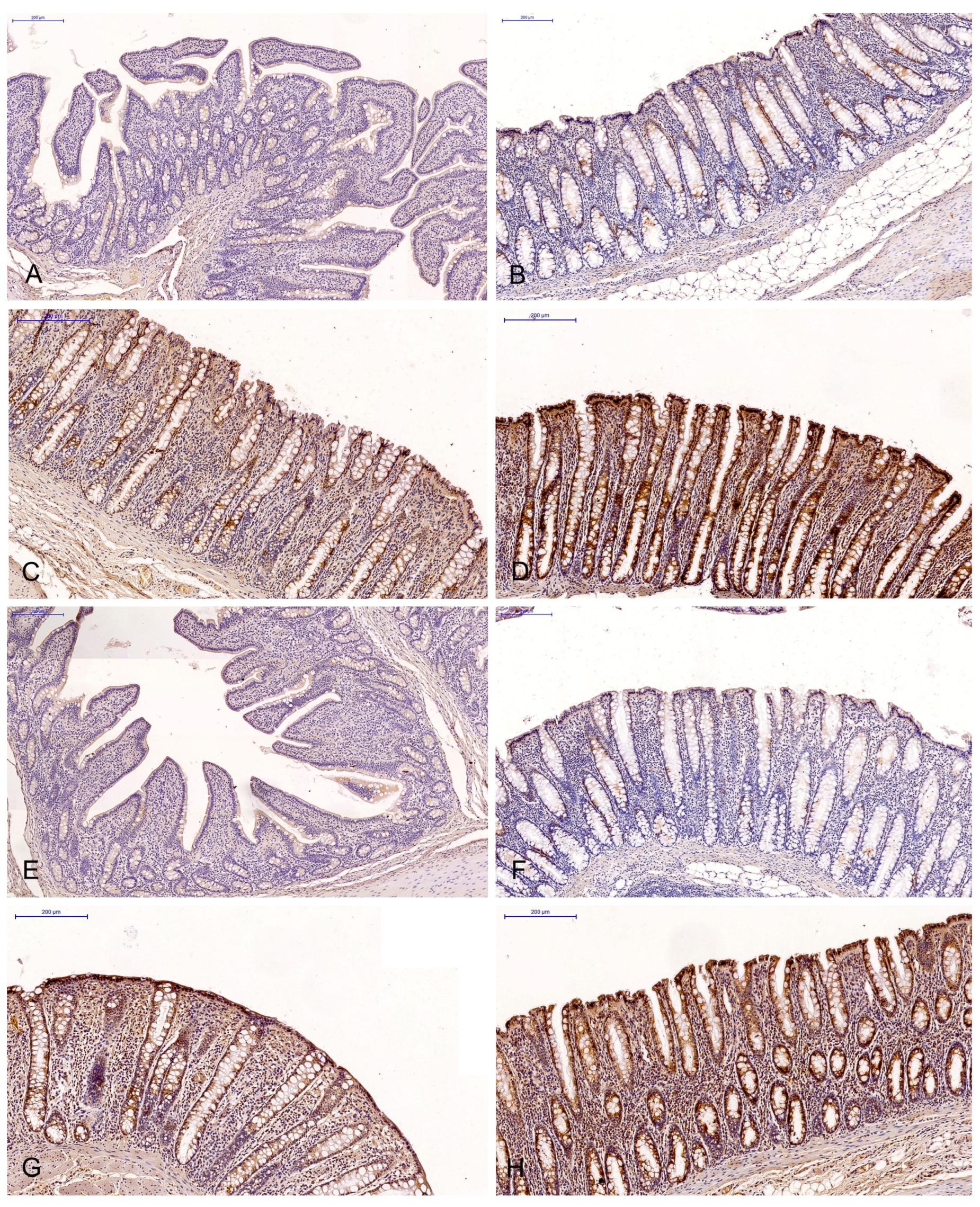
Figure 1.
Scanned slides showing the IE of ERα in the descending colon in group C ((A)—0; (B)—+; (C)—++; (D)—+++) and group E ((E)—0; (F)—+; (G)—++;. (H)—+++). HE.
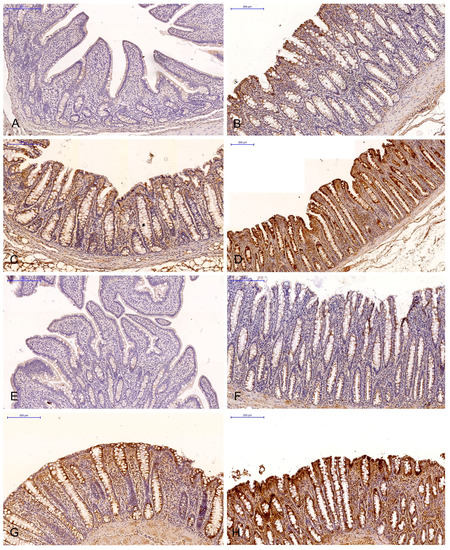
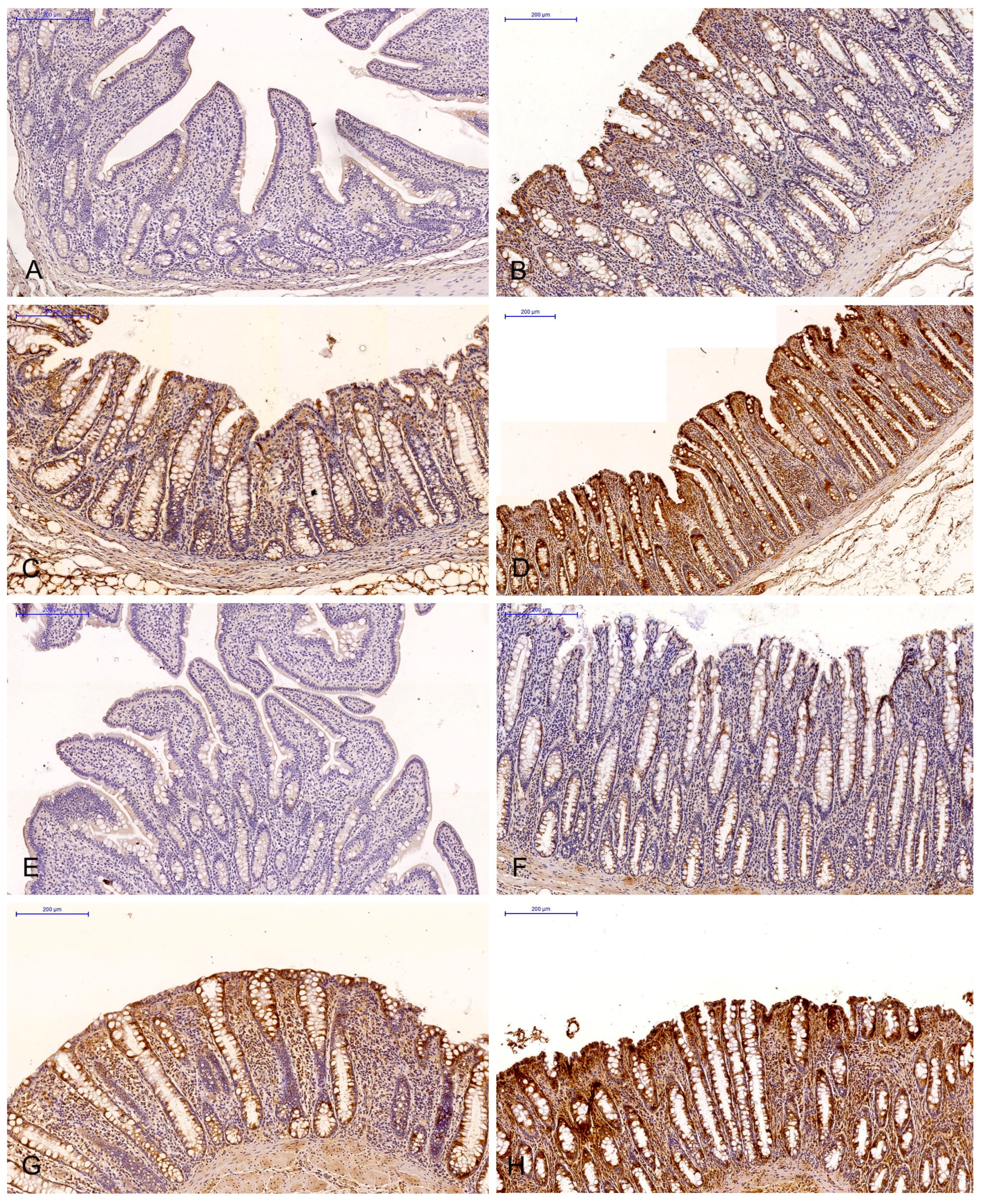
Figure 2.
