Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis hypogea L.) Grown in Kenya
Abstract
1. Introduction
2. Results
2.1. Agronomic Performance of the Six Varieties
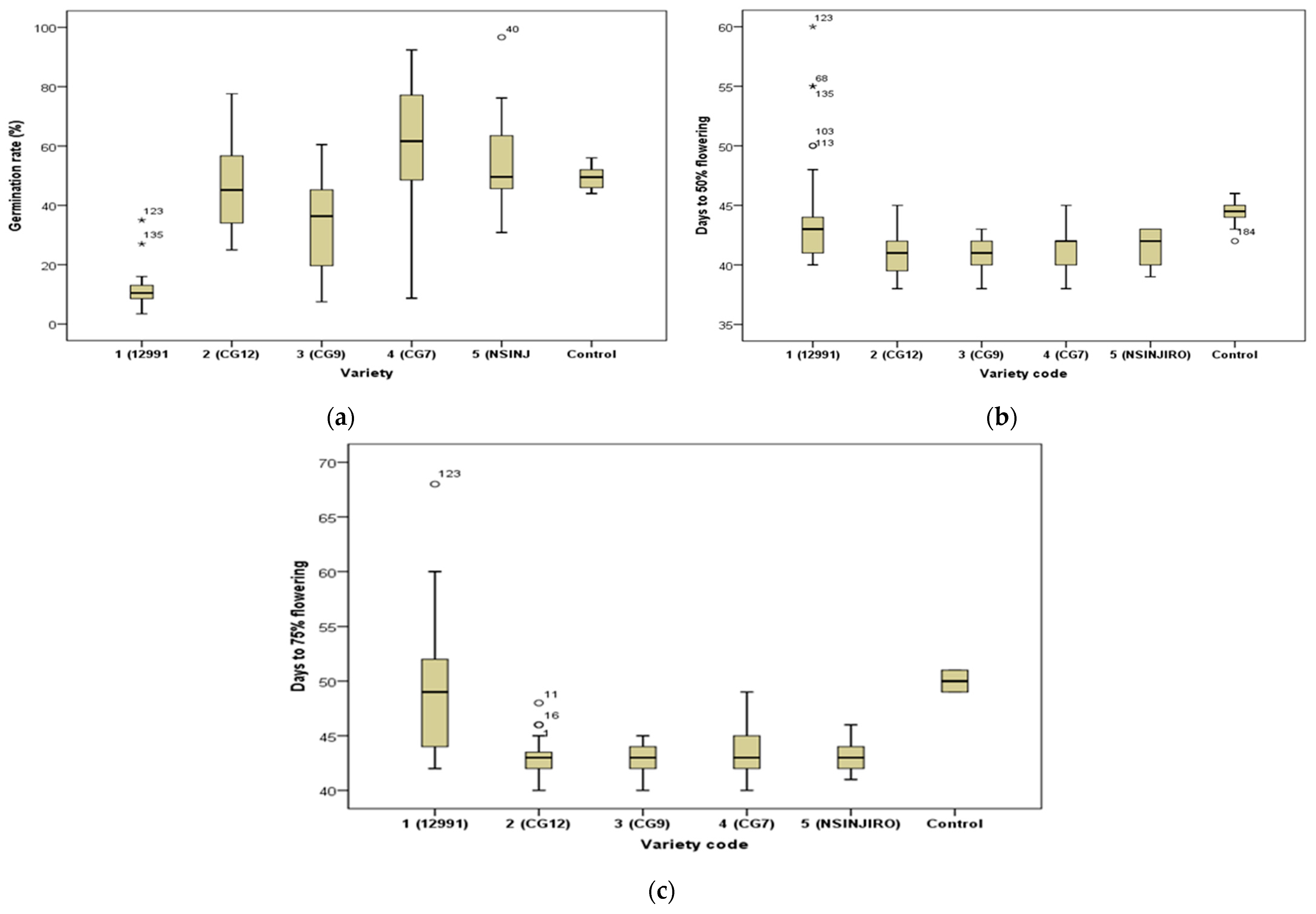
2.1.1. Germination
2.1.2. Flowering
2.2. Disease Incidence among the Six Varieties
2.3. Yield Performance of the Six Varieties
2.3.1. Total Pod Weight
2.3.2. Biomass
2.3.3. Harvest Index
2.3.4. 100-Pod Weight
2.3.5. 100-Seed Weight
2.3.6. Pods per Plant
2.3.7. Shelling Percentage
2.4. Aflatoxin Accumulation among the Five Varieties
3. Discussion
3.1. Agronomic Performance
3.2. Diseases
3.3. Yield Performance
3.4. Aflatoxins
4. Conclusions and Recommendations
5. Materials and Methods
5.1. Field Location
5.2. Experimental Design and Treatments
5.3. Determination of Agronomic Performance
5.4. Disease Scoring
5.5. Fungal Disease Analysis
5.6. Identifying Fungal Isolates Using Morphological Methods
5.7. Yield Parameters: Total Pod Weight, Biomass, Harvest Index, 100-Seed Weight, 100-Pod Weight, Shelling Percentage
5.8. Aflatoxin Analysis
5.8.1. Aflatoxin Sample Preparation
5.8.2. Extraction of Aflatoxin in Peanut
5.8.3. Analysis Using Ultrahigh-Performance Liquid Chromatography
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belay, F.; Meresa, H.; Syum, S. Variation and association for kernel yield and yield related traits of released groundnut (Arachis hypogaea L.) varieties in abergelle district, northern Ethiopia. J. Med. Plants 2018, 6, 265–271. [Google Scholar]
- Janila, P.; Nigam, S.N.; Pandey, M.K.; Nagesh, P.; Varshney, R.K. Groundnut improvement: Use of genetic and genomic tools. Front. Plant Sci. 2013, 4, 23. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Mutegi, C.; Karanja, L.; Kimani, J.; Christie, M.E. Fungal species isolated from peanuts in major Kenyan markets: Emphasis on Aspergillus section Flavi. Crop Prot. 2013, 52, 1–9. [Google Scholar] [CrossRef]
- Fávero, A.P.; De Moraes, S.A.; Garcia, A.A.F.; Valls, J.F.M.; Vello, N.A. Characterization of rust, early and late leaf spot resistance in wild and cultivated peanut germplasm. Sci. Agric. 2009, 66, 110–117. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Nawaz, N.; Yousuf, M.; Khan, M.A.; Mirza, M.Y.; Mohmand, A.S.; Sher, M.A.; Masood, M.A. Stability performance for pod yield in groundnut. Pak. J. Agric. Res. 2009, 22, 116–119. [Google Scholar]
