Abstract
Piperine is a plant-derived promising piperamide candidate isolated from the black pepper (Piper nigrum L.). In the last few years, this natural botanical product and its derivatives have aroused much attention for their comprehensive biological activities, including not only medical but also agricultural bioactivities. In order to achieve sustainable development and improve survival conditions, looking for environmentally friendly pesticides with low toxicity and residue is an extremely urgent challenge. Fortunately, plant-derived pesticides are rising like a shining star, guiding us in the direction of development in pesticidal research. In the present review, the recent progress in the biological activities, mechanisms of action, and structural modifications of piperine and its derivatives from 2020 to 2023 are summarized. The structure-activity relationships were analyzed in order to pave the way for future development and utilization of piperine and its derivatives as potent drugs and pesticides for improving the local economic development.
Keywords:
piperine; piperine derivatives; biological activity; mechanism of action; structural modification; structure-activity relationship Key Contribution:
This review presents an overview on the biological activities, mechanisms of action, structural modifications, and structure-activity relationships of piperine and its derivatives.
1. Introduction
Natural alkaloids are a huge library of promising lead compounds, exhibiting a variety of biological activities, which can provide multiple strategies for the development of drugs and pesticides. For instance, camptothecin, a well-known antitumor agent first isolated from Camptotheca acuminata Decne. in the mid-1960s, belongs to quinoline alkaloids [1,2]. The botanical products were utilized as pesticides to prevent pests in ancient China as early as the 7–5th centuries BC [3]. Nowadays, industrial chemical pesticides still occupy the dominant position of pesticides, but their high toxicity and environmental pollution have aroused many controversies among the public worldwide. Thus, plant secondary metabolites have become a major focus of research due to their potential application in developing new pesticides. Since 1985, neem-based insecticide Margosan-O, the first commercial pesticide with azadirachtin as the main ingredient, has been approved for registration in the United States, which triggered global enthusiasm for research on natural-based products from plants [4,5]. Plant-derived pesticides usually have the characteristics that their constituents are formed over the long period evolution process in nature and possess an integrated degradation mechanism. Due to their unique properties, they are difficult to accumulate and do not easily harm crops or promote resistance. It is estimated that more than 400,000 species of bioactive compounds in plants have been found, nevertheless, only 10% of the plant species have been investigated. Therefore, plant-derived products have a wide application prospect in the field of agriculture for the management of pests [6].
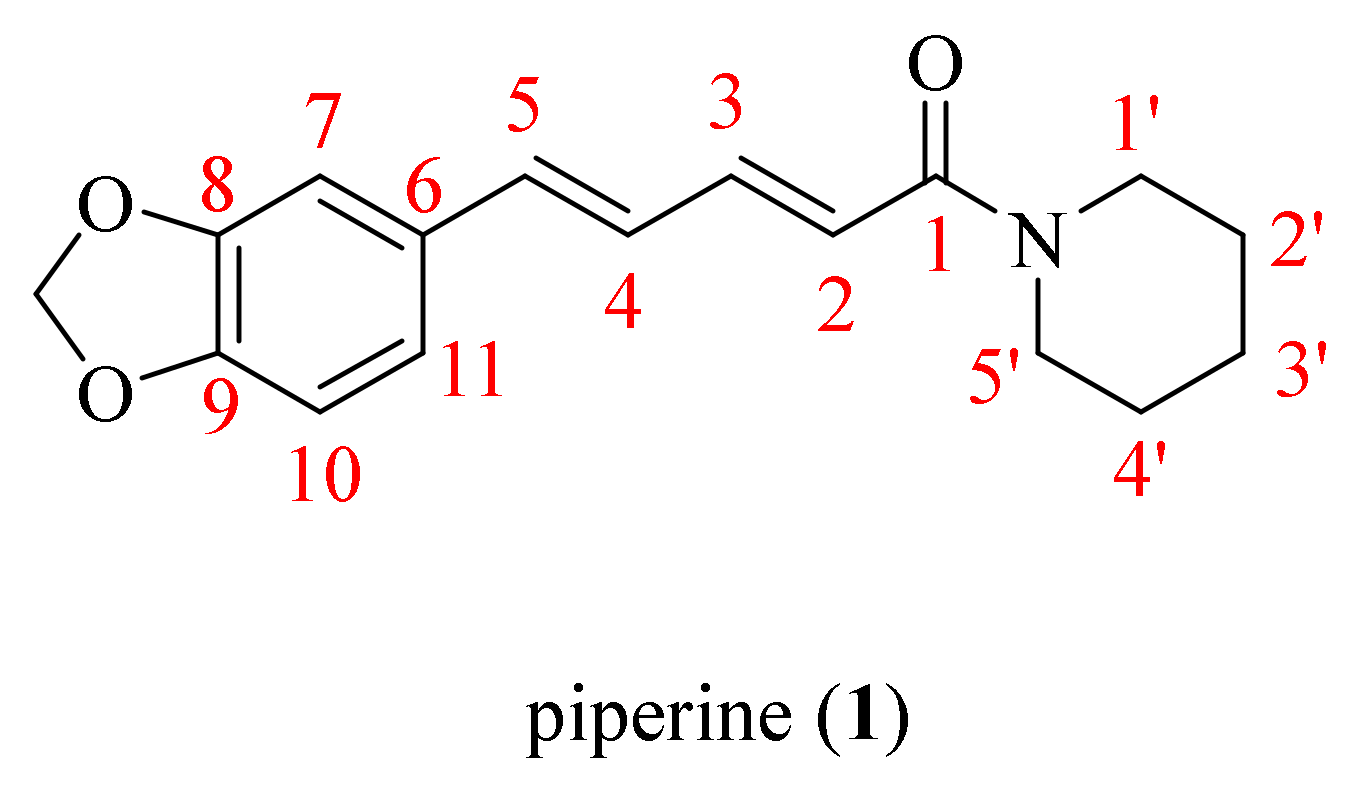
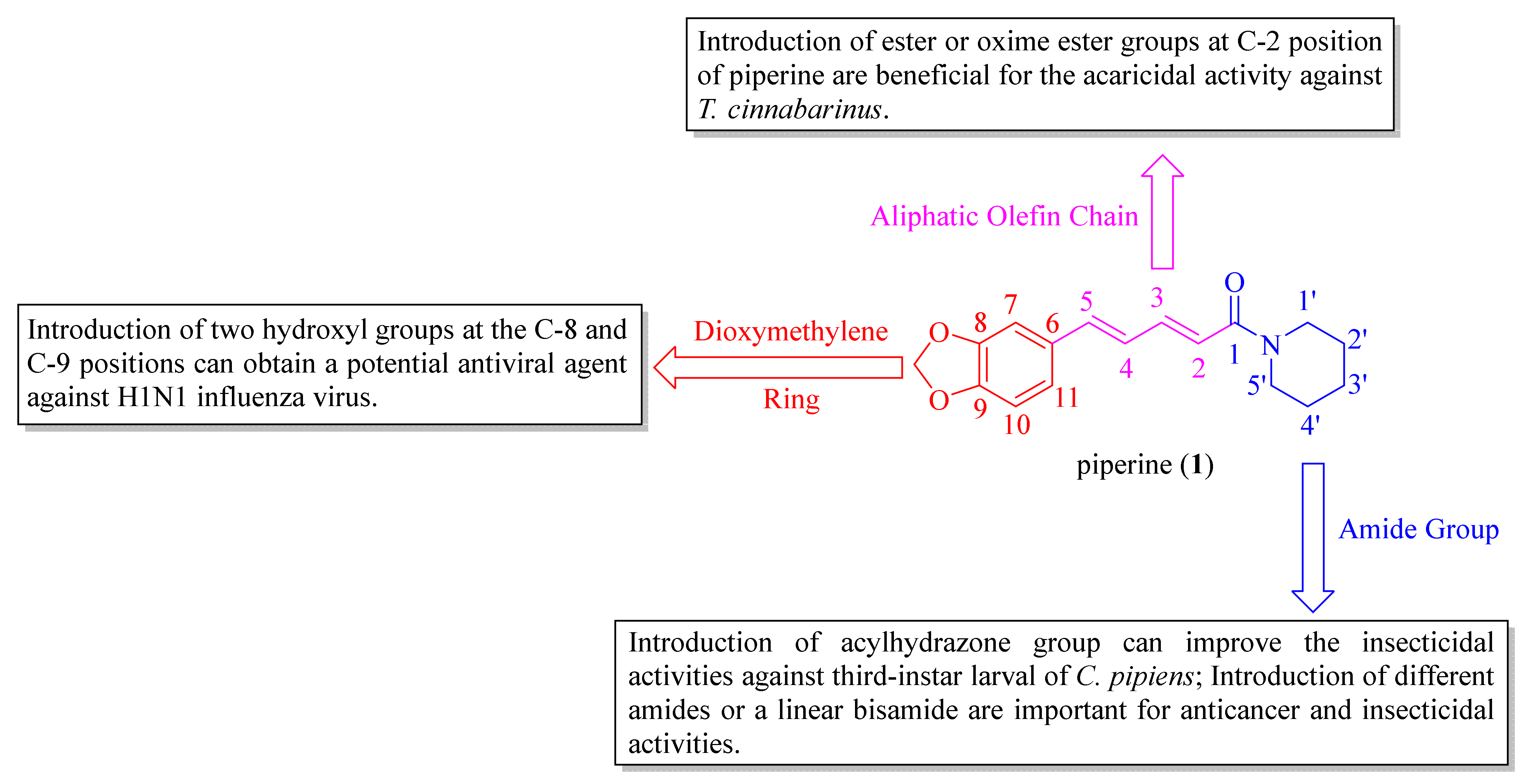
Black pepper (Piper nigrum L.), a family member of Piperaceae, is considered one of the most valuable spices and a potential candidate in natural product research, widely cultivated in lots of tropical and subtropical districts [7,8]. In addition, P. nigrum is acknowledged as the king of spices and consists of diverse bioactive compounds, such as sterols, fatty acids, terpenes, amides, and other phytoconstituents with the characteristics of various biological activities [9]. Piperine (1, Figure 1) is one of the major alkaloids isolated from the black pepper, which also occurs in the ripe fruits of Piper longum L. and the roots of Piper sarmentosum Roxb. [10,11]. The pepper processing industry participates in a huge number of various wastes that contain lots of piperine. To effectively utilize the by-products, people investigated the conditions of pressure and temperature on the method of supercritical CO2 (SC-CO2) extraction for two steps (100 bar, 60 °C for 1 h and 300 bar, 60 °C for 2 h) to obtain piperamide piperine in a gentle and efficient way [12]. A recent study reported that PFPE-CH, a dietary supplement made by mixing a low piperine fractional Piper nigrum extract (PFPE) with cold-pressed coconut oil and honey in distilled water, can help decrease the risk of tumor formation and reduce the side effects of chemotherapeutic drugs during breast cancer treatment. This is promising news for those undergoing treatment for breast cancer [13]. In the early 1970s, a piperine analogue Ilepcimide was originally extracted from a Chinese folk remedy, which was discovered as a safe and effective antiepileptic drug through structural characterization. In addition, the hospital of Peking University (Beijing, China) has been clinically implemented in more than 100,000 patients, and the results showed that the effective rate of Ilepcimide was 95.6% [14,15]. By 2020, the actual cultivated area of pepper in Yunnan Province had reached 39.4 square kilometers, the yield was 1070.8 tons, and the output values had achieved 42.8 million RMB [16]. The piperine molecular contains a dioxymethylene ring, an aliphatic olefin chain, and an amide group. Piperine and its derivatives possess many biological activities, including anticancer [17], antitumor [18], antiviral [19], anti-inflammatory [20], antimalarial [21], antioxidant [22,23], anti-Alzheimer’s disease [24,25,26], and anti-Parkinson disease properties [27]. The characteristics of the structure of piperine allow for modification at multiple sites so as to obtain more candidates with better activities, which are widely used in the agricultural field. For instance, Zhang et al. synthesized a series of bisamide-type piperine derivatives and evaluated them for insecticidal activity against Plutella xylostella (L.). The study results revealed that most of the desired compounds displayed better insecticidal activity compared to piperine. In particular, the molecular docking results indicated that a synthetic compound could act on γ-aminobutyric acid receptors [28]. Recently, our studies synthesized two novel series of ester and oxime ester piperine-type derivatives, some compounds displayed more than 100 times the acaricidal activity of piperine against Tetranychus cinnabarinus (Bois.). Moreover, the scanning electron microscope (SEM) demonstrated that their potential acaricidal activities may be related to the destruction of the construction of the cuticle layer crest of T. cinnabarinus [29,30]. Wang et al. found that the ester derivative prepared from piperine exhibited particularly significant broad-spectrum fungicidal activity against Rhizoctonia solani Kühn, Fusarium graminearum Schwabe, Alternaria tenuis Nees, Gloeosporium theae-sinensis I. Miyake, Phytophthora capsica Leonian, and Phomopsis adianticola [31].
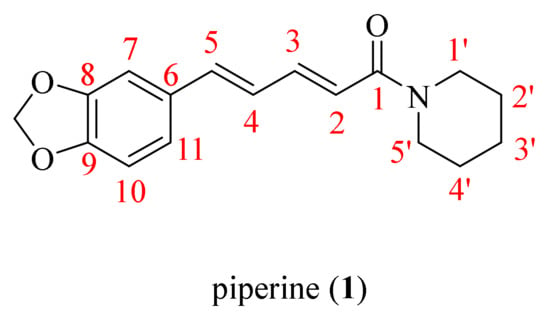
Figure 1.