Scanned slides showing the IE of ERβ in the descending colon in group C ((A)—0; (B)—+; (C)—++; (D)—+++) and group E ((E)—0; (F)—+; (G)—++; (H)—+++). HE.
The effect of six-week exposure to ZEN on the expression levels of the selected ERs was determined in selected segments of the gastrointestinal tract (GI) of gilts in the control and experimental groups using a four point scale (negative—0; weak and homogeneous—1; mild or moderate and homogeneous—2; intense or strong and homogeneous—3) (Figure 3 and Figure 4). Expression levels were compared between the dates of sample collection in specific sections of the intestines. Meaningful differences in the IE of ERα were not observed in the descending colon in the control group and in the ascending colon and descending colon in the experimental group. Meaningful differences in the IE of ERβ were not noted in the caecum and ascending colon in group C, and in the duodenal cap, the third section of the duodenum and the caecum in group E. The intestinal sections where no significant differences were found are not presented graphically.
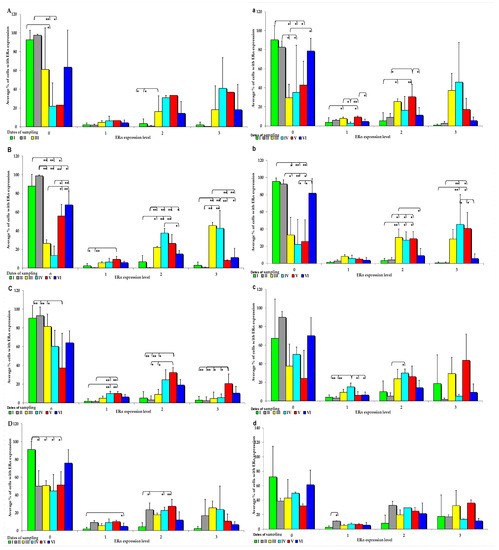
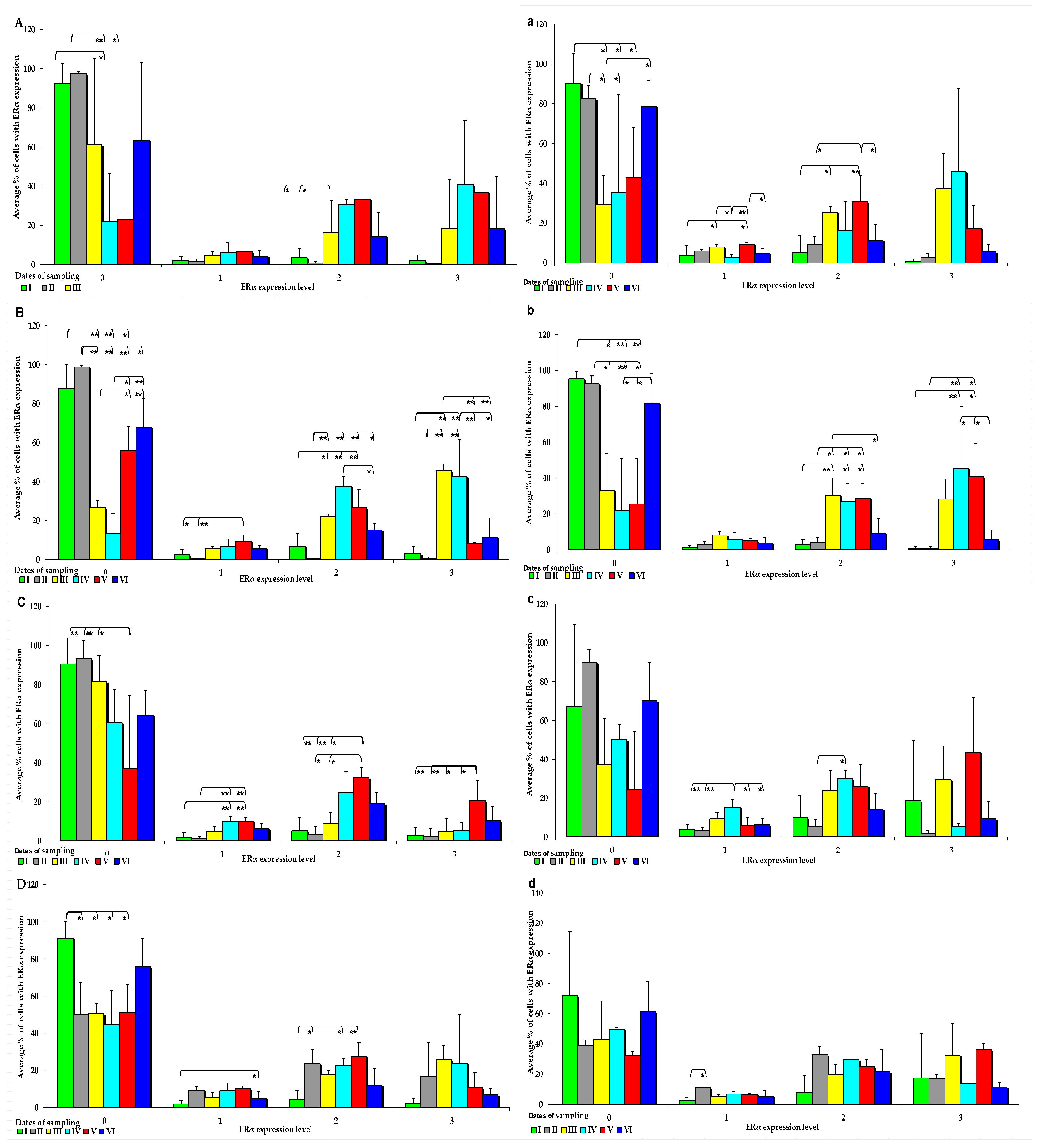
Figure 3.