- Arungaa, E.E.; Ochuodhob, J.; Woyengoc, V.W.; Owuoched, J.; Embu, K.; Egerton, K. Incidence and Prevalence of Groundnut Diseases in Western Kenya. In Proceedings of the Kibabii University 3rd Interdisciplinary International Scientific Conference, Bungoma, Kenya, 12–14 June 2018; Volume 12, p. 14. [Google Scholar]
- Ashish, J.; Nadaf, H.L.; Gangadhara, K. Genetic analysis of rust and late leaf spot in advanced generation recombinant inbred lines of groundnut (Arachis hypogaea L.). Int. J. Genet. Eng. Biotechnol. 2014, 5, 109–114. [Google Scholar]
- Noriega Cantu, D.H.; Pereyra-Hernandez, J.; Joaquin Torres, I.C.; Mora Aguilera, G.; Nieto Angel, D.; Cantu Almaguer, M.A.; Gomez Montiel, N.O. Epidemiology of late leaf spot and rust of groundnut in Guerrero, Mexico. Int. Arachis Newsl. 2000, 20, 40. [Google Scholar]
- Mutegi, C.; Cotty, P.; Bandyopadhyay, R. Prevalence and mitigation of aflatoxins in Kenya (1960-to date). World Mycotoxin J. 2018, 11, 341–357. [Google Scholar] [CrossRef]
- Omara, T.; Kiprop, A.K.; Wangila, P.; Wacoo, A.P.; Kagoya, S.; Nteziyaremye, P.; Peter Odero, M.; Kiwanuka Nakiguli, C.; Baker Obakiro, S. The scourge of aflatoxins in Kenya: A 60-year review (1960 to 2020). J. Food Qual. 2021, 2021, 8899839. [Google Scholar] [CrossRef]
- Ochola, C.O.; Obuoyo, J.A. Effect of Socio-Economic Characteristics of Resource Poor Farmers on Food Security in Semi-Arid AgroEcological Zones of Nyakach, Kenya. Int. J. Sci. Res. Publ. 2015, 5, 510–513. [Google Scholar]
- Ajuang, C.O.; Abuom, P.O.; Bosire, E.K.; Dida, G.O.; Anyona, D.N. Determinants of climate change awareness level in upper Nyakach Division, Kisumu County, Kenya. Springerplus 2016, 5, 1015. [Google Scholar] [CrossRef] [PubMed]
- Bakal, H.; Arioglu, H. Determination of some agronomic and quality traits of peanut varieties with different pod characteristics at different harvesting times in main crop growing season. Turk. J. Field Crops 2021, 26, 79–87. [Google Scholar] [CrossRef]
- Gulluoglu, L.; Bakal, H.; Onat, B.; Kurt, C.; Arioglu, H. Comparison of agronomic and quality characteristics of some peanut (Arachis hypogaea L.) varieties grown as main and double crop in mediterranean region. Turk. J. Field Crops 2017, 22, 166–177. [Google Scholar] [CrossRef]