The chemical structure of piperine (1).
Generally, with the characteristics of easy modification and lots of bioactivities, piperine and its derivatives have received much attention in the fields of synthetic organic chemistry and medicinal chemistry. In this review, many works concerning piperine and its derivatives have been conducted, we widely summarized the advances in the construction and structural modification of piperine and its derivatives from 2020 to 2023. Meanwhile, the latest biological activities, mechanisms of action, and structure-activity relationships of piperine and its derivatives are also presented.
2. Bioactivities of Piperine and Its Derivatives
2.1. Anticancer Activity
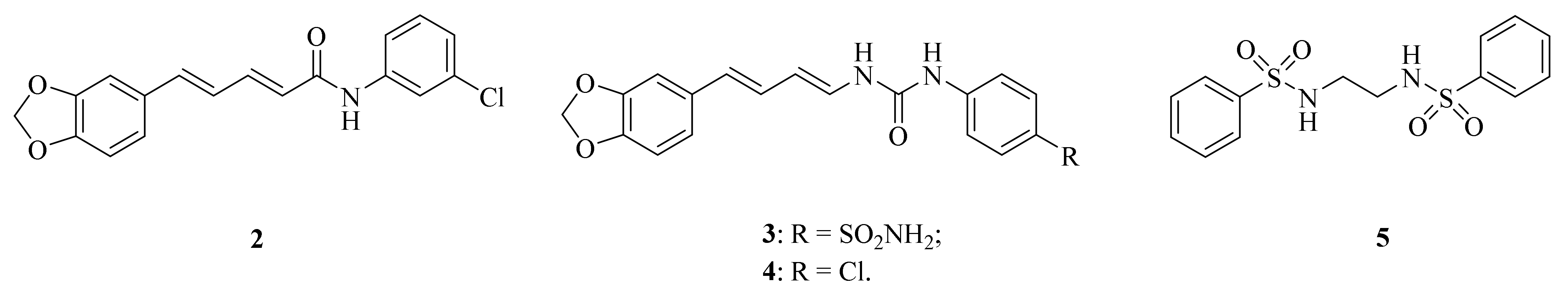
Cancer still poses the biggest danger to human health. For example, breast cancer is an increasing global health challenge. According to the World Health Organization (WHO), in 2020, approximately 2.3 million women were diagnosed with breast cancer and there were 685,000 deaths reported worldwide [32]. Rajarajan et al. reported that the dietary piperine represented a potential candidate that could suppress obesity-associated breast cancer growth and metastasis by regulating the miR-181c-3p/PPARα Axis [33]. Furthermore, Jeong et al. also found that piperine has anticancer properties such as growth inhibition, anti-migration, and anti-invasion of cancer cells [34]. In order to application of piperine derivatives as more potential anticancer drugs, structural modification has received much attention. For example, compound 2 (Figure 2) was evaluated for cytotoxicity and inhibition of TrxR (thioredoxin reductase) activity, paving the way for further exploration of piperine derivatives as TrxR inhibitors [35]. Elimam et al. examined the piperine-based sulfonamide 3 (Figure 2) for its anticancer activity towards the breast MCF-7 cancer cell line. The results indicated that it is a promising lead compound for developing efficient anticancer candidates with potent carbonic anhydrase (CA) inhibitory activity [36]. In addition, to validate the in silico results against triple-negative breast cancer (TNBC) MDA-MB-231, an in vitro vascular endothelial growth factor receptor-2 (VEGFR-2) inhibition assay was conducted. Compound 4 (Figure 2) exhibited powerful inhibitory activity with a half-maximal inhibitory concentration (IC50) value of 231 nM [37]. Pandya et al. found that piperine analogue 5 (Figure 2) may serve as an anticancer therapeutic seeing it affects c-myc oncogene expression via G-quadruplex mediated mechanism [38].
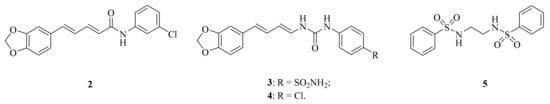
Figure 2.
The chemical structures of compounds 2–5.
2.2. Antiviral Activity
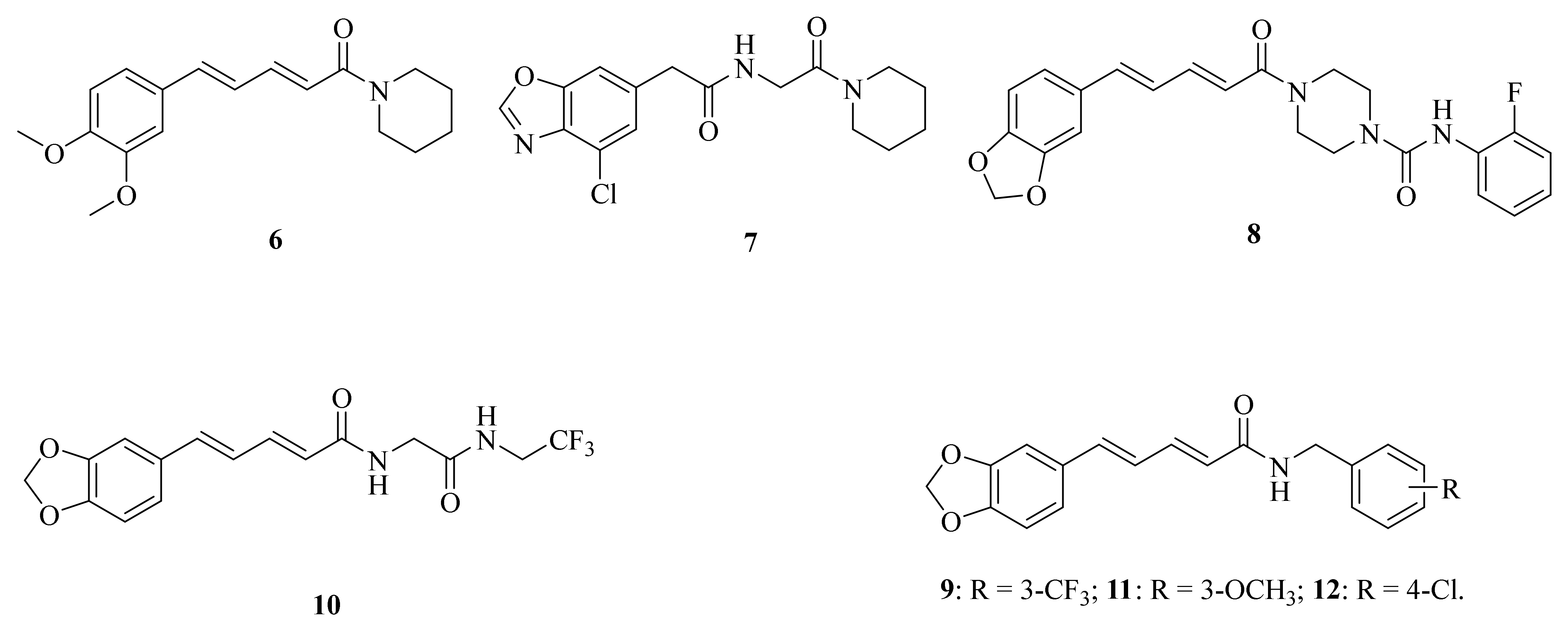
The respiratory illness caused by the pandemic H1N1 influenza virus has become a significant global health concern. Mohammed et al. conducted a study in which they screened a piperine derivative 6 (Figure 3) for its antiviral activity and compared it with other strains in vitro on the MDCK cell line. The adsorption assay demonstrated a dose-dependent reduction of viral plaque with an EC50 value of 0.33 μM [39]. Over the past five years, the COVID-19 (SARS-CoV-2) epidemic has spread around the world, bringing immeasurable damage to people’s lives and health. However, some potential molecules showed promising activities against coronavirus, a published research illustrated that the plant alkaloid piperine could inhibit the vesicle fusion mediated by SARS-CoV-2 peptides and reduce the titer of SARS-CoV-2 progeny in vitro in Vero cells [40]. In addition, largemouth bass virus (LMBV) is a systemic viral pathogen that affects cultivated largemouth bass, causing high mortality rates. Wang et al. investigated the antiviral activity of piperine against LMBV in vitro and in vivo, and the results showed that piperine could act as a therapeutic and preventative drug for further study against LMBV infection [41].
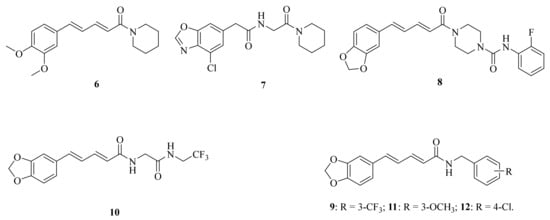
Figure 3.
The chemical structures of compounds 6–12.
2.3. Anti-Inflammatory Activity
Ultraviolet (UV) rays from sunlight are one of many environmental insults that harm the skin. Jaisin et al. discovered that piperine may effectively treat skin inflammation caused by UV irradiation [42]. Acute pancreatitis (AP) is a common acute abdominal disease characterized by pancreatic acinar cell death and inflammation. Huang et al. confirmed that piperine can target the endoplasmic reticulum autophagy (ER-phagy), providing a new insight into the pharmacological mechanism of piperine in treating AP [43]. Psoriasis is a common chronic inflammatory skin disease that is prone to relapse and difficult to cure. Studies have shown that piperine can help alleviate psoriasis by reducing epidermal hyperplasia, inflammatory cell infiltration, and the expression of psoriasis-characteristic cytokines, chemokines, and proteins in IMQ-induced psoriasiform dermatitis. Therefore, piperine has the potential to become an effective agent for psoriasis and may provide new strategies for clinical intervention [44]. Additionally, sciatica is a kind of combined pain caused by stimulation and compression of various factors, resulting in stabbing, burning, and dull pain along the route of the sciatic nerve and in the surrounding areas, which brings great physical and psychological pain to patients. Wang et al. confirmed the regulatory relationship between miR-520a and p65. In addition, they investigated the impact of miR-520a/P65 on cytokine levels following piperine stimulation to determine its therapeutic role in sciatica. As a result, they found that piperine can help alleviate pain. Specifically, piperine can promote the expression of miR-520a, which directly targets and inhibits the expression of P65, down-regulate pro-inflammatory factors IL-1β and TNF-α, while up-regulating the effects of anti-inflammatory factors IL-10 and TGF-β1. As a result, piperine may as a treatment for sciatica [45]. Duan et al. suggested that piperine may have anti-inflammatory properties by regulating the key factors of the NF-κB and MAPK signaling pathways [46]. Yang et al. developed a novel piperine derivative 7 (Figure 3) and demonstrated that it exhibited potent therapeutic effects against neuroinflammation, which might be partly attributed to its inhibitory activity on kelch-like ECH-associated protein (Keap1)-nuclear factor erythroid-2-related factor 2 (Nrf2) protein-protein interaction [47]. Tian et al. synthesized a potent inhibitor 8 (Figure 3) of fatty acid amide hydrolase (FAAH) with an IC50 value of 0.65 μM. And the inhibitor was found to attenuate the lipopolysaccharide (LPS)-induced activation of BV2 cells, showing a significant anti-inflammatory activity [48].
2.4. Insecticidal Activity
In 2022, Oliveira et al. found that piperine loaded into nanostructured systems might be an effective drug to improve larvicidal activity against Aedes aegypti L. [49]. Yang et al. identified that compound 9 (Figure 3) was the most effective multichitinase inhibitor and exhibited higher insecticidal activity against Ostrinia furnacalis (Guenée) (Asian corn borer) than dual- or single-chitinase inhibitors. Results of molecular mechanism studies showed that compound 9 can interact with two conserved TRP and TYR of three chitinases in identical ways through hydrogen bonds, hydrophobic, and π-π interactions. Furthermore, the microinjection experiment revealed that the agent displayed significant sublethal effects against O. furnacalis by regulating its growth and development [50]. Zhang et al. synthesized a series of piperine derivates containing a linear bisamide. Among them, compound 10 (Figure 3) gave rise to 90% mortality at 1.0 mg/mL concentration against P. xylostella [28]. In order to investigate the effects of potential trehalase inhibitors in Spodoptera frugiperda (Smith), compounds 11 (Figure 3) and 12 (Figure 3) were synthesized by Zhong et al. The results showed that compound 12 significantly reduced trehalase activity. Additionally, compound 11 not only can reduce membraned-bound trehalase activity, but also inhibit the expression of SfTRE2, SfCHS2, and SfCHT, thus affecting the chitin metabolism, providing a theoretical basis for the application of trehalase inhibitors in the control of agricultural pests [51].