IE of ERα (based on a 4-point grading scale: negative—0; weak and homogeneous—1; mild or moderate and homogeneous—2; intense or strong and homogeneous—3) in the intestines of sexually immature gilts from the control group: (A) in the duodenal cap on selected dates of exposure; (B)—in the third section of the duodenum on selected dates of exposure; (C) in the jejunum on selected dates of exposure; (D) in the caecum on selected dates of exposure. In the intestines of sexually immature gilts from the experimental group: (a) in the duodenal cap on selected dates of exposure; (b) in the third section of the duodenum on selected dates of exposure; (c) in the jejunum on selected dates of exposure only in the weak(1) and mild (2) grades; (d) in the caecum on selected dates of exposure only in the weak grade (1). Expression was presented as ± (confidence interval) and SE (standard error) for some samples. * p ≤ 0.05 and ** p ≤ 0.01 compared with the residual groups.
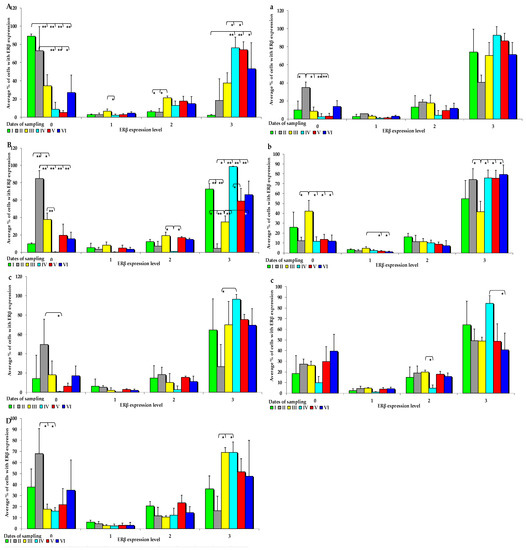
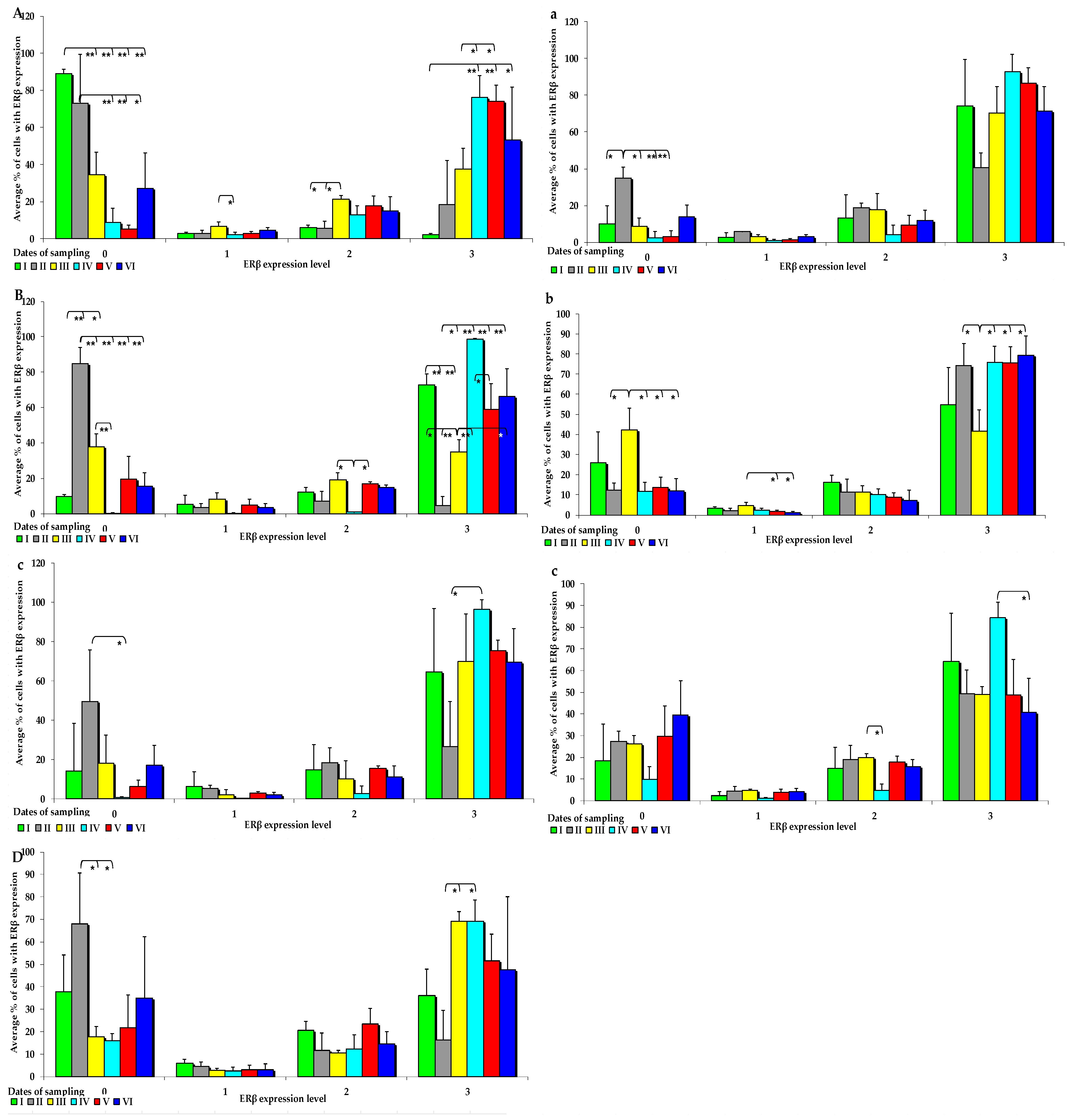
Figure 4.
IE of ERβ (based on a 4-point grading scale: negative—0; weak and homogeneous—1; mild or moderate and homogeneous—2; intense or strong and homogeneous—3) in the intestines of sexually immature gilts from the control group: (A) in the duodenal cap on selected dates of exposure; (B) in the third section of the duodenum on selected dates of exposure; (C) in the jejunum on selected dates of exposure only in the negative (0) and intense (3) grades; (D) in the descending colon on selected dates of exposure only in the negative (0) and intense (3) grades; in the intestines of sexually immature gilts from the experimental group: (a) in the jejunum on selected dates of exposure only in the negative grade (0); (b) in the ascending colon on selected dates of exposure; (c) in the descending colon on selected dates of exposure only in the mild (2) and intense (3) grades. Expression was presented as ± (confidence interval) and SE (standard error) for some samples. * p ≤ 0.05 and ** p ≤ 0.01 compared with the residual groups.
On each date of analysis, ERα was more highly expressed in the control group than in the experimental group, especially at absorbance level 0 (Figure 3A–D). Significant differences in ERα expression were found in the control group at different absorption levels, but absorption was significantly more pronounced on dates I, II, and VI. Significant differences in ERα expression were also observed at other absorption levels, but the noted values were much lower than at absorption level 0, and they were only found in the small intestine (Figure 3A–D). In the control group, the average ERα expression was highest at absorbance level 0, and it increased when the digesta entered the caudal segment of the small intestine.
An analysis of the IE of ERα revealed that it was suppressed in most intestinal segments on all dates in group E (0 points on a 4-point scale), but significant differences were detected only on dates I, II, and VI (Figure 3a). ERα was more highly expressed in the ascending and descending colon at absorption level 3 in the experimental group than in the control group. However, in group E, ERα expression was suppressed at all absorption levels (Figure 3a–d). Differences in the ERα expression were noted in the control group, but only in selected segments of the small intestine, particularly in both parts of the duodenum examined in the study (Figure 3a,b). Similarly to group C, ERα expression was induced in the experimental group at absorbance level 0, whereas at absorbance level 3, the levels of ERα expression in the analysed intestinal segments were higher in the experimental group than in the control group.
In group C, the IE of ERβ was suppressed in both segments of the duodenum, jejunum, and descending colon (Figure 4A–D). The average values of ERß expression in the control group and in the experimental group followed a certain trend. In group E, ERß expression was observed at absorbance level 3, and ERβ was more strongly expressed in all analysed tissues, but its expression was more suppressed at absorbance level 0. However, these differences were not significant. An immunohistochemical analysis of ERß expression in the examined intestinal segments, compared with ERα expression, revealed completely different results. In group E, ERβ was more strongly expressed, especially at absorption level 3 and, interestingly, in the jejunum and colon (Figure 4a–c). However, significant differences between the groups were found only on dates I, II, and III, especially in the examined segments of the duodenum, which can be explained by the fact that ERβ saturation was lower in the duodenum than in the other intestinal segments.
2.3. The Prognostic Value of the ERs Expression Profile
A total of 432 samples were analysed to determine the ER expression indicator (P-ERs). In many of the analysed samples, there were no significant differences in ER expression. The mean values of P-ERs were 42 ± 27 for ERα and 38 ± 26 for ERβ. P-ERs values were not normally distributed (Table 1).

Table 1.
ERα and ERß expression at various absorption levels in the analysed sections of the GI tract in pre-pubertal gilts.
2.3.1. P-ER Values for ERα
In group C, the P-ERα value was 42, reaching 15 in the lower quartile and 69 in the upper quartile. An analysis of the median and the upper and lower quartiles revealed that the expression values could be divided into four subgroups: A—very low P-ERα (P-ERα <15), B—low P-ERα (15 ≤ P -ERα < 42), C—high P-ERα (42 ≤ P-ERα < 69) and D—very high P-ERα (P-Erα ≥69) (Table 1). In group C, very low (A), low (B), high (C), and very high (D) P-ERα values were found in 11 (46%), seven (29%), five (21%), and one (4%) cases, respectively. The statistical analysis was conducted for different means, medians, upper and lower quartiles of the separation points, but no meaningful differences were observed.
The results of the analyses involving the uptake of only Erα or ERβ are difficult to interpret. The values of P-ERs (Table 1) provide new information on the presence of a low ZEN dose in the diet. These were very similar in both groups, but at absorption level 3, an increase in P-ERs was observed in group E, resulting in a shift from quartile A to quartile B from the jejunum directly to the descending colon. The results described above and previous research findings suggest that ZEN may compensate for E2 deficiency by triggering ERα [27].
2.3.2. P-ER Values for ERβ
In group C, the P-ERβ worth was 35, reaching 9 in the lower quartile and 61 in the upper quartile. Based on the average value of the median, and the upper and lower quartiles, expression values were divided into four subgroups: A—very low P-ERβ (P—ERβ ≤ 9), B—low P-ERβ (9 ≤ P-ERβ < 35), C—high P-ERβ (35 ≤ P-ERβ < 61), and D—very high P-ERβ (P-Erβ ≥ 61) (Table 1). In group C, very low (A), low (B), high (C), and very high (D) levels of P-ERβ were found in six (25%), 11 (46%), 6 (25%), and one (4%) cases, respectively. The statistical analysis was carried out for different means, medians, upper and lower quartiles, but no meaningful differences were found.
In group E, the P-ERβ value was 38, reaching 12 in the lower quartile and 64 in the upper quartile. Based on the values of the median, the upper and lower quartiles and expression values were divided into four subgroups: A—very low P-ERβ (P-Rβ < 12), B—low P-ERβ (12 ≤ P-ERβ < 38), C—high P-ERβ (38 ≤ P-ERβ < 64) and D—very high P-ERβ (P-Erβ ≥ 64) (Table 1). In the experimental group, very low (A), low (B), high (C), and very high (D) P-ERβ values were known in eight (33%), 10 (42%), three (12%), and three (12%) cases, respectively. The statistical analysis was carried out for different means, medians, upper and lower quartiles, but no meaningful differences were found.
The values of P-ERβ (Table 1) shifted to the right from quartile C to quartile D at absorption level 3 in the caecum and the ascending colon. An analysis of the expression of both receptors demonstrated that the P-ERα levels shifted significantly to the lower quartiles (to the left) in animals exposed to low ZEN doses.
3. Discussion
This study confirmed our recent observations that low ZEN doses improve somatic [36] and reproductive health (our previous mechanistic studies) [2,19,37]. On the first day of exposure, ZEN exerted a stimulatory effect on the body, with the exception of the reproductive system [18,38]. This effect was minimised after the second or third day of exposure, probably due to: (i) the negative effects of extragonadal compensation for oestrogen synthesis [39,40] by androgen conversion or the acquisition of exogenous oestrogens or oestrogen-like substances [2,9,41]; (ii) adaptive mechanisms [37]; (iii) higher energy and protein utilisation, indicating more efficient feed conversion (productivity in group E) [41,42,43]; or (iv) detoxification processes (biotransformation) [3]. The last argument is difficult to confirm since an analysis of the carry-over factor in the GI tract of the same animals did not reveal the inherence of α- ZEL or β- ZEL (ZEN metabolites) in the intestinal walls or that the registered levels were below the detection limit [20,25]. According to López-Calderero et al. [44], a higher ERα/ERβ ratio indicates that proliferative processes are stimulated or silenced, and it is unrelated to apoptosis [38]. Similar observations were made by Cleveland et al. [45] and Williams et al. [46]. These results suggest that low levels of ZEN in the diet stimulate proliferative processes in the gastrointestinal tract of prepubertal gilts, especially in the colon. In sexually mature animals, this is a good predictor of weight gain or the time needed to reach slaughter weight [41], and it suggests that the gastrointestinal tract regulates somatic health [9,38]. Thus, the digestive system acts as a “second brain” [47] as it performs numerous functions including a modulatory role between the intestinal contents and tissues vis. the central nervous system [48]. These findings also suggest that ZEN and endogenous oestrogens control growth, differentiation and other important functions in tissues including in the gastrointestinal tract [2] of prepubertal gilts with supraphysiological oestrogen levels [18]. The above also suggests that oestrogen signalling (e.g., ZEN and its metabolites), regardless of its origin, is the major regulator of genomic mechanisms. Oestrogen receptors play a special role: (i) they are activated by ligand-dependent and ligand-independent pathways; (ii) they act as transcription factors that activate and trigger the expression of all sensitive genes; and (iii) the feedback loop regulated by oestrogens contributes to the maintenance or modification of all genomic processes.
3.1. Oestrogen Receptors
The biological effects of oestrogens are determined by the type of ERs including the classical nuclear ERα and ERβ as well as the G-protein-coupled ERs (GPER; its expression has not been analysed). Therefore, the levels of different ERs determine the effects of endogenous and exogenous oestrogens on cells (tissues).
3.1.1. Oestrogen Receptor Alpha
The expression of ERα in the control group could be attributed to the physiological deficiency of E2 in the gilts before puberty [4,24,49], which could point to supraphysiological hormone levels rather than hypoestrogenism [18,50]. Zearalenone mycotoxicosis contributes to an increase in steroid levels (endogenous steroids such as E2, progesterone, and testosterone as well as exogenous steroids such as ZEN), which may restore or enhance ER signalling in cells [18,51], but only in relation to hormone-dependent ERs [27]. As a result, ERα expression is not stimulated but deregulated [51]. Most importantly, circulating steroid hormones are bioavailable (not bound to carrier proteins) and their cellular effects are observed at very low concentrations of approximately 0.1–9 pg/mL E2 [49]. The concentrations of active hormones are determined by the age and health status of animals [2,8,18,24,52].
Various conclusions can be drawn from the observations of the role of ERα in mammals and the results of the experimentally induced ZEN mycotoxicosis. According to Suba [38], both high and low levels of E2 stimulate the expression and transcriptional activity of ERs to restore or enhance ER signalling in cells, which was not observed in the current study. However, the IE of ERα was suppressed to a greater extent. Low ZEN doses in the diet decrease the IE of ERα, which directly affects the somatic (higher weight gain) [41] and reproductive health (delayed sexual maturity [53]) of animals. It should also be noted that low serum E2 levels may induce compensatory effects to increase the expression and transcriptional activity of ERs, while increased synthesis of endogenous E2 may compensate for low ER signalling [54]. However, it remains uncertain as to whether low ZEN doses are sufficient to meet the requirements of sexually immature gilts. The present findings suggest that this may be the case, with positive implications for pig farmers.
3.1.2. Oestrogen Receptor Beta
According to the literature, intense ERβ expression or a high level of absorption (3 points on a 4-point grading scale) contributes significantly to gut health, especially colon health, and intensifies metabolic processes [55,56]. In turn, ERβ silencing increases the risk of duodenal inflammation and enhances oncogenesis not only in the gastrointestinal tract, but also in the reproductive system [22,40,45,46,57]. Deletion processes suggest that ERβ has anti-inflammatory and anti-carcinogenic properties, and exerts chemopreventive effects in the colon [58], which was confirmed in a study of low-dose ZEN mycotoxicosis [59].
Apart from the previously published research on the effects of E2 deficiency in prepubertal animals, another issue should be addressed. Williams et al. [46] and Gajęcka et al. [59] reported that selected phytoestrogens (silymarin and silibinin) and mycoestrogens (ZEN) have a selective affinity for ERβ [60,61]. This is the result of the increased expression of the ERβ gene, suggesting that natural exogenous dietary oestrogens may have anti-inflammatory properties [35]. These oestrogens also exert chemopreventive effects [22], and they can reverse minor carcinogenic changes in the colon [62]. Calabrese et al. [63] found that a mixture of phytoestrogens and lignans reduced the size and number of duodenal polyps and exerted therapeutic effects in this segment of the gastrointestinal tract [64].
As stated in the research objective, this study was conducted to determine if low ZEN doses naturally occurring in feeds could produce similar effects, and the present results suggest that it is possible. This conclusion is also consistent with the results of previous studies conducted as part of the same research project [2,19,29,31,32,33,34,35,36,52].
3.1.3. ER Expression Indicator
In animals exposed to ZEN, the P-ER levels differed between quartiles. In group E, the P-ERα values shifted from quartile A to quartile B, while the P-ERβ values shifted from quartiles B and C to quartiles A and D. The expression levels of ERα confirm that low ZEN doses can exert oestrogenic effects on the studied ERs.
The endogenous ligand that triggers ERβ [27] and the cells that are activated by specific receptors could not be identified based on the existing knowledge. For this reason, the influence of ZEN on ERβ is difficult to interpret. It seems that E2 does not bind to ERα and ERβ with equal affinity, but it binds to oestrogen response elements. However, ERβ is a much weaker transcriptional activator than ERα. In turn, the oestrogen response element activator protein-1 is responsible for the proliferation processes induced by E2. Nevertheless, E2 has no effect on ERβ, which may indicate that ERβ can modulate ERα activity in cells where both receptors are co-expressed. However, in many cells, ERβ is expressed in the absence of ERα, and in these cells, ERβ remains active independently of ERα [56]. This is the case in epithelial cells of the colon [65], where ERβ-driven enhanced metabolic processes occur [55].
Preclinical models have shown that ERα activity can be modulated by ERβ, which inhibits oestrogen-dependent proliferation and promotes apoptosis [66]. There is evidence that uncontrolled proliferation, progression, and/or failure to respond to treatment may disrupt oestrogen signalling. ERα may be associated with proliferative disorders, and it can be used to determine the efficacy of hormone therapy. In contrast, ERβ is present in healthy colonic mucosa and its expression is significantly delayed in colonic proliferative disorders [44,56].
3.1.4. Summary
The observed silencing of ERs indicates that: (i) low monotonic doses of ZEN elicited the strongest responses on analytical dates III, IV, and VI, whereas on the last date, the prepubertal gilts developed tolerance to the analysed undesirable substance; (ii) ERα expression was increased in the duodenum and ERβ expression was increased in the descending colon; (iii) the opposite was observed in the caecum and the ascending colon; and (iv) the gastrointestinal tract of sexually immature gilts was adapted to the presence of ZEN in the feed after the first two exposure dates. Due to the very low concentrations of E2, ZEN was bound to ERs and triggered qualitative changes in ERs during the successive weeks of the experiment (activation?). Qualitative changes were manifested by a shift in the ER expression levels from absorption level 0 to 3, especially ERβ expression in the descending colon. The observed shift in ERβ expression suggests that zearalenone and its metabolites are involved in the control of proliferation and apoptosis in enterocytes.
4. Materials and Methods
4.1. Experimental Animals
The experiment was carried out at the Department of Veterinary Prevention and Feed Hygiene of the Faculty of Veterinary Medicine of the University of Warmia and Mazury in Olsztyn, Poland, on 36 clinically healthy gilts with an initial body weight (BW) of 25 ± 2 kg. Pre-puberty gilts were kept in groups and had ad lib access to water.
4.2. Experimental Feed
The feed administered to animals (Table 2) was analysed for the presence of ZEN and DON. Mycotoxin content was determined by standard separation techniques using immunoaffinity columns (Zearala-TestTM Zearalenone Testing System, G1012, VICAM, Watertown, MA, USA; DON-TestTM DON Testing System, VICAM, Watertown, MA, USA) and high-performance liquid chromatography (HPLC) (Hewlett Packard, type 1050 and 1100) [67] with fluorescence and/or ultraviolet detection techniques. The detection limit was 3.0 ng/g for ZEN [19] and 1.0 ng/g for DON [36].

Table 2.
Mixture of diets for pre-pubertal gilts (first stage of rearing).
4.3. Experimental Design
The animals were allocated to an experimental group (E = ZEN; n = 18) and a control group (C, n = 18) [68,69]. The animals in group E were orally administered ZEN at a dose of 40 μg/kg BW (Table 3). The pigs in group C were given a placebo. At the time when this test was designed, the above value complied with the recommendations of the European Food Safety Authority (CR 2006/576/EC—2006 [70]) and No-Observed-Adverse-Effect Level (NOAEL) dose. The mycotoxin was administered every morning before feeding, in gel capsules that dissolved in the stomach. In group C, pigs received identical gel capsules, but without the mycotoxin.

Table 3.
Diurnal feed intake in a restricted feeding regime (kg/day) and the average zearalenone concentration per kg feed (μg ZEN/kg feed).
Zearalenone was biosynthesised at the Faculty of Chemistry at the University of Life Sciences in Poznań. The trial lasted 42 days. Zearalenone doses were adapted to the BW of gilts. Zearalenone was served in capsules to avoid potential problems resulting from unequal feed intake. Zearalenone samples were dissolved in 500 μL 96% C2H5OH (96% ethyl SWW 2442-90, Polskie Odczynniki Chemiczne SA, Poland) to obtain the required dose (converted to BW). The solutions were kept at 20 °C for twelve hours. The gilts were weighed at weekly intervals to adjust the ZEN dose of each animal. Three gilts from each group (six animals in total) were euthanised on days 7 (date I), 14 (date II), 21 (date III), 28 (date IV), 35 (date V), and 42 (date VI) by intravenous administration of sodium pentobarbital (Fatro, Ozzano Emilia BO, Italy). Directly after cardiac arrest, part of the intestinal tissue were taken and prepared for analysis.
4.4. Reagents
ZEN was obtained from the Faculty of Chemistry, University of Life Sciences in Poznań based on an earlier developed methodology [71,72] presented in other studies [73].
4.5. Chemicals and Equipment
The chromatographic analysis of ZEN was conducted at the Faculty of Chemistry, University of Biosciences in Poznań based on an earlier developed methodology [73].
4.6. Tissue Samples
On each experimental day, intestinal tissue samples (approx. 1 × 1.5 cm) were collected from the succeeding segments of the GI tract of gilts: the duodenum—the first part and the third section; the jejunum and ileum—the middle part; the large intestine—the middle parts of the ascending colon, transverse colon and descending colon; and the caecum—1 cm from the ileocecal valve. The samples were rinsed with phosphate buffer.
4.7. Immunohistochemistry
4.7.1. Localisation of ERα and ERβ
Tissue samples were fixed in four percent paraformaldehyde and embedded in paraffin. Two samples from each test section were stained to determine the ERα and ERβ expression. In the negative control, the primary antibody was omitted. To unmask the antigens, the sections were placed in citrate buffer (Sigma-Aldrich, Saint Louis, MO, USA) and cooked for 20 min in a microwave oven at 800 W. The sections were coated with ready-to-use DAKO REALTM Peroxidase Blocking Solution (DAKO, Glostrup, Denmark) and reacted for 15 min. Non-specific antigen binding areas were blocked with 2.5% normal goat serum solution. The sections were reacted overnight at a temperature of 6 °C with the following primary antibodies: Mouse Anti-Human Oestrogen Receptor α (Clone: 1D5, DAKO Santa Clara, CA, USA) and Mouse Anti-Oestrogen Receptor β (Clone: 14C8, Abcam, Cambridge, UK), diluted to 1:60 and 1:20, respectively. After the reaction, the specimens were rinsed three times with PBS (Sigma-Aldrich, Saint Louis, MO, USA) at five-minute intervals. Secondary antibodies conjugated with horseradish peroxidase-labelled micropolymer (ImmPRESS™ HRP Universal Antibody, Vector Laboratories, Burlingame, CA, USA) were applied to the specimens. The sections were coloured by incubation with DAB (DAKO, Glostrup, Denmark) for 3 min, and H2O2 was added to visualise the activity of the bound enzyme (brown colour). The sections were washed with water and contrast stained with Mayer’s haematoxylin solution (Sigma-Aldrich, Saint Louis, MO, USA). The primary antibody was ignored in the negative control. Negative controls (solvent-coated slides only, no primary antibody) and positive controls were converted together with the slides [74]. The pig’s ovary was used as a positive control for ERβ [75].
4.7.2. Scanning of the Coloured Slides
The expressions of ERα and ERβ were analysed on the scanned slides (Pannoramic MIDI scanner, 3DHISTECH, Budapest, H) using the NuclearQuant programme (3DHISTECH, H). The slides were converted into digital images (Figure 1 and Figure 2). The profile of nuclear detection and staining intensity were as previously described [59].
4.8. Statistical Analysis
The activity of ERα and ERβ in the GI tract of pigs was presented on the basis of ± and SD for each sample. The results were compiled using the Statistica programme (StatSoft Inc., USA). Based on the applied ZEN dose and the duration of its application, the arithmetic means for systems with repeatable measurements were compared using one-way analysis of variance. The homogeneity of variance in the compared groups was checked with the Brown–Forsythe test. Differences between groups were analysed using Tukey’s honestly significant difference test (p < 0.05 or p < 0.01).
Author Contributions
The experiments were designed and planned by M.G. and M.T.G. The experiments were conducted by I.O.-D., P.B., S.L.-Ż. and M.G. The data were analysed and interpreted by M.D. and M.G. The manuscript was written by M.G. and critically revised by Ł.Z. and M.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Polish Ministry of Science and Higher Education as part of Project No. 12-0080-10/2010. The project was financially supported by the Minister of Education and Science under the program entitled “Regional Initiative of Excellence” for the years 2019–2023, Project No. 010/RID/2018/19, amount of funding PLN 12,000,000.
Institutional Review Board Statement
All experimental procedures involving animals were carried out in compliance with Polish regulations setting forth the terms and conditions of animal experimentation for 2010–2013 (opinion no. 88/2009 of the local Ethics Committee for Animal Experimentation at the University of Warmia and Mazury in Olsztyn, Poland of 16 Dec 2009). All of the investigators are authorised to perform experiments on animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Activity of zearalenone in the porcine intestinal tract. Molecules 2017, 22, 18. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Hampl, R.; Kubatova, J.; Starka, L. Steroids and endocrine disruptors -History, recent state of art and open questions. J. Steroid Biochem. Mol. Biol. 2016, 155, 217–223. [Google Scholar] [CrossRef]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female reproductive disorders: The roles of endocrinedisrupting compounds and developmental timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef]
- Yurino, H.; Ishikawa, S.; Sato, T.; Akadegawa, K.; Ito, T.; Ueha, S.; Inadera, H. Endocrine disrupters (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol. Sci. 2004, 81, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Phar. 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Sun, L.; Dai, J.; Xu, J.; Yang, J.; Zhang, D. Comparative Cytotoxic Effects and Possible Mechanisms of Deoxynivalenol, Zearalenone and T-2 Toxin Exposure to Porcine Leydig Cells In Vitro. Toxins 2022, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; Kotelevtsev, Y.; Mason, J.I. Steroid promiscuity: Diversity of enzyme action. J. Steroid Biochem. 2015, 151, 1–2. [Google Scholar] [CrossRef]
- Barton, M. Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER, Review Article. Steroids 2016, 111, 37–45. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sřrlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.M.; Zhang, L.; Zhao, Z.M.; Zhao, J.; Peng, S.Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signalling. Mol. Cell. Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Shoorei, H.; Dinger, M.E.; Ghafouri-Fard, S. Perspectives on the Role of Non-Coding RNAs in the Regulation of Expression and Function of the Estrogen Receptor. Cancers 2020, 12, 2162. [Google Scholar] [CrossRef] [PubMed]
- Adibnia, E.; Razi, M.; Malekinejad, H. Zearalenone and 17 β-estradiol induced damages in male rats reproduction potential; evidence for ERα and ERβ receptors expression and steroidogenesis. Toxicon 2016, 120, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, F.; Caloni, F.; Schreiber, N.B.; Cortinovis, C.; Spicer, L.J. In vitro effects of deoxynivalenol and zearalenone major metabolites alone and combined, on cell proliferation, steroid production and gene expression in bovine small-follicle granulose cells. Toxicon 2016, 109, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, C.; Damiano, S.; Ferrara, G.; Montagnaro, S.; Meucci, V.; Intorre, L.; Bacci, S.; Esposito, L.; Piscopo, N.; Rubino, A.; et al. Zearalenone (ZEN) and Its Metabolite Levels in Tissues of Wild Boar (Sus scrofa) from Southern Italy: A Pilot Study. Toxins 2023, 15, 56. [Google Scholar] [CrossRef]
- Rykaczewska, A.; Gajęcka, M.; Onyszek, E.; Cieplińska, K.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Obuchowski, A.; Gajęcki, M.T.; Zielonka, Ł. Imbalance in the Blood Concentrations of Selected Steroids in Prepubertal Gilts Depending on the Time of Exposure to Low Doses of Zearalenone. Toxins 2019, 11, 561. [Google Scholar] [CrossRef]
- Zielonka, Ł.; Gajęcka, M.; Żmudzki, J.; Gajęcki, M. The effect of selected environmental Fusarium mycotoxins on the ovaries in the female wild boar (Sus scrofa). Pol. J. Vet. Sci. 2015, 18, 391–399. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, L.; Leng, B.; Li, Y.; Jiao, N.; Jiang, S.; Yang, W.; Yuan, X. Zearalenone Affect the Intestinal Villi Associated with the Distribution and the Expression of Ghrelin and Proliferating Cell Nuclear Antigen in Weaned Gilts. Toxins 2021, 13, 736. [Google Scholar] [CrossRef]
- Obremski, K.; Gonkowski, S.; Wojtacha, P. Zearalenone—Induced changes in lymphoid tissue and mucosal nerve fibers in the porcine ileum. Pol. J. Vet. Sci. 2015, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Hwang, K.A.; Choi, K.C. Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis. Toxins 2016, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. Position paper: The membrane estrogen receptor GPER—Clues and questions. Steroids 2012, 77, 935–942. [Google Scholar] [CrossRef]
- Fruhauf, S.; Novak, B.; Nagl, V.; Hackl, M.; Hartinger, D.; Rainer, V.; Labudová, S.; Adam, G.; Aleschko, M.; Moll, W.D.; et al. Biotransformation of the Mycotoxin Zearalenone to its Metabolites Hydrolyzed Zearalenone (HZEN) and Decarboxylated Hydrolyzed Zearalenone (DHZEN) Diminishes its Estrogenicity In Vitro and In Vivo. Toxins 2019, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Mycotoxins an Gut Microbiota Interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- Warner, M.; Gustafsson, J.A. DHEA—A precursor of ERß ligands. J. Steroid Biochem. 2015, 145, 245–247. [Google Scholar] [CrossRef]
- Soler, L.; Oswald, I.P. The importance of accounting for sex in the search of proteomic signatures of mycotoxin exposure. J. Proteomics 2018, 178, 114–122. [Google Scholar] [CrossRef]
- Song, T.; Zhou, X.; Ma, X.; Jiang, Y.; Yang, W.; Liu, F.; Liu, M.; Huang, L.; Jiang, S. Zearalenone Promotes Uterine Development of Weaned Gilts by Interfering with Serum Hormones and Up-Regulating Expression of Estrogen and Progesterone Receptors. Toxins 2022, 14, 732. [Google Scholar] [CrossRef]
- Wan, B.; Yuan, X.; Yang, W.; Jiao, N.; Li, Y.; Liu, F.; Liu, M.; Yang, Z.; Huang, L.; Jiang, S. The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts. Toxins 2021, 13, 626. [Google Scholar] [CrossRef]
- Lewczuk, B.; Przybylska-Gornowicz, B.; Gajęcka, M.; Targońska, K.; Ziółkowska, N.; Prusik, M.; Gajęcki, M.T. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Exp. Toxicol. Pathol. 2016, 68, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Śliżewska, K.; Gajęcka, M.; Piotrowska, M.; Żakowska, Z.; Zielonka, Ł.; Gajęcki, M. The genotoxicity of caecal water from gilts following experimentally induced Fusarium mycotoxicosis. Vet. Med. 2015, 60, 133–140. [Google Scholar] [CrossRef]
- Piotrowska, M.; Śliżewska, K.; Nowak, A.; Zielonka, Ł.; Żakowska, Z.; Gajęcka, M.; Gajęcki, M. The effect of experimental Fusarium mycotoxicosis on microbiota diversity in porcine ascending colon contents. Toxins 2014, 6, 2064–2081. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Gornowicz, B.; Tarasiuk, M.; Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Zielonka, Ł.; Gajęcki, M.; Gajęcka, M. The Effects of Low Doses of Two Fusarium Toxins, Zearalenone and Deoxynivalenol, on the Pig Jejunum. A Light and Electron Microscopic Study. Toxins 2015, 7, 4684–4705. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz-Kosińska, M.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The Effects of Deoxynivalenol and Zearalenone on the Pig Large Intestine. A Light and Electron Microscopic study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef]
- Mendel, M.; Karlik, W.; Latek, U.; Chłopecka, M.; Nowacka-Kozak, E.; Pietruszka, K.; Jedziniak, P. Does Deoxynivalenol Affect Amoxicillin and Doxycycline Absorption in the Gastrointestinal Tract? Ex Vivo Study on Swine Jejunum Mucosa Explants. Toxins 2022, 14, 743. [Google Scholar] [CrossRef]
- Gajęcka, M.; Tarasiuk, M.; Zielonka, Ł.; Dąbrowski, M.; Nicpoń, J.; Baranowski, M.; Gajęcki, M.T. Changes in the metabolic profile and body weight of pre-pubertal gilts during prolonged monotonic exposure to low doses of zearalenone and deoxynivalenol. Toxicon 2017, 125, 32–43. [Google Scholar] [CrossRef]
- Suba, Z. Key to estrogen’s anticancer capacity. Acad. Lett. 2021. submitted. [Google Scholar]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef]
- Suba, Z. Estrogen Withdrawal by Oophorectomy as Presumed Anticancer Means is a Major Medical Mistake. J. Family Med. Community Health 2016, 3, 1081. [Google Scholar]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and Zearalenone—Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Hickey, G.L.; Craig, P.S.; Luttik, R.; de Zwart, D. On the quantification of intertest variability in ecotoxicity data with application to species sensitivity distributions. Environ. Toxicol. Chem. 2012, 31, 1903–1910. [Google Scholar] [CrossRef]
- López-Calderero, I.; Carnero, A.; Astudillo, A.; Palacios, J.; Chaves, M.; Benavent, M. Prognostic relevance of estrogen receptor-α Ser167 phosphorylation in stage II-III colon cancer patients. Hum. Pathol. 2014, 45, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.G.; Oikarinen, S.I.; Bynoté, K.K.; Marttinen, M.; Rafter, J.J.; Gustafsson, J.Å.; Roy, S.K.; Pitot, H.C.; Korach, K.S.; Lubahn, D.B.; et al. Disruption of estrogen receptor signaling enhances intestinal neoplasia in Apc(Min/+) mice. Carcinogenesis 2009, 30, 1581–1590. [Google Scholar] [CrossRef]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J.Å. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K. The gastrointestinal tract in growth and reproduction. Sci. Am. 1989, 261, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Ziaja, M.; Piastowska-Ciesielska, A.W. Intestinal Barrier, Claudins and Mycotoxins. Toxins 2021, 13, 758. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endoc. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Lawrenz, B.; Melado, L.; Fatemi, H. Premature progesterone rise in ART-cycles. Reprod. Biol. 2018, 18, 1–4. [Google Scholar] [CrossRef]
- Suba, Z. Amplified crosstalk between estrogen binding and GFR signaling mediated pathways of ER activation drives responses in tumors treated with endocrine disruptors. Recent Pat. Anticancer Drug Discov. 2018, 13, 428–444. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.H.; Oh, S.Y.; Cho, D.H.; Kim, S.; Jo, I. Zearalenone-Induced Interaction between PXR and Sp1 Increases Binding of Sp1 to a Promoter Site of the eNOS, Decreasing Its Transcription and NO Production in BAECs. Toxins 2020, 12, 421. [Google Scholar] [CrossRef]
- Bayala, B.; Zoure, A.A.; Baron, S.; de Joussineau, C.; Simpore, J.; Lobaccaro, J.M.A. Pharmacological Modulation of Steroid Activity in Hormone-Dependent Breast and Prostate Cancers: Effect of Some Plant Extract Derivatives. Int. J. Mol. Sci. 2020, 21, 3690. [Google Scholar] [CrossRef]
- Suba, Z. Diverse pathomechanisms leading to the breakdown of cellular estrogen surveillance and breast cancer development: New therapeutic strategies. Drug Design Devel. Ther. 2014, 8, 1381–1390. [Google Scholar] [CrossRef]
- Savva, C.; Korach-André, M. Estrogen Receptor beta (ERβ) Regulation of Lipid Homeostasis—Does Sex Matter? Metabolites 2020, 10, 116. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, M.; Li, Z.; Li, T.; Xu, X. Dual effects of bisphenol A on wound healing, involvement of estrogen receptor β. Ecotoxic. Environ. Saf. 2022, 231, 113207. [Google Scholar] [CrossRef] [PubMed]
- Giroux, V.; Lemay, F.; Bernatchez, G.; Robitaille, Y.; Carrier, J.C. Estrogen receptor β deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int. J. Cancer 2008, 123, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Saleiro, D.; Murillo, G.; Benya, R.V.; Bissonnette, M.; Hart, J.; Mehta, R.G. Estrogen receptor-β protects against colitis-associated neoplasia in mice. Int. J. Cancer 2012, 131, 2553–2561. [Google Scholar] [CrossRef]
- Jakimiuk, E.; Radwińska, J.; Woźny, M.; Pomianowski, A.; Brzuzan, P.; Wojtacha, P.; Obremski, K.; Zielonka, Ł. The Influence of Zearalenone on Selected Hemostatic Parameters in Sexually Immature Gilts. Toxins 2021, 13, 625. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; Hegazy, S.; El-Desoky, K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine 2010, 17, 116–125. [Google Scholar] [CrossRef]
- Barone, M.; Tanzi, S.; Lofano, K.; Scavo, M.P.; Pricci, M.; Demarinis, L.; Papagni, S.; Guido, R.; Maiorano, E.; Ingravallo, G.; et al. Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis 2010, 31, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Pratico, C.; Calafiore, A.; Coscia, M.; Gentilini, L.; Poggioli, G.; Gionchetti, P.; Campieri, M.; Rizzello, F. Eviendep (R) reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch-anal anastomosis. World J. Gastroenterol. 2013, 19, 5671–5677. [Google Scholar] [CrossRef]
- Pasternak, J.A.; Aiyer, V.I.A.; Hamonic, G.; Beaulieu, A.D.; Columbus, D.A.; Wilson, H.L. Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed. Toxins 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Wada-Hiraike, O.; Imamov, O.; Hiraike, H.; Hultenby, K.; Schwend, T.; Omoto, Y.; Warner, M.; Gustafsson, J.Å. Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Oturkar, C.C.; Gandhi, N.; Rao, P.; Eng, K.H.; Miller, A.; Singh, P.K.; Zsiros, E.; Odunsi, K.O.; Das, G.M. Estrogen Receptor-Beta2 (ERβ2)–Mutant p53–FOXM1 Axis: A Novel Driver of Proliferation, Chemoresistance, and Disease Progression in High Grade Serous Ovarian Cancer (HGSOC). Cancers 2022, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Zwierzchowski, W.; Gajęcki, M.; Obremski, K.; Zielonka, Ł.; Baranowski, M. The occurrence of zearalenone and its derivatives in standard and therapeutic feeds for companion animals. Pol. J. Vet. Sci. 2004, 7, 289–293. [Google Scholar]
- Heberer, T.; Lahrssen-Wiederholt, M.; Schafft, H.; Abraham, K.; Pzyrembel, H.; Henning, K.J.; Schauzu, M.; Braeunig, J.; Goetz, M.; Niemann, L.O.; et al. Zero tolerances in food and animal feed-Are there any scientific alternatives? A European point of view on an international controversy. Toxicol. Lett. 2007, 175, 118–135. [Google Scholar] [CrossRef]
- Smith, D.; Combes, R.; Depelchin, O.; Jacobsen, S.D.; Hack, R.; Luft, J. Optimising the design of preliminary toxicity studies for pharmaceutical safety testing in the dog. Regul. Toxicol. Pharm. 2005, 41, 95–101. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Recommendation 2006/576/EC, of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Off. J. Eur. Union Series L 2006, 229, 7–9. [Google Scholar]
- Kostecki, M.; Goliński, P.; Chełkowski, J. Biosynthesis, isolation, separation and purification of zearalenone, deoxynivalenol and 15-acetyldeoxynivalenol. Mycotoxin Res. 1991, 7, 156–159. [Google Scholar] [CrossRef]
- Kostecki, M.; Goliński, P.; Chełkowski, J. Biosynthesis, isolation, separation and purification of nivalenol, fusarenone-X and zearalenone. Mycotoxin Res. 1991, 7, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Waśkiewicz, A.; Zielonka, Ł.; Golinski, P.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Gajęcki, M.T. Mycotoxin levels in the digestive tissues of immature gilts exposed to zearalenone and deoxynivalenol. Toxicon 2018, 153, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Norrby, M.; Madej, A.; Ekstedt, E.; Holm, L. Effects of genistein on oestrogen and progesterone receptor, proliferative marker Ki-67 and carbonic anhydrase localisation in the uterus and cervix of gilts after insemination. Anim. Reprod. Sci. 2013, 138, 90–101. [Google Scholar] [CrossRef]
- Słomczyńska, M.; Woźniak, J. Differential distribution of estrogen receptor-beta and estrogen receptor-alpha in the porcine ovary. Exp. Clin. Endocrinol. Diabetes 2001, 109, 238–244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).