- Sudini, H.; Upadhyaya, H.D.; Reddy, S.V.; Naga Mangala, U.; Rathore, A.; Vijay Krishna Kumar, K. Resistance to late leaf spot and rust diseases in ICRISAT’s mini core collection of peanut (Arachis hypogaea L.). Australas. Plant Pathol. 2015, 44, 557–566. [Google Scholar]
- Menza, N.C.; Margaret, M.W.; Lucy, K.M. Incidence, Types and Levels of Aflatoxin in Different Peanuts Varieties Produced in Busia and Kisii Central Districts, Kenya. Open J. Med. Microbiol. 2015, 05, 209–221. [Google Scholar] [CrossRef]
- Gajjar, K.N.; Mishra, G.P.; Radhakrishnan, T.; Dodia, S.M.; Rathnakumar, A.L.; Kumar, N.; Kumar, A. Validation of SSR markers linked to the rust and late leaf spot diseases resistance in diverse peanut genotypes. Aust. J. Crop Sci. 2014, 8, 927–936. [Google Scholar]
- Matloob, A.A.; Juber, K.S. First report of peanut crown rot disease caused by Aspergillus niger in Iraq and its biocontrol. J. Exp. Biol. 2014, 2, 2. [Google Scholar]
- Demirhan, B.E.; Demirhan, B. Investigation of Twelve Significant Mycotoxin Contamination in Nut-Based Products by the LC–MS/MS Method. Metabolites 2022, 12, 120. [Google Scholar] [CrossRef]
- Abdulrauf, L. (Ed.) Aflatoxins: Occurrence, Detoxification, Determination and Health Risks; BoD–Books on Demand: Norderstedt, Germany, 2022. [Google Scholar]
- Singh, F.; Oswalt, D.L. Major Diseases of Groundnut; ICRISAT: Patanche, India, 1992. [Google Scholar]
- Taufiq, M.M.J.; Darah, I. Fungal endophytes isolated from the leaves of a medicinal plant, Ocimum sanctum Linn and evaluation of their antimicrobial activities. Afr. J. Microbiol. Res. 2018, 12, 616–622. [Google Scholar] [CrossRef]
- Tong, W.Y.; Darah, I.; Latiffah, Z. Antimicrobial activities of endophytic fungal isolates from medicinal herb Orthosiphon stamineus Benth. J. Med. Plants Res. 2011, 5, 831–836. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; Volume 519, p. 388. [Google Scholar]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, S.Y.; Salari, M.; Pirnia, M.; Sabbagh, S.K. Original Article. Identification of Cercospora species in southwestern Iran. J. Plant Prot. Res. 2016, 57, 50–55. [Google Scholar] [CrossRef]
- Hubert, J.; Fourrier-Jeandel, C.; Nakamura, E.; Hugot, N.; Ioos, R. First report of peanut rust caused by Puccinia arachidis on Arachis hypogaea in New Caledonia. New Dis. Rep. 2022, 46, e12104. [Google Scholar] [CrossRef]
- Okoth, S.; Nyongesa, B.; Ayugi, V.; Kang’Ethe, E.; Korhonen, H.; Joutsjoki, V. Toxigenic Potential of Aspergillus Species Occurring on Maize Kernels from Two Agro-Ecological Zones in Kenya. Toxins 2012, 4, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC official method 994.08, Aflatoxins in corn, almonds, Brazil nuts, peanuts, and pistachio nuts—Multifunctional Column (mycosep) method. J. AOAC Int. 1994, 77, 1512–1513. [Google Scholar] [CrossRef]
- AOAC. AOAC official method 2005.08, Aflatoxins in corn, raw peanuts, and peanut butter—Liquid chromatography with post-column photochemical derivatization. J. AOAC Int. 2006, 89, 678–679. [Google Scholar]
| Varieties | Disease Incidence (Mean ± SD) (%) | |||||
|---|---|---|---|---|---|---|
| Rust | ELS | LLS | Groundnut Rosette Virus | Aspergillus Crown Rot | Mean Disease Incidence per Variety | |
| 12991 | 1.78 ± 0.62 ab | 4.79 ± 0.67 c | 4.29 ± 1.88 b | 8.29 ± 1.61 c | 1.34 ± 0.40 a | 5.41 ± 8.31 |
| CG7 | 0.52 ± 0.18 a | 1.78 ± 0.17 a | 1.21 ± 0.12 a | 1.90 ± 0.21 a | 0.17 ± 0.07 a | 1.53 ± 2.13 |
| CG9 | 0.44 ± 0.17 a | 3.15 ± 0.50 abc | 2.03 ± 0.37 ab | 3.7 ± 0.39 ab | 0.31 ± 0.13 a | 2.60 ± 4.17 |
| CG12 | 0.69 ± 0.02 a | 2.08 ± 0.25 ab | 1.23 ± 0.24 a | 2.30 ± 0.33 a | 0.38 ± 0.12 a | 1.85 ± 2.74 |
| (ICGV-SM 90704) | 0.22 ± 0.16 a | 1.68 ± 0.33 a | 0.86 ± 0.21 a | 1.62 ± 0.38 a | 0.34 ± 0.16 a | 1.31 ± 1.75 |
| Control | 2.99 ± 0.48 b | 4.13 ± 0.78 bc | 8.92 ± 1.10 c | 7.64 ± 1.77 bc | 9.21 ± 2.50 b | 7.41 ± 1.88 |
| All Varieties | 0.88 ± 0.15 | 2.79 ± 0.20 | 2.34 ± 0.24 | 4.01 ± 0.44 | 0.75 ± 0.21 | |
| Variety Code | Total Average Pod Weight per Row (kg) (Mean ± SD) | Average Biomass per Row (kg) (Mean ± SD) | Average Harvest Index per Row (%) (Mean ± SD) |
|---|---|---|---|
| 12991 | 1.60 ± 0.28 c | 5.45 ± 0.54 c | 22.00 ± 0.16 c |
| CG12 | 7.29 ± 0.56 a | 17.67 ± 2.63 ab | 25.31 ± 1.22 ab |
| CG9 | 4.50 ± 0.39 b | 14.34 ± 1.17 bc | 23.89 ± 1.12 b |
| CG7 | 8.86 ± 1.45 a | 25.06 ± 2.47 ab | 26.12 ± 0.91 a |
| ICGV-SM 90704 | 9.22 ± 1.19 a | 27.21 ± 5.05 a | 29.21 ± 0.72 a |
| Control | 1.50 ± 1.11 c | 6.60 ± 1.20 c | 18.52 ± 1.44 c |
| Yield Parameters (Mean ± SD) | ||||
|---|---|---|---|---|
| Variety Code | 100-Seed Weight (g) | 100 Pod-Weight (g) | Pods per Plant (Pods Plant−1) | Shelling Percentage (%) |
| 12991 | 78.71 ± 3.17 a | 181.46 ± 6.44 bc | 36.10 ± 3.02 bc | 43.66 ± 1.33 a |
| CG12 | 77.69 ± 1.83 a | 205.25 ± 5.80 b | 72.19 ± 3.53 a | 38.62 ± 1.19 a |
| CG9 | 118.55 ± 6.03 b | 259.37 ± 6.40 a | 53.19 ± 2.76 b | 49.21 ± 5.79 a |
| CG7 | 111.43 ± 2.12 b | 260.84 ± 4.60 a | 57.60 ± 1.94 ab | 43.28 ± 0.96 a |
| (ICGV-SM 90704) | 115.71 ± 15.24 b | 262.93 ± 10.8 a | 62.88 ± 5.73 a | 43.86 ± 4.54 a |
| Control | 84.40 ± 4.52 a | 150.80 ± 11.4 c | 24.47 ± 1.15 d | 43.60 ± 1.16 a |
| Variety | Mean ± SD (ug kg−1) | ||||
|---|---|---|---|---|---|
| Total Aflatoxins | AFB1 | AFB2 | AFG1 | AFG2 | |
| 12991 | 3.38 ± 0.70 a | 0.46 ± 0.65 a | 0.31 ± 0.43 a | 1.15 ± 0.11 a | 1.46 ± 0.27 a |
| CG7 | 2.81 ± 1.99 b | 0.83 ± 0.97 a | 0.00 ± 0.00 b | 0.85 ± 1.01 a | 1.12 ± 0.55 a |
| CG9 | 2.96 ± 3.26 b | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 2.25 ± 3.62 b | 0.72 ± 0.85 b |
| CG12 | 3.36 ± 3.68 a | 0.80 ± 0.92 a | 0.21 ± 0.42 b | 1.19 ± 1.11 a | 0.16 ± 1.56 b |
| ICGV-SM 90704 | 1.82 ± 1.41 b | 0.31 ± 0.62 a | 0.00 ± 0.00 b | 0.50 ± 0.57 a | 1.01 ± 0.43 a |
| Control | 7.11 ± 3.25 c | 0.00 ± 0.00 a | 0.56 ± 0.28 a | 6.29 ± 3.16 c | 0.27 ± 0.37 b |
| Variety | Range of Aflatoxins (ug kg−1) | ||||
|---|---|---|---|---|---|
| Total Aflatoxins | AFB1 | AFB2 | AFG1 | AFG2 | |
| 12991 | 2.88–3.87 | 0.00–0.9 | 0.00–0.61 | 1.07–1.23 | 1.27–1.65 |
| CG7 | 0.61–5.38 | 0.00–1.82 | 0.00–0.00 | 0.00–2.01 | 0.61–1.86 |
| CG9 | 0.00–7.58 | 0.00–0.00 | 0.00–0.00 | 0.00–7.58 | 0.00–1.69 |
| CG12 | 0.25–2.88 | 0.00–1.70 | 0.00–0.84 | 0.00–2.62 | 0.00–3.46 |
| ICGV–SM 90704 | 0.00–2.85 | 0.00–0.1.24 | 0.00–0.00 | 0.00–1.01 | 0.67–1.6 |
| Control | 4.81–9.41 | 0.00–0.00 | 0.36–0.76 | 4.05–8.52 | 0.00–0.53 |
| Agricultural Practices | Varieties | |
|---|---|---|
| 12991, CG7, CG9, CG12, ICGV-SM 90704 | Control | |
| Ploughing | ||
| Dates (2021) | 5–12 February | 5–12 February |
| 24–26 February | 24–26 February | |
| Planting | 27 March | |
| Space inter-row (m) | 0.50 | Broadcasted |
| Space between plants within row (m) | 0.10 | Broadcasted |
| Manure application | ||
| Dates (2021) | 17–19 February | No manure |
| Rates (t ha−1) | 14,830 | No manure |
| Fungicide application | ||
| Specifications | Ridomil | None |
| No. of applications | 3 | None |
| Days after planting | 45, 59, and 73 | None |
| Rates (g/L of water/plot) | 50/20/plot | None |
| Insecticide application | ||
| Specifications | Duduthrin | None |
| N. of applications | 3 | None |
| Days after planting | 45, 59, and 73 | None |
| Rates (mL/L of water/plot) | 65/20/plot | None |
| Molding | ||
| N. of interventions | 1 | 1 |
| Growth stage | Flowering | Flowering |
| Manual weeding | ||
| N. of interventions | 2 | 1 |
| Growth stage | Germination, flowering | Flowering |
| Toxin | Calibration Standards Linearity | Spike Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | Level 1 7.5 ng/g | Level 2 15 ng/g | Level 3 30 ng/g | Average Spike Recovery | Spike Recovery Standard Deviation | LOD (ug kg−1) | LOQ (ug kg−1) | |
| AFB1 | 0.52–104.1 | 77.6 | 74.01 | 74.43 | 75.36 | 1.63 | 0.79 | 2.64 |
| AFB2 | 0.52–103.9 | 74.06 | 75.65 | 74.14 | 74.62 | 0.73 | 0.56 | 1.86 |
| AFG1 | 0.52–103.9 | 102.32 | 84.18 | 76.52 | 87.67 | 10.82 | 0.26 | 0.88 |
| AFG2 | 0.52–104.1 | 58.16 | 75.20 | 80.92 | 71.42 | 9.67 | 0.40 | 1.32 |
| Total aflatoxins | 1.03 | 3.42 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njoki, L.; Okoth, S.; Wachira, P.; Ouko, A.; Mwololo, J.; Rizzu, M.; Oufensou, S.; Amakhobe, T. Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis hypogea L.) Grown in Kenya. Toxins 2023, 15, 111. https://doi.org/10.3390/toxins15020111
Njoki L, Okoth S, Wachira P, Ouko A, Mwololo J, Rizzu M, Oufensou S, Amakhobe T. Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis hypogea L.) Grown in Kenya. Toxins. 2023; 15(2):111. https://doi.org/10.3390/toxins15020111
Chicago/Turabian StyleNjoki, Loise, Sheila Okoth, Peter Wachira, Abigael Ouko, James Mwololo, Margherita Rizzu, Safa Oufensou, and Truphosa Amakhobe. 2023. "Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis hypogea L.) Grown in Kenya" Toxins 15, no. 2: 111. https://doi.org/10.3390/toxins15020111
APA StyleNjoki, L., Okoth, S., Wachira, P., Ouko, A., Mwololo, J., Rizzu, M., Oufensou, S., & Amakhobe, T. (2023). Evaluation of Agronomic Characteristics, Disease Incidence, Yield Performance, and Aflatoxin Accumulation among Six Peanut Varieties (Arachis hypogea L.) Grown in Kenya. Toxins, 15(2), 111. https://doi.org/10.3390/toxins15020111