2.5. Antibacterial and Antifungal Activities
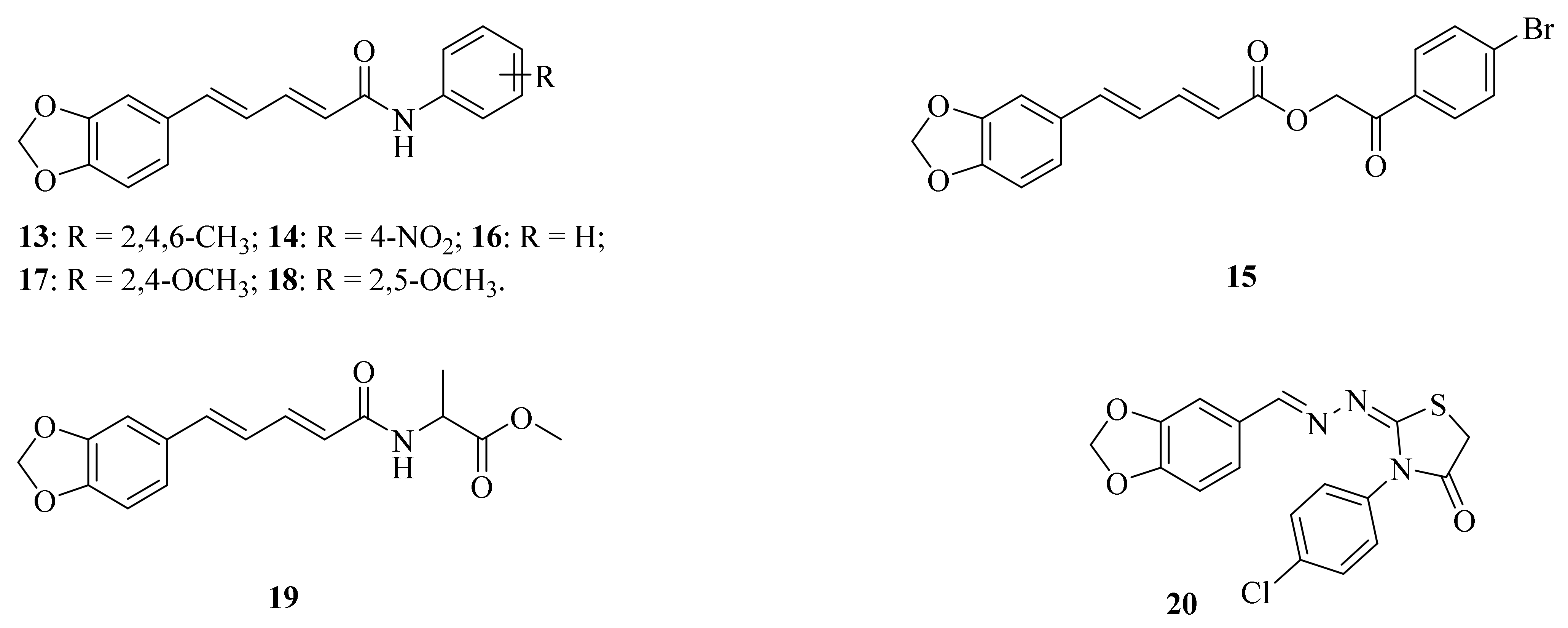
A series of piperine amide derivatives were synthesized by Sivashanmugam et al. Of all the derivates, compounds 13 (Figure 4) and 14 (Figure 4) worked exceptionally well against Gram-negative bacteria (Escherichia coli and Acinetobacter baumannii) and Gram-positive bacteria (Staphylococcus aureus, Escherichia faecalis, and Staphylococcus epidermidis) [52]. Das et al. found that piperine exhibited potent antibiofilm activity against Pseudomonas aeruginosa by accumulating reactive oxygen species, affecting cell surface hydrophobicity, and quorum sensing. This research suggested that piperine can effectively disrupt the biofilm formation of P. aeruginosa, offering a sustainable solution for protecting public health [53]. The emergence and rapid spread of multidrug-resistant (MDR) bacteria, such as Vibrio cholerae, is a major global public health concern. Piperine has been found to show a dose-dependent bactericidal effect on V. cholerae growth, regardless of their biotypes and serogroups at the concentrations of 200 and 300 μg/mL, respectively. It also can inhibit the growth of multidrug-resistant (MDR) strains of P. aeruginosa and E. coli isolated from poultry, and enterohemorrhagic/enteroaggregative E. coli O104 in 200 μg/mL. The results demonstrated that piperine has antimicrobial properties against pathogenic bacteria, including MDR strains, making it a potential therapeutic and preventative agent against infections [54]. Souza, Jr. et al. synthesized the compound 15 (Figure 4) and evaluated its antifungal activity against Candida, Trichophyton, and Microsporum strains. As a result, compound 15 exhibited 70% inhibition in seven tested strains, such as Candida albicans ATCC 76645, LM-111, LM-122 and Candida krusei LM-656, LM-13 and Microsporum canis LM-12, and Microsporum gypseum LM-512, with a minimum inhibitory concentration (MIC) ranging from 1.23–2.46 μmol/mL and a minimum fungicide concentration (MFC) ranging from 9.84–19.68 μmol/mL [55].
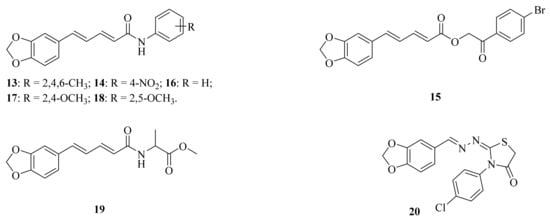
Figure 4.
The chemical structures of compounds 13–20.
2.6. Other Activities
A series of N-aryl amide derivatives of piperine (16–18, Figure 4) were prepared by semi-synthesis, and these compounds were examined for their antitrypanosomal, antimalarial, and anti-SARS-CoV-2 main protease activities. Among them, compound 18 exhibited the most robust biological activities with no cytotoxicity against mammalian cell lines Vero and Vero E6, its IC50 values for antitrypanosomal activity against Trypanosoma brucei rhodesiense was 15.46 ± 3.09 μM, and its antimalarial activity against the 3D7 strain of Plasmodium falciparum was 24.55 ± 1.91 μM, which were 4-fold and 5-fold higher than that of piperine, respectively. Furthermore, compound 18 inhibited the activity of 3C-like main protease (3CLPro) toward anti-SARS-CoV-2 activity with the IC50 value of 106.9 ± 1.2 μM. Docking and molecular dynamic simulation indicated that the potential binding of compound 18 in the 3CLpro active site had improved binding interaction and stability [21]. Peroxisome proliferator-activated receptor γ (PPARγ) plays a key role in glucose, which is a ligand-mediated transcription factor. The lipid homeostasis always serves as a pharmacological target for new drug discovery and development. Wang et al. synthesized and evaluated some compounds for their agonistic activities of PPARγ. In particular, compound 19 (Figure 4) presented itself as a potential PPARγ agonist with an IC50 value of 2.43 μM, and the molecular docking studies indicated that compound 19 stably interacts with the amino acid residues of the PPARγ complex active site [56]. Piperine derivate 20 (Figure 4) containing benzodioxole molecule was identified as the promising antiparasitic candidate against Leishmania amazonensis [57]. Hsieh et al. investigated the protective effects of piperine on nerve growth factor (NGF) signaling in a kainic acid (KA) rat model of excitotoxicity. The results showed that piperine can protect hippocampal neurons against KA-induced excitotoxicity by enhancing the NGF/TrkA/Akt/GSK3β signaling pathways [58]. Alsareii et al. conducted an in vivo study and histopathological examination, which revealed early and intrinsic healing of wounds with the piperine-containing bioactive hydrogel system compared to the bioactive hydrogel system without piperine. These findings established that the piperine-containing bioactive hydrogel system is a promising therapeutic approach for wound healing applications [59].
3. Structural Modifications of Piperine and Its Derivatives
3.1. Structural Modifications at the Dioxymethylene Ring or the Aliphatic Olefin Chain of Piperine
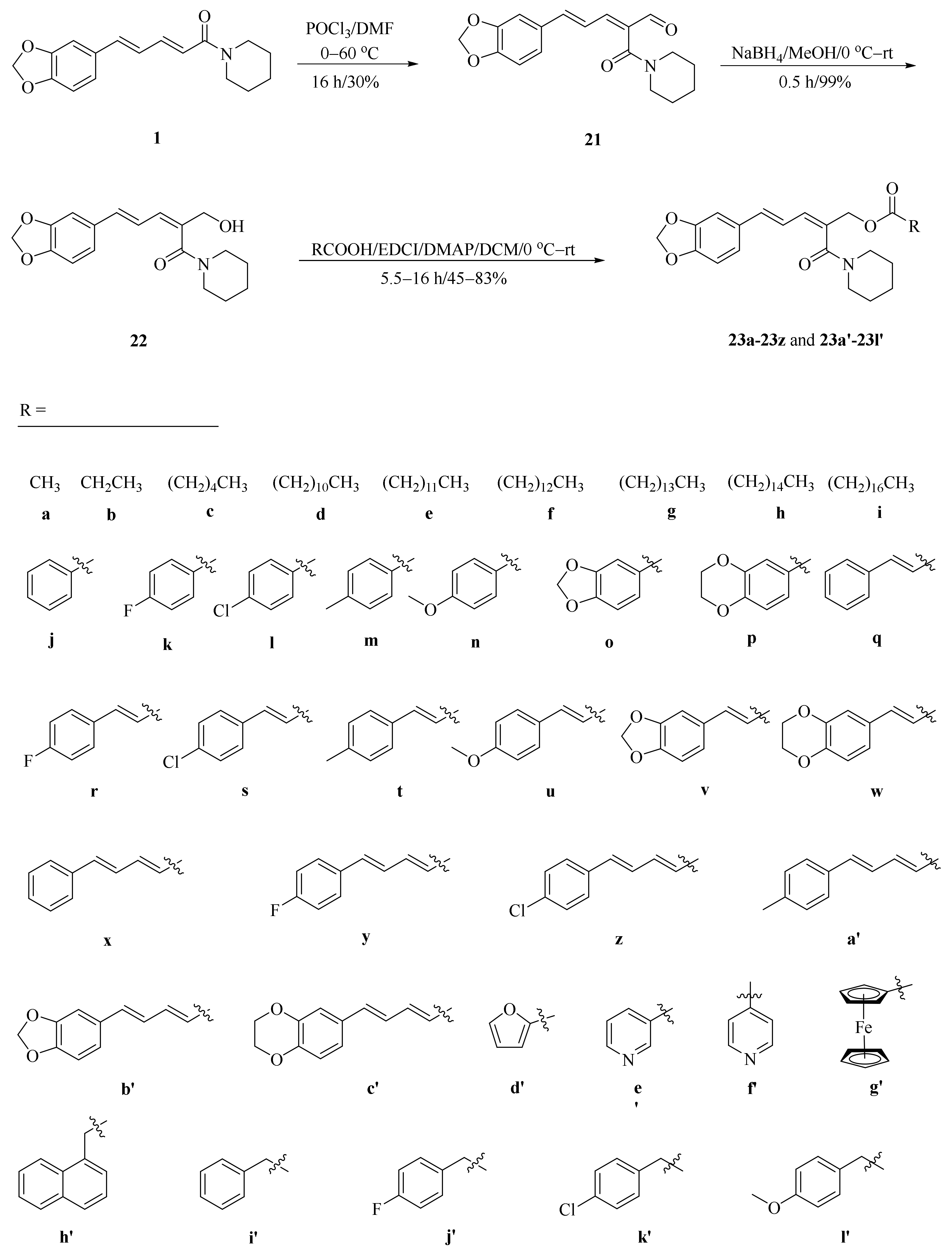
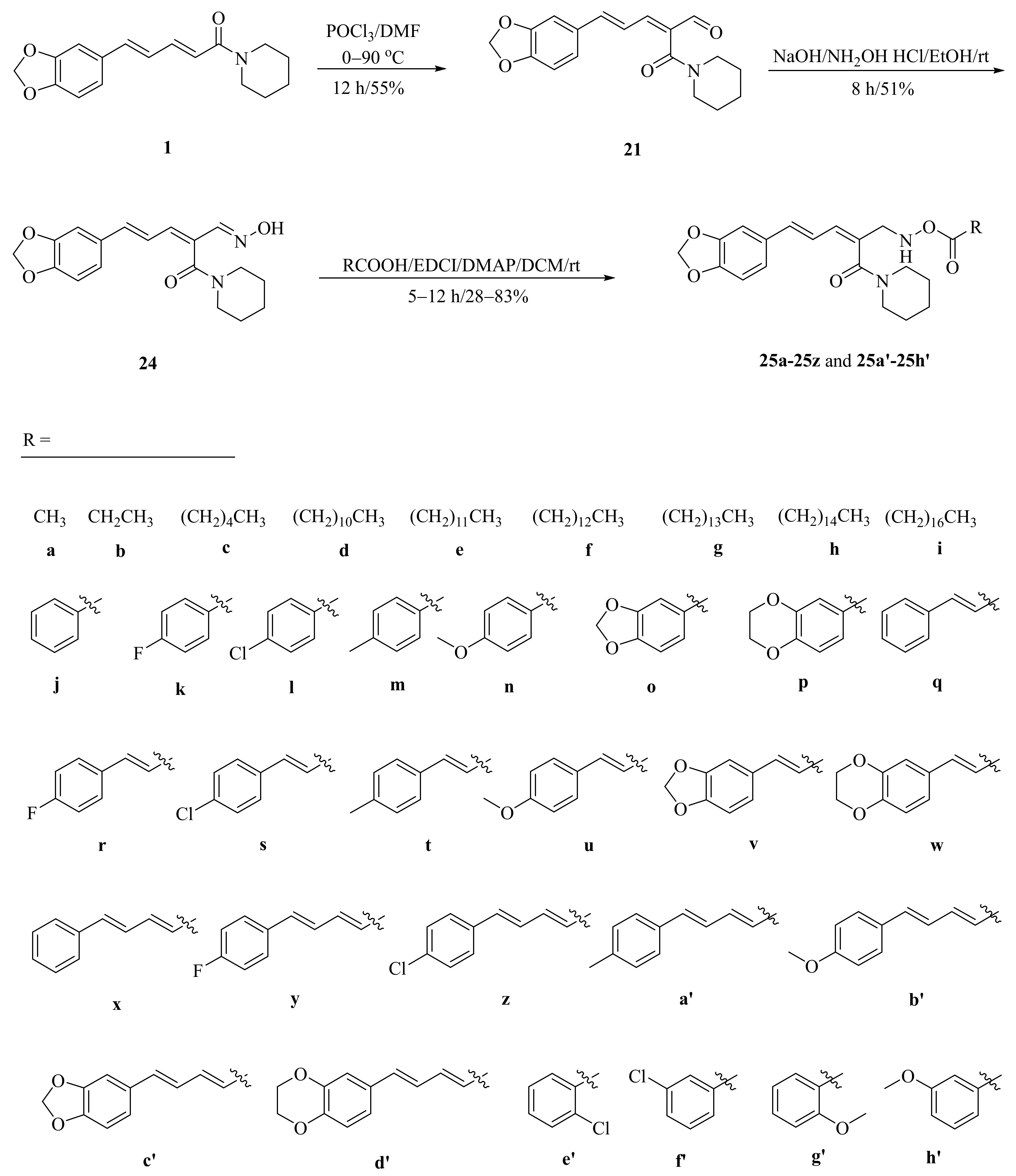
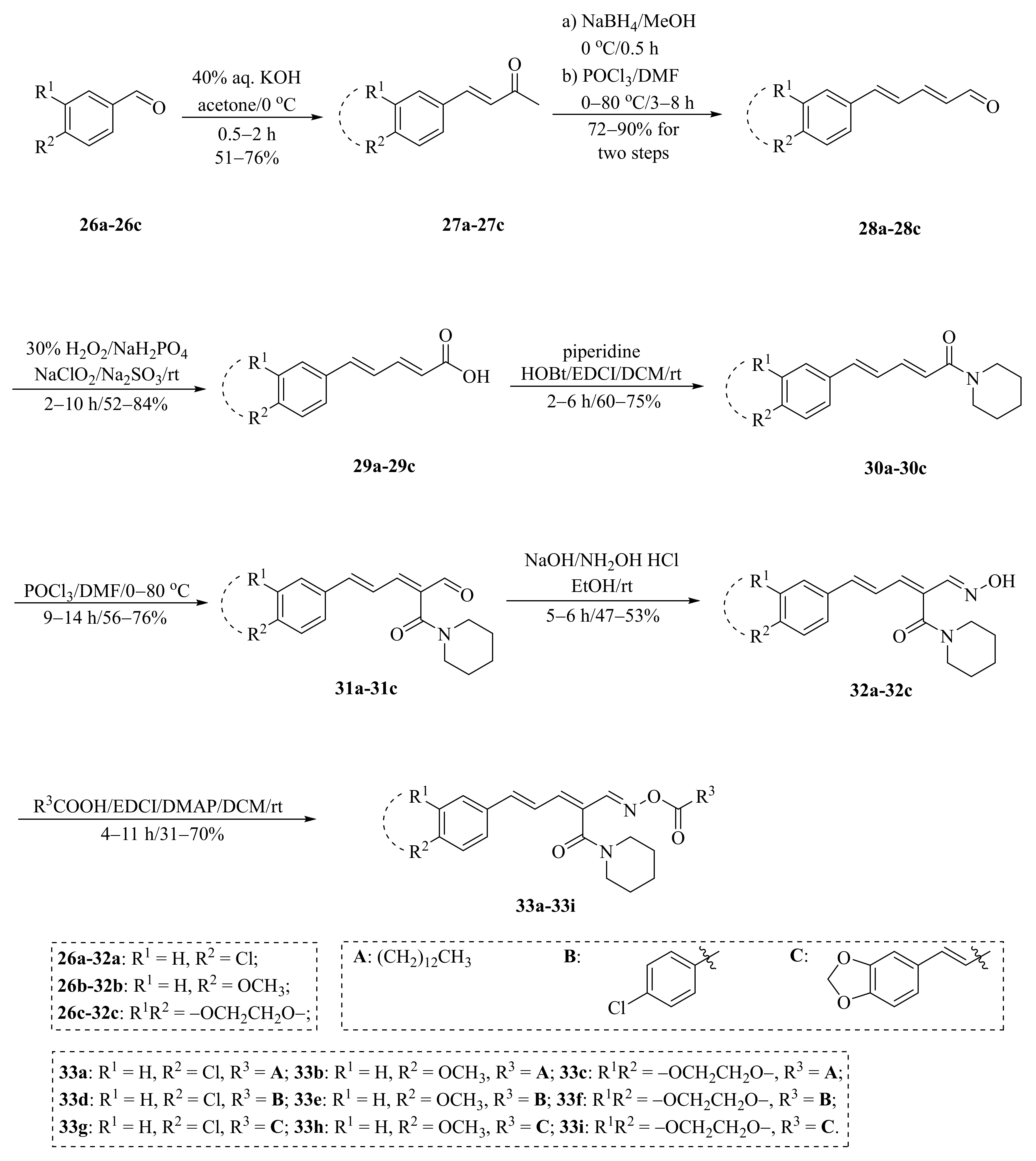
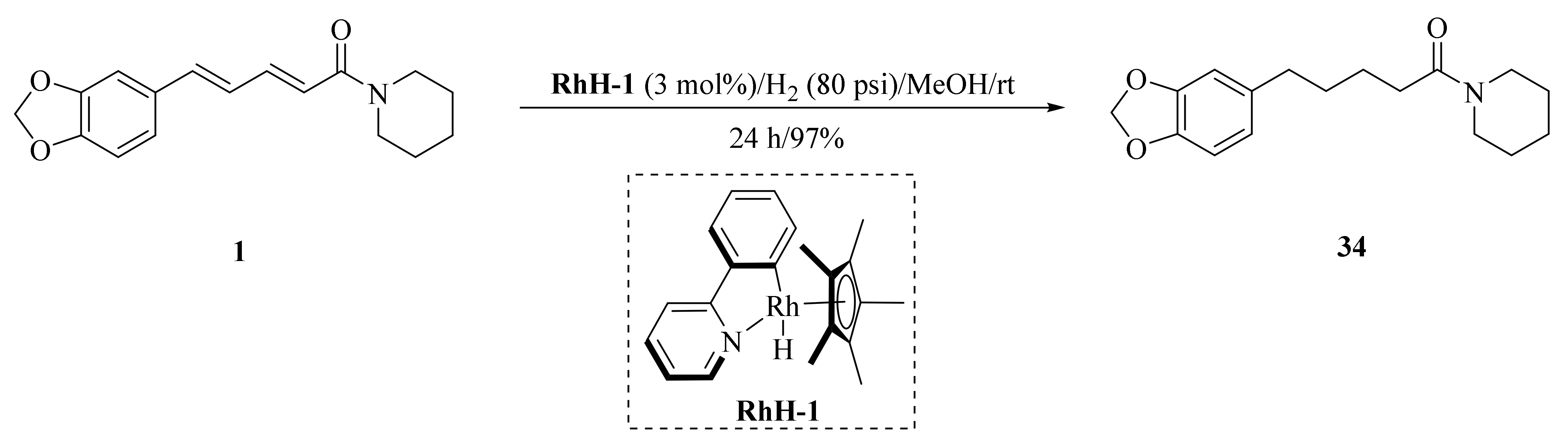
As described in Scheme 1 and Scheme 2, Li et al. and Lv et al. regio- and stereo-selectively synthesized a series of piperine-type ester derivates 23a–23z and 23a’–23l’, and oxime ester derivates 25a–25z and 25a’–25h’, respectively, by using the Vilsmeier–Haack–Arnold (VHA) reaction. Meanwhile, as shown in Scheme 3, by changing the substituent groups of dioxymethylene ring, compounds 33a–33i were also prepared by Lv et al. All the synthetic compounds were evaluated for their acaricidal activities against T. cinnabarinus, among them, compounds 23e, 23f, 23u, 23v, 25f, 25l, 25u, and 25v showed significant acaricidal activities with the LC50 values ranging from 0.12 to 0.19 mg/mL (Table 1), which were comparable to that of the commercial acaricidal agent spirodiclofen (LC50: 0.12 mg/mL), and displayed almost 100-fold higher acaricidal activities than piperine (LC50: 14.20 mg/mL) [28,29,60]. As shown in Scheme 4, Gu et al. used the catalyst RhH-1 to reduce the aliphatic olefin chain of piperine under a mild condition with a high yield to obtain analogue 34 [61].
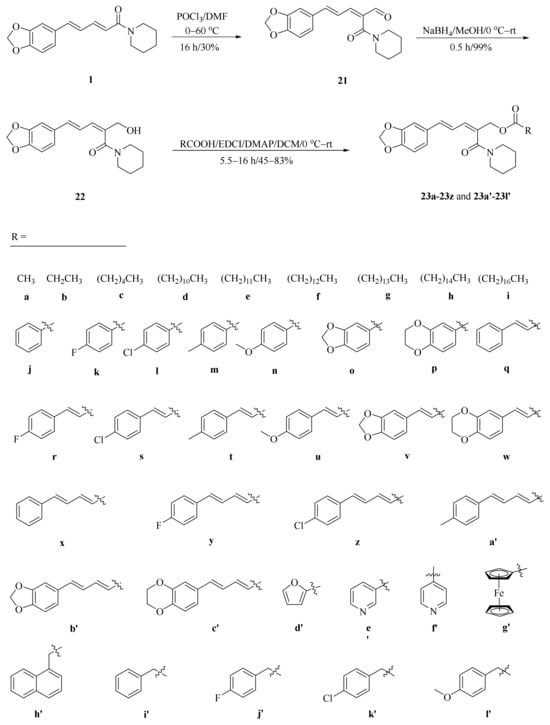
Scheme 1.
Synthesis of piperine ester derivates 23a–23z and 23a’–23l’.
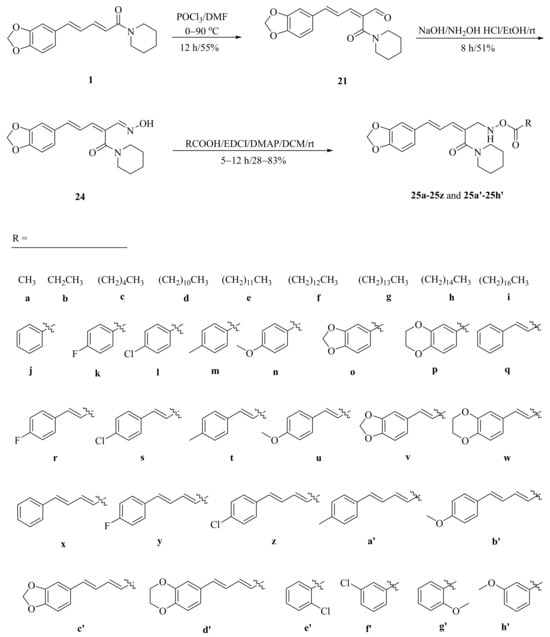
Scheme 2.
Synthesis of piperine oxime ester derivates 25a–25z and 25a’–25h’.
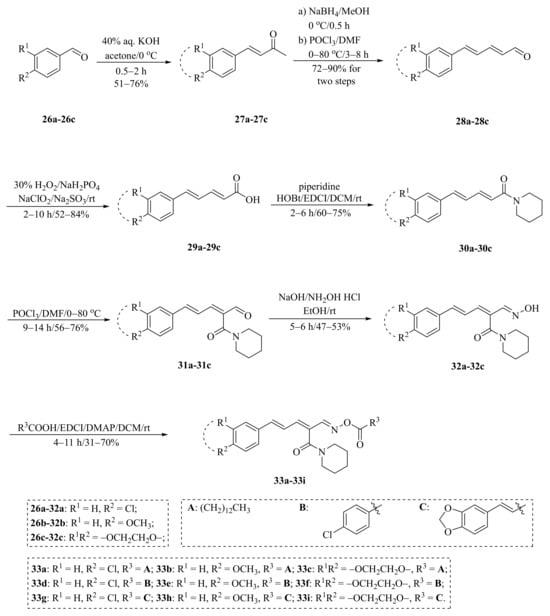
Scheme 3.
Synthesis of piperine analogs type oxime ester derivates 33a–33i.

Table 1.
LC50 values of some potent compounds against T. cinnabarinus at 72 h.
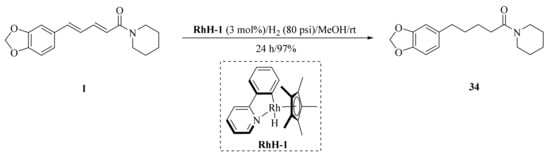
Scheme 4.
Synthesis of piperine analogue 34.
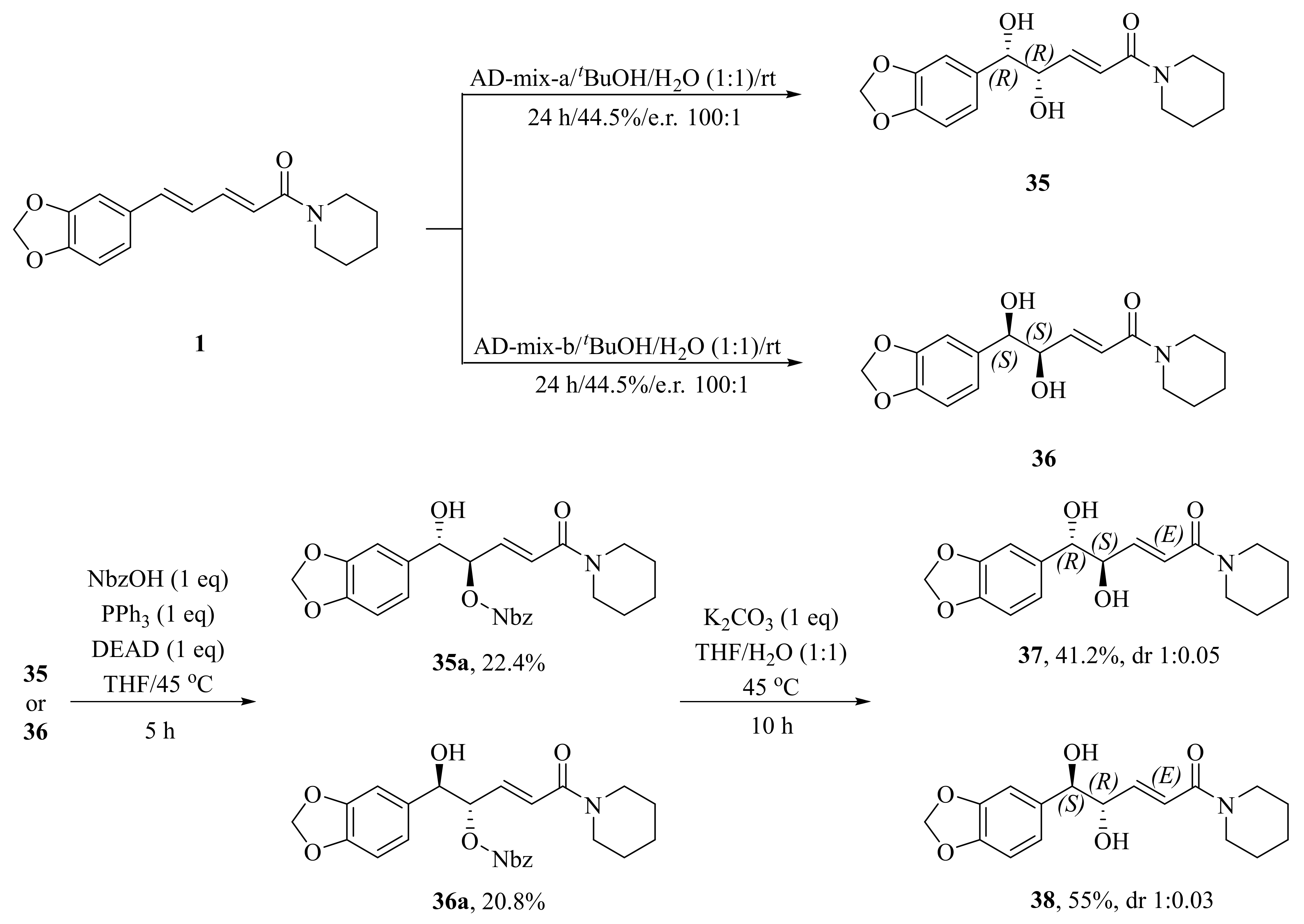
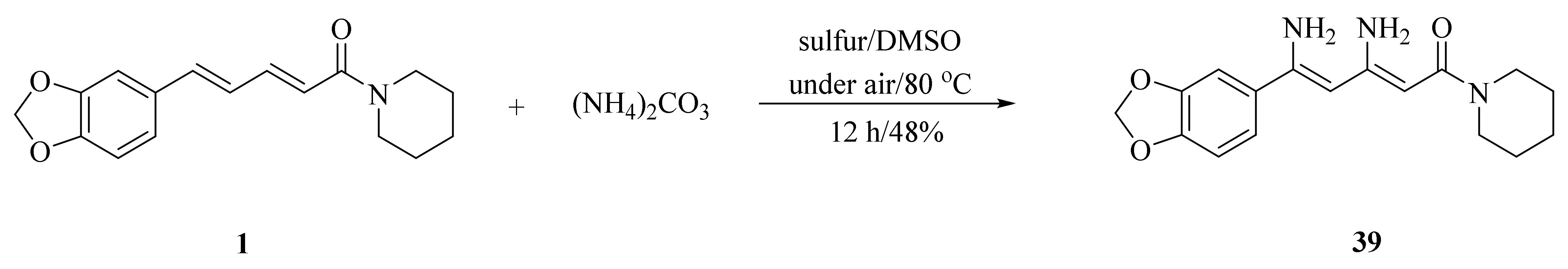
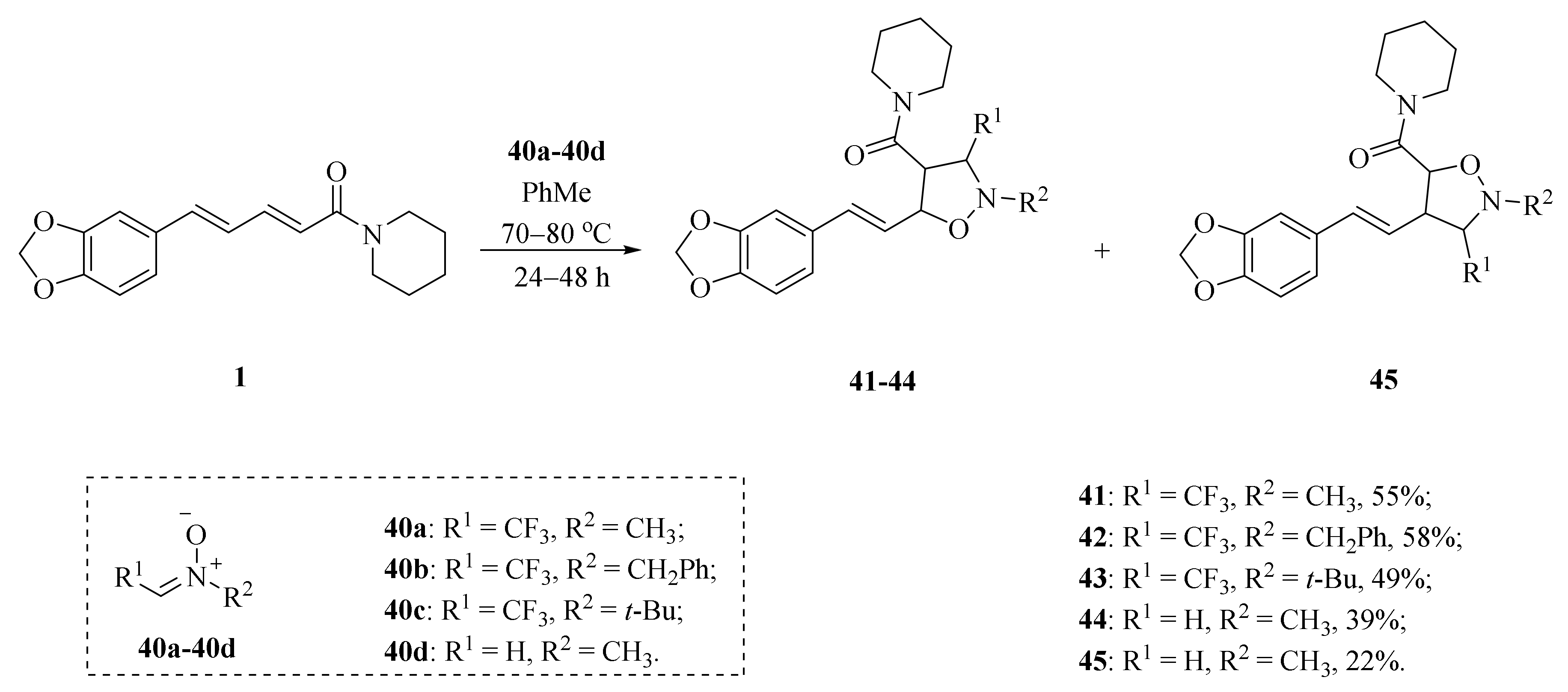
As illustrated in Scheme 5, Luo et al. synthesized four piperine derivates 35 (4R,5R), 36 (4S,5S), 37 (4S,5R), and 38 (4R,5S), which were established by NMR, optical rotation, and CD spectra [62]. As demonstrated in Scheme 6, the sample amine groups were introduced at the aliphatic olefin chain of piperine through a trisulfur-radical-anion-triggered C(sp2)-H amination of α,β-unsaturated carbonyl to obtain derivate 39 [63]. As presented in Scheme 7, Wojtowicz-Rajchel et al. synthesized five novel corresponding cycloadducts 41–45 in moderate yields by the reaction of prochiral N-alkyltrifluoromethyl-methylene nitrones with piperine under non-catalyzed condition [64].
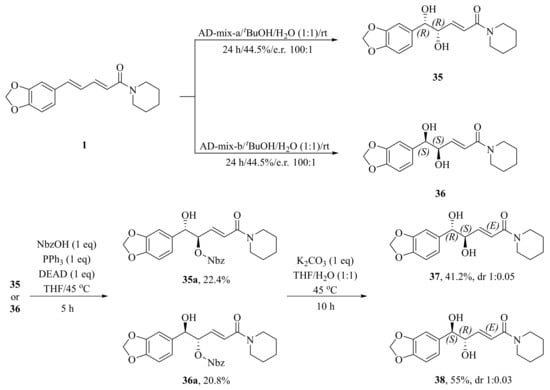
Scheme 5.
Synthesis of piperine derivates 35–38.
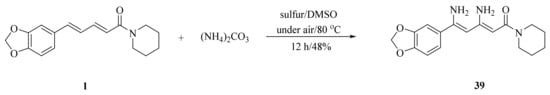
Scheme 6.
Synthesis of piperine derivate 39.
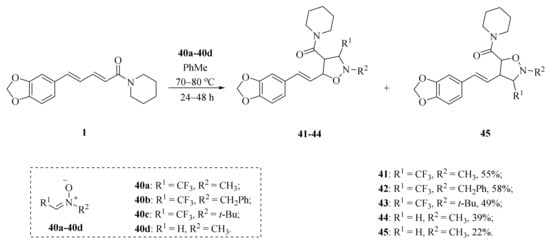
Scheme 7.
Synthesis of piperine derivates 41–45.
3.2. Structural Modifications at the Amide Group of Piperine
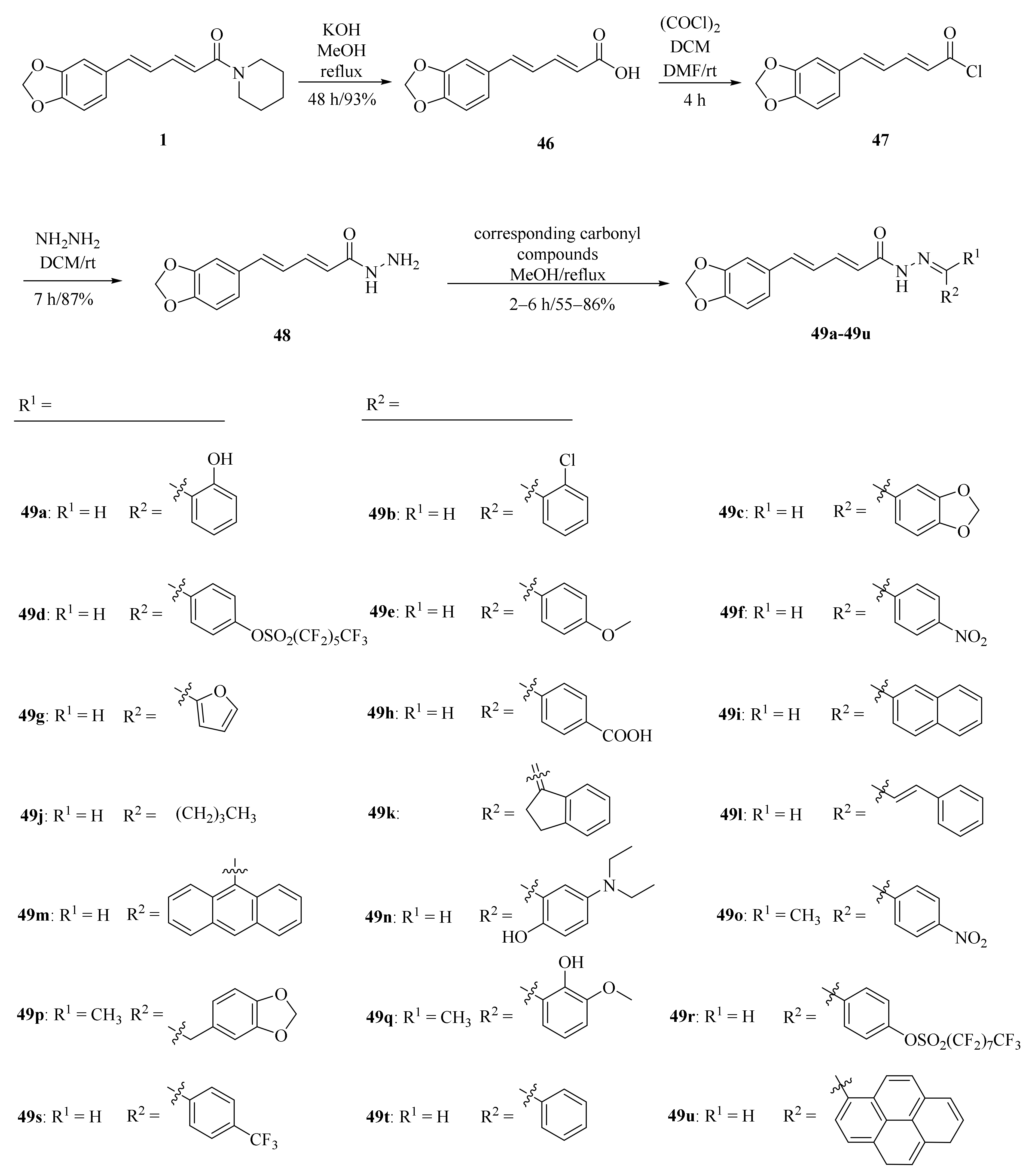
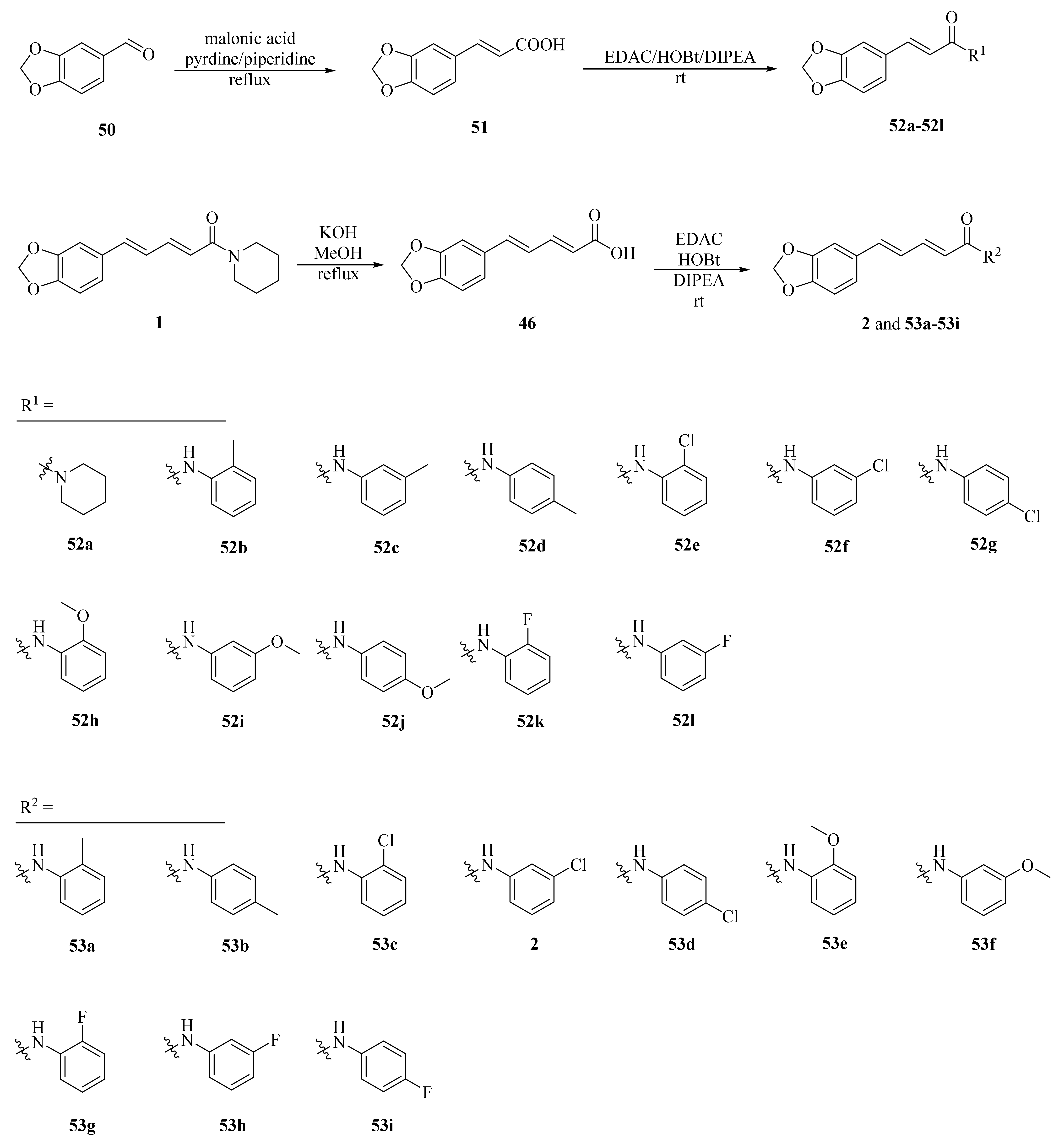
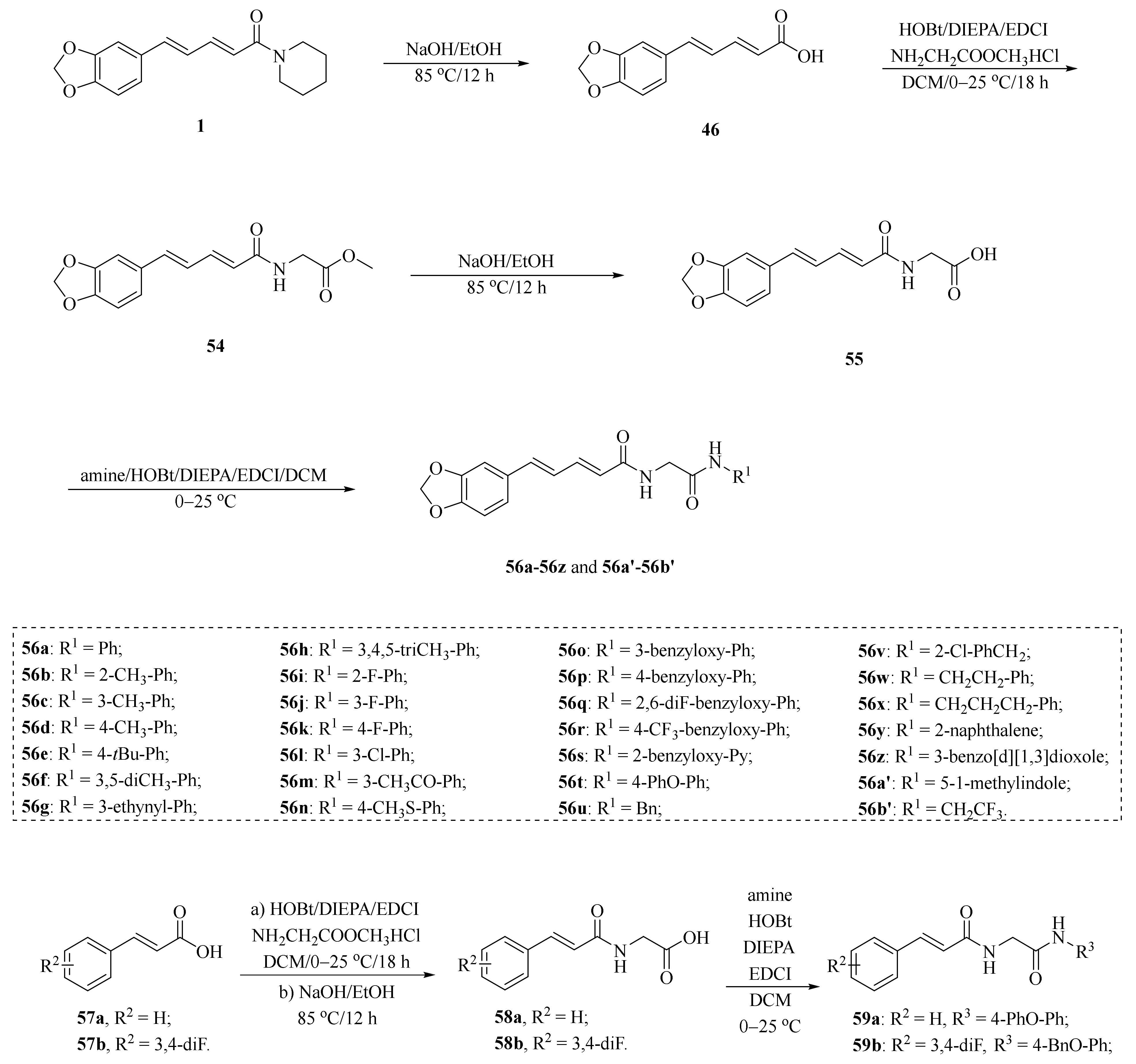
As shown in Scheme 8 and Table 2, Tantawy et al. designed and synthesized a series of piperine-based dienehydrazide derivatives 49a–49u and evaluated their insecticidal activities against third-instar larval of Culex pipiens L. Among all synthetic derivates, compounds 49a, 49b, 49f, 49g, 49m, 49n, 49o, 49p, and 49u displayed better activities than piperine and deltamethrin (a commercial positive control). Molecular modeling revealed several interactions between derivates and acetylcholinesterase (AChE) substrate binding sites responsible for binding and inhibition [65]. As shown in Scheme 9, twenty-two piperine derivates 2, 52a–52l, and 53a–53i were synthesized by Zhong et al. and evaluated for their anticancer properties [34]. As shown in Scheme 10, a series of novel piperine derivates 56a–56z, 56a’–56b’, and 59a–59b containing a linear bisamide were synthesized and evaluated for their insecticidal activities against P. xylostella [50].
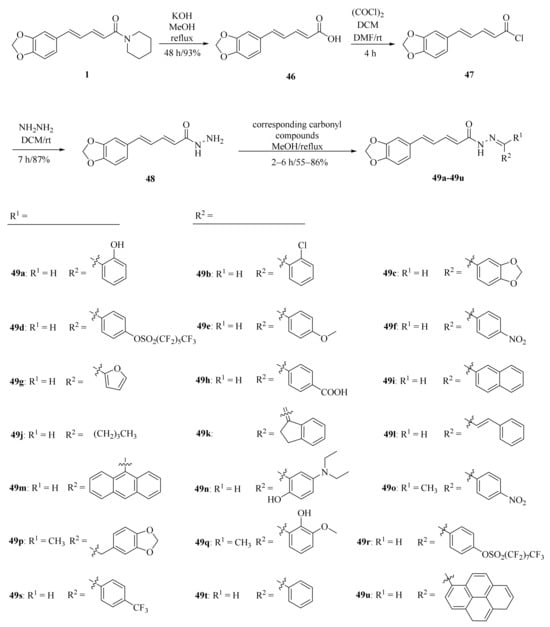
Scheme 8.
Synthesis of piperine derivates 49a–49u.

Table 2.
LC50 values of some potent compounds against 3rd larval instar of C. pipiens [65].
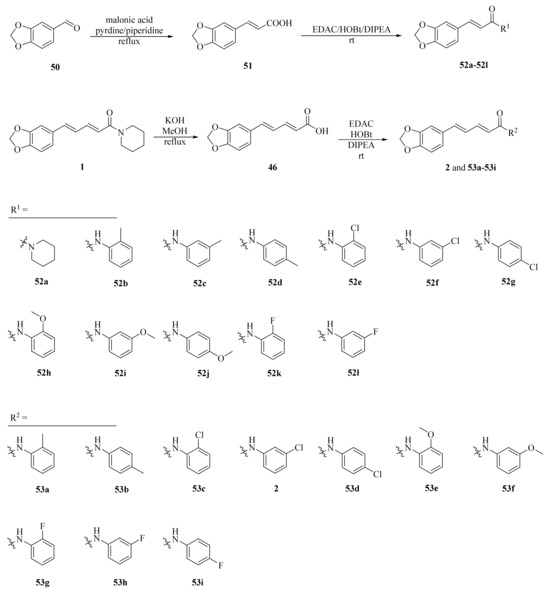
Scheme 9.
Synthesis of piperine derivates 2, 52a–52l, and 53a–53i.
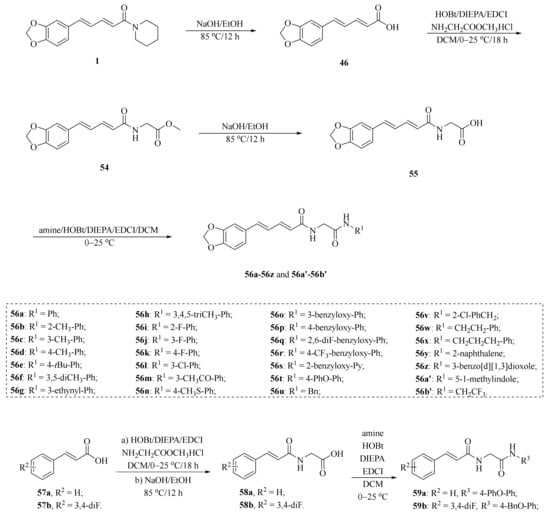
Scheme 10.
Synthesis of piperine derivates 56a–56z, 56a’–56b’, and 59a–59b.
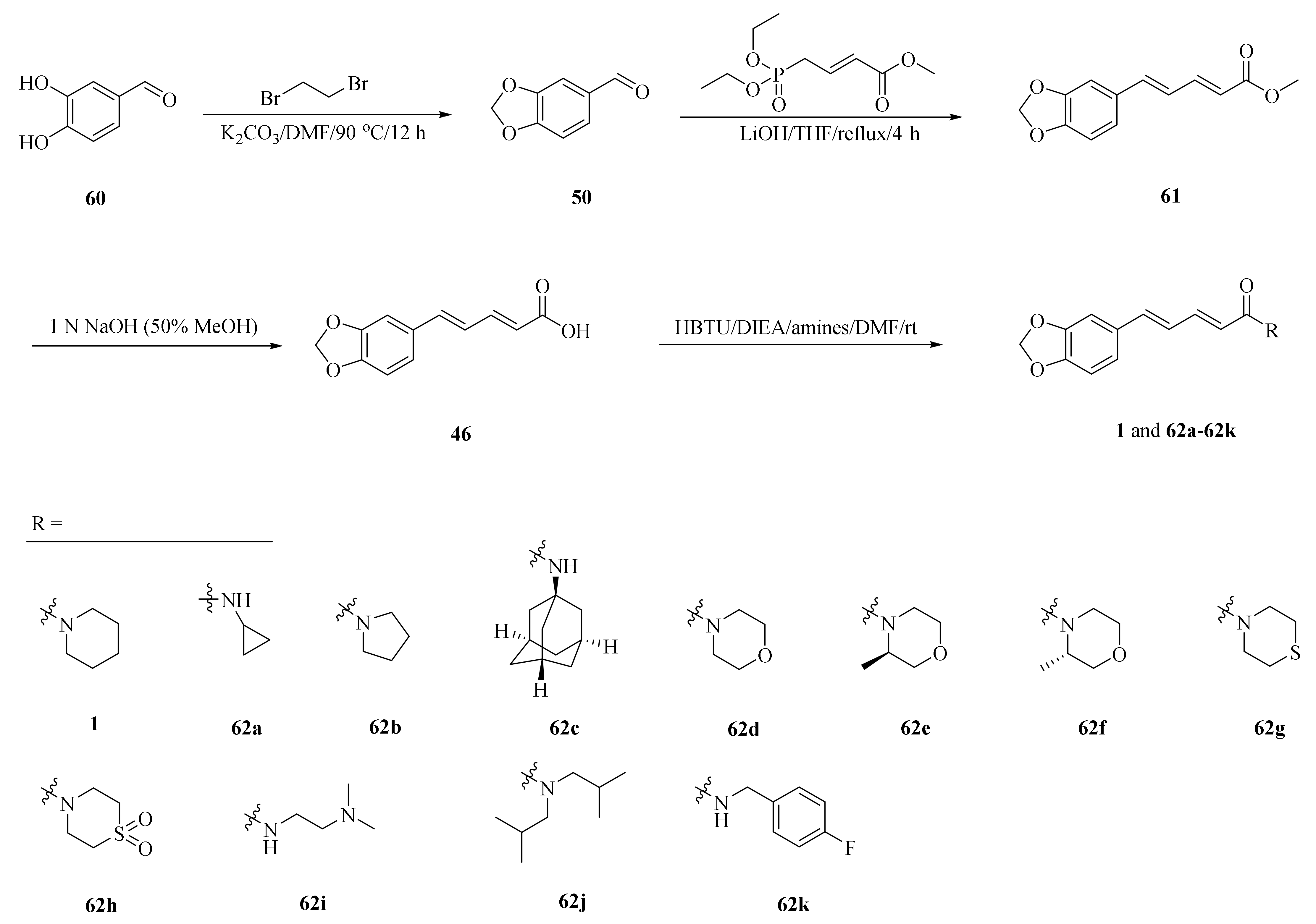
Human Cytochrome P450 2J2 (CYP2J2) plays an important role in metabolizing polyunsaturated fatty acids (PUFAs). As described in Scheme 11, a series of piperine derivatives 62a–62k were designed and synthesized based on the underlying interactions of piperine with CYP2J2. Among them, compounds 62j and 62k were developed as much stronger inhibitors and their inhibition activities increased approximately 10-fold higher than piperine with the IC50 values of 40 and 50 nM, respectively (Table 3) [66].
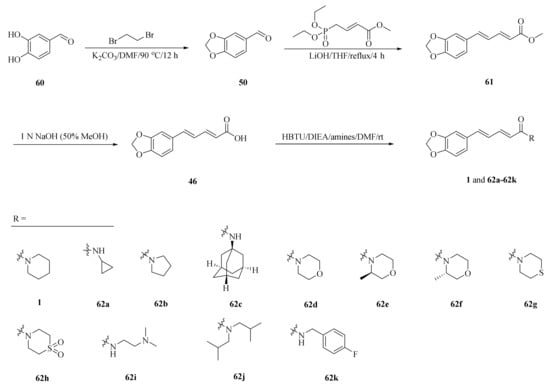
Scheme 11.
Synthesis of piperine (1) and its derivatives 62a–62k.

Table 3.
The CYP2J2 inhibitory activities of compounds 62a–62k [66].
4. Mechanisms of Action of Piperine and Its Derivatives
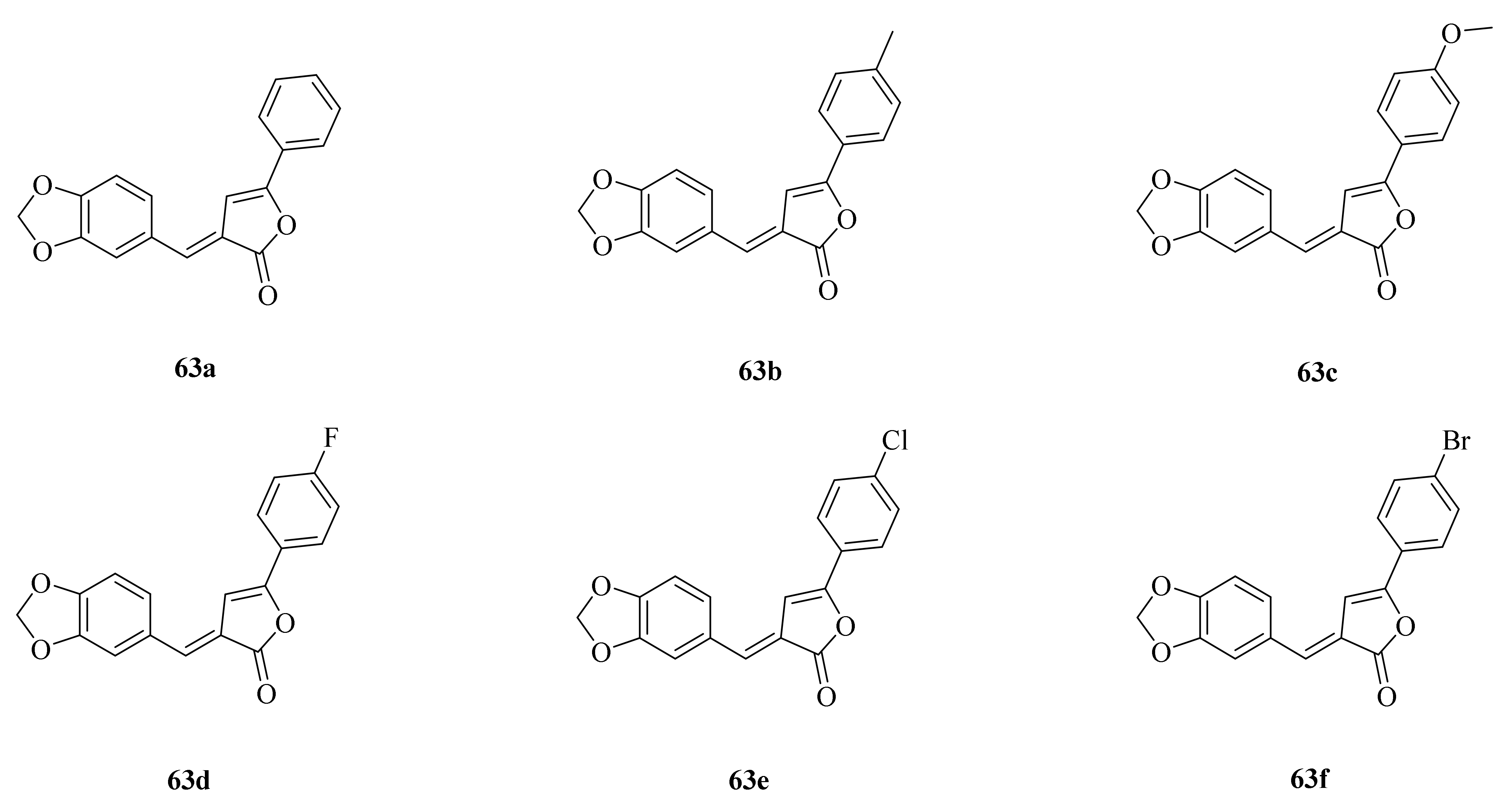
The natural product piperine (1) and its derivates exhibit wide biological activity in the fields of medicine and agriculture. For example, piperine and its derivates have anticancer properties such as growth-inhibition, anti-migration, and anti-invasion of cancer cells by regulating the miR-181c-3p/PPARα Axis [32] and inhibiting thioredoxin reductase and carbonic anhydrase [34,35]. In addition, piperine can target endoplasmic reticulum autophagy (ER-phagy) in treating acute pancreatitis [42]. On the other hand, the insect chitinase OfChtI from the agricultural pest O. furnacalis is a promising target for green insecticide design. Han et al. first found that piperine can inhibit the insect chitinase from O. furnacalis. Piperine derivates 63a–63f (Figure 5) were designed and synthesized by introducing a butenolide scaffold into the lead compound piperine. The results of the enzymatic activity assay revealed that the synthetic compounds (Ki = 1.03–2.04 μM) were approximately 40–80 times more effective in inhibiting OfChtI than the lead compound piperine (Ki = 81.45 μM). The inhibitory mechanism demonstrated that the introduced butenolide skeleton improved the binding affinity to OfChtI [67].
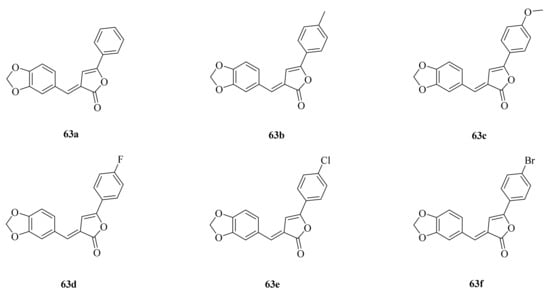
Figure 5.
The chemical structures of compounds 63a–63f.
5. Structure-Activity Relationships of Piperine and Its Derivatives
The SARs analysis of piperine and its derivatives are described in Figure 6, and the detailed descriptions are as follows: (i) introduction of hydroxyl groups at C-8 and C-9 positions can obtain a potent antiviral candidate [38]. Replacing the dioxymethylene ring with a 2-oxazoline heterocyclic ring and introducing a Cl atom at the C-10 position can produce an anti-inflammatory agent, which has potent therapeutic effects against neuroinflammation [46]; (ii) introduction of ester and oxime ester groups at C-2 position of piperine is important for improving acaricidal activity. In particular, the long aliphatic chain esters or oxime esters, and a very crucial prerequisite is to retain the dioxymethylene ring [28,29,60]; (iii) introduction of the dienehydrazide groups can obtain potent insecticidal compounds against third-instar larval of C. pipiens [65]. The introduction of different substituted amides is beneficial for anticancer activity and can obtain a potential inhibitor against CYP2J2 [34,67]. Piperine derivates containing a linear bisamide exhibited significant insecticidal activity against P. xylostella, and they can be further studied as lead pesticidal agents [50].
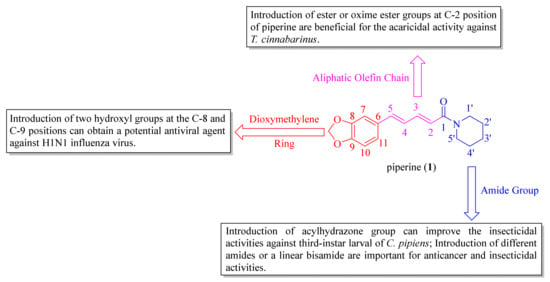
Figure 6.
The structure-activity relationships of piperine and its derivatives.
6. Conclusions
In recent years, research and development of plant-derived pesticides in China have been steadily improving. The commercialization of plant-derived pesticides should capitalize on their benefits and prioritize sustainability. In the future, plant-derived pesticides will play a significant role in food security and the agricultural field for improving the local economic development. This review has summarized the recent developments from 2020 to 2023 on biological activities, structural modifications, and structure-activity relationships of piperine and its derivatives, in addition to their mechanisms of action. The structural characteristics of piperine implied that it had various sites for structural modifications, making it a high-value-added lead compound. It is prospective that this paper can provide essential information and proposals for further design and development of novel piperine derivatives as potent natural-product-based drugs and pesticides for improving the local economic development in the future.
Author Contributions
All persons who have made substantial contributions to present work are named in the manuscript. J.H. (Jin Han), T.L., S.Z. and J.H. (Jun He) designed the work, analyzed the data, and wrote and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TrxR | Thioredoxin reductase |
| VEGFR | Vascular endothelial growth factor receptors |
| TNF-α | Tumor necrosis factor-α |
| TGF-β | Transforming growth factor-β |
| NF-κB | Nuclear factor-κB |
| MAPK | Mitogen-activated protein kinase |
| LC50 | Lethal concentration |
| DMF | N,N-Dimethylformamide |
| t-Bu | tert-Butyl |
| EDCI | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| HOBt | 1-Hydroxybenzotriazole |
| DMAP | 4-Dimethylaminopyridine |
References
- Lazareva, N.F.; Baryshok, V.P.; Lazarev, I.M. Silicon-containing analogs of camptothecin as anticancer agents. Arch. Pharm. 2018, 351, e1700297. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, J.F.; Saltz, L.B. The camptothecins. Lancet 2003, 361, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.Q.; Feng, J.T.; Wu, H.; Han, L.R. Review on research and development of botanical pesticides. Chin. J. Biol. Control 2015, 31, 685–698. [Google Scholar]
- Tunce, C.; Aliniazee, M.T. Acute and chronic effects of neem on Myzocallis coryli (Homoptera: Aphididae). Int. J. Pest Manag. 1998, 44, 53–58. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xi, H.C.; Chang, W.C.; Huang, L.L.; Chen, X. Current situation of commercialized application of plant-derived pesticides in China and suggestions for industrial development. World Pestic. 2020, 42, 6–15. [Google Scholar]
- Guo, Y.J.; Han, J.Y.; Li, Z.Q.; Yang, S.B. Research and application of plant-derived pesticides. Heilongjiang Agric. Sci. 2019, 4, 131–133. [Google Scholar]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch. Carbohyd. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative quantification of the phenolic compounds, piperine content, and total polyphenols along with the antioxidant activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Xia, J.; Wu, Z.; Lei, J.; Yu, J. Anti-inflammatory and antitumour activity of various extracts and compounds from the fruits of Piper longum L. J. Pharm. Pharmacol. 2019, 71, 1162–1171. [Google Scholar] [CrossRef]
- Ee, G.C.L.; Lim, C.M.; Lim, C.K.; Rahmani, M.; Shaari, K.; Bong, C.F.J. Alkaloids from Piper sarmentosum and Piper nigrum. Nat. Prod. Res. 2009, 23, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Kittl, T.; Minceva, M. Supercritical CO2 extraction of spices: A systematic study with focus on terpenes and piperamides from black pepper (Piper nigrum L.). Food Chem. 2023, 406, 135090. [Google Scholar] [CrossRef] [PubMed]
- Mad-adam, N.; Madla, S.; Lailerd, N.; Hiransai, P.; Graidist, P. Piper nigrum extract: Dietary supplement for reducing mammary tumor incidence and chemotherapy-induced toxicity. Foods 2023, 12, 2053. [Google Scholar] [CrossRef]
- Wood, A.J.L.; Weise, N.J.; Frampton, J.D.; Dunstan, M.S.; Hollas, M.A.; Derrington, S.R.; Lloyd, R.C.; Quaglia, D.; Parmeggiani, F.; Leys, D.; et al. Adenylation activity of carboxylic acid reductases enables the synthesis of amides. Angew. Chem. Int. Ed. 2017, 56, 14498–14501. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, D.Y.; Zhang, Z.H.; Zuo, C.H.; Zhang, Y.; Pei, Y.Q.; Lo, Y.Q. Trial of antiepilepsirine (AES) in children with epilepsy. Brain Dev. 1999, 21, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.B. Pepper-the characteristic industry in Yunnan Province. China Rural. Sci. Technol. 2020, 304, 74–75. [Google Scholar]
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.G.M.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400. [Google Scholar] [CrossRef]
- Qi, Y.B.; Yang, W.; Si, M.; Nie, L. Wnt/β-catenin signaling modulates piperine-mediated antitumor effects on human osteosarcoma cells. Mol. Med. Rep. 2020, 21, 2202–2208. [Google Scholar] [CrossRef]
- Amperayani, K.R.; Varadhi, G.; Oruganti, B.; Parimi, U.D. Molecular dynamics and absolute binding free energy studies of piperine derivatives as potential inhibitors of SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2023. [Google Scholar] [CrossRef]
- Priscilla, J.; Arul Dhas, D.; Hubert Joe, I.; Balachandran, S. Spectroscopic, quantum chemical, hydrogen bonding, reduced density gradient analysis and anti-inflammatory activity study on piper amide alkaloid piperine and wisanine. J. Mol. Struct. 2021, 1225, 129146. [Google Scholar] [CrossRef]
- Wansri, R.; Lin, A.C.K.; Pengon, J.; Kamchonwongpaisan, S.; Srimongkolpithak, N.; Rattanajak, R.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Hengphasatporn, K.; et al. Semi-synthesis of N-aryl amide analogs of piperine from Piper nigrum and evaluation of their antitrypanosomal, antimalarial, and anti-SARS-CoV-2 main protease activities. Molecules 2022, 27, 2841. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wu, D.; Tang, L.; Zhang, J.; Zeng, Z.; Geng, F.; Li, H. Binding mechanism and antioxidant activity of piperine to hemoglobin. Food Chem. 2022, 394, 133558. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Malik, N.; Khatkar, A. Natural based piperine derivatives as potent monoamine oxidase inhibitors: An in silico ADMET analysis and molecular docking studies. BMC Chem. 2020, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Nazifi, M.; Oryan, S.; Esfahani, D.E.; Ashrafpoor, M. The functional effects of piperine and piperine plus donepezil on hippocampal synaptic plasticity impairment in rat model of Alzheimer’s disease. Life Sci. 2021, 265, 118802. [Google Scholar] [CrossRef]
- Yang, X.; Zhi, J.; Leng, H.; Chen, Y.; Gao, H.; Ma, J.; Ji, J.; Hu, Q. The piperine derivative HJ105 inhibits Aβ1-42-induced neuroinflammation and oxidative damage via the Keap1-Nrf2-TXNIP axis. Phytomedicine 2021, 87, 153571. [Google Scholar] [CrossRef]
- Jaipea, S.; Saehlim, N.; Sutcharitruk, W.; Athipornchai, A.; Ingkaninan, K.; Saeeng, R. Synthesis of piperine analogues as AChE and BChE inhibitors for the treatment of Alzheimer’s disease. Phytochem. Lett. 2023, 53, 216–221. [Google Scholar] [CrossRef]
- Li, R.; Lu, Y.; Zhang, Q.; Liu, W.; Yang, R.; Jiao, J.; Liu, J.; Gao, G.; Yang, H. Piperine promotes autophagy flux by P2RX4 activation in SNCA/α-synuclein-induced Parkinson disease model. Autophagy 2022, 18, 559–575. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, Q.; Li, Y. Design, synthesis, and insecticidal activity evaluation of piperine derivatives. Front. Chem. 2022, 10, 973630. [Google Scholar] [CrossRef]
- Lv, M.; Li, S.; Wen, H.; Wang, Y.; Du, J.; Xu, H. Expedient discovery of novel oxime ester derivatives of piperine/piperine analogs as potent pesticide candidates and their mode of action against Tetranychus cinnabarinus Boisduval. Pest Manag. Sci. 2023, 79, 3459–3470. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Wang, J.; Wang, Z.; Xu, J.; Fang, S.; Xu, H. High value-added application of natural plant products in crop protection: Construction and pesticidal activities of piperine-type ester derivatives and their toxicology study. J. Agric. Food Chem. 2022, 70, 16126–16134. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Xiong, H.; Song, D.; Cao, X. Natural phenolic derivatives based on piperine scaffold as potential antifungal agents. BMC Chem. 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Anderson, B.O.; Cameron, D.; Cardoso, F.; Horton, R.; Knaul, F.M.; Mutebi, M.; Lee, N.; Abraham, J.E.; Anderson, B.O.; et al. The Lancet Breast Cancer Commission: Tackling a global health, gender, and equity challenge. Lancet 2022, 399, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Rajarajan, D.; Natesh, J.; Penta, D.; Meeran, S.M. Dietary piperine suppresses obesity-associated breast cancer growth and metastasis by regulating the miR-181c-3p/PPARα Axis. J. Agric. Food Chem. 2021, 69, 15562–15574. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Ryu, J.H.; Lee, H.J. In vitro inhibition of Piper nigrum and piperine on growth, migration, and invasion of PANC-1 human pancreatic cancer cells. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, L.; Tao, Y.; Zhao, J.; Chang, B.; Zhang, F.; Tu, J.; Cai, W.; Zhang, B. Synthesis and evaluation of piperine analogs as thioredoxin reductase inhibitors to cause oxidative stress-induced cancer cell apoptosis. Bioorg. Chem. 2023, 138, 106589. [Google Scholar] [CrossRef] [PubMed]
- Elimam, D.M.; Elgazar, A.A.; Bonardi, A.; Abdelfadil, M.; Nocentini, A.; El-Domany, R.A.; Abdel-Aziz, H.A.; Badria, F.A.; Supuran, C.T.; Eldehna, W.M. Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 225, 113800. [Google Scholar] [CrossRef] [PubMed]
- Elimam, D.M.; Elgazar, A.A.; El-Senduny, F.F.; El-Domany, R.A.; Badria, F.A.; Eldehna, W.M. Natural inspired piperine-based ureas and amides as novel antitumor agents towards breast cancer. J. Enzym. Inhib. Med. Chem. 2022, 37, 39–50. [Google Scholar] [CrossRef]
- Pandya, N.; Kumar, A. Piperine analogs arrest c-myc gene leading to downregulation of transcription for targeting cancer. Sci. Rep. 2021, 11, 22909. [Google Scholar] [CrossRef]
- Mohammed, A.; Velu, A.B.; Al-Hakami, A.M.; Meenakshisundaram, B.; Esther, P.; Abdelwahid, S.A.; Irfan, A.; Prasanna, R.; Anantharam, D.; Harish, C.C. Novel piperine compound AB05 (N-5-(3,4-dimethoxyphenyl)-2E,4E pentadienylpiperidine) inhibits H1N1 influenza virus propagation in vitro. Trop. Biomed. 2020, 37, 1062–1073. [Google Scholar]
- Shekunov, E.V.; Efimova, S.S.; Yudintceva, N.M.; Muryleva, A.A.; Zarubaev, V.V.; Slita, A.V.; Ostroumova, O.S. Plant alkaloids inhibit membrane fusion mediated by Calcium and fragments of MERS-CoV and SARS-CoV/SARS-CoV-2 fusion peptides. Biomedicines 2021, 9, 1434. [Google Scholar] [CrossRef]
- Wang, M.; Yang, B.; Ren, Z.; Liu, J.; Lu, C.; Jiang, H.; Ling, F.; Wang, G.; Liu, T. Inhibition of the largemouth bass virus replication by piperine demonstrates potential application in aquaculture. J. Fish Dis. 2023, 46, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Jin, W.; Yang, J.; Yu, G.; Shi, H.; Shi, K. Piperine alleviates acute pancreatitis: A possible role for FAM134B and CCPG1 dependent ER-phagy. Phytomedicine 2022, 105, 154361. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gong, H.; Du, J.; Gao, W.; Xu, J.; Cai, X.; Yang, Y.; Xiao, H. Piperine ameliorates psoriatic skin inflammation by inhibiting the phosphorylation of STAT3. Int. Immunopharmacol. 2023, 119, 110221. [Google Scholar] [CrossRef]
- Yu, J.W.; Li, S.; Bao, L.D.; Wang, L. Piperine treating sciatica through regulating inflammation and MiR-520a/P65 pathway. Chin. J. Nat. Med. 2021, 19, 412–421. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine derived from Piper nigrum L. inhibits LPS-induced inflammatory through the MAPK and NF-κB signalling pathways in RAW264.7 Cells. Foods 2022, 11, 2990. [Google Scholar] [CrossRef]
- Yang, X.; Ji, J.; Liu, C.; Zhou, M.; Li, H.; Ye, S.; Hu, Q. HJ22, a novel derivative of piperine, attenuates ibotenic acid-induced cognitive impairment, oxidativestress, apoptosis and inflammation via inhibiting the protein-protein interaction of Keap1-Nrf2. Int. Immunopharmacol. 2020, 83, 106383. [Google Scholar] [CrossRef]
- Tian, M.; Tian, Z.; Yao, D.; Ning, J.; Deng, S.; Feng, L.; Huo, X.; Tian, X.; Zhang, B.; Wang, C.; et al. A NIR fluorescent probe for fatty acid amide hydrolase bioimaging and its application in development of inhibitors. J. Mater. Chem. B 2021, 9, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.G.; Pilz-Júnior, H.L.; de Lemos, A.B.; da Silva da Costa, F.A.; Fernandes, M.; Gonçalves, D.Z.; Variza, P.F.; de Moraes, F.M.; Morisso, F.D.P.; Magnago, R.F.; et al. Polymer-based nanostructures loaded with piperine as a platform to improve the larvicidal activity against Aedes aegypti. Acta Trop. 2022, 230, 106395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shi, D.; Li, H.; He, D.; Zhu, K.; Li, J.; Zi, Y.; Xu, Z.; Huang, J.; Duan, H.; et al. Rational design and identification of novel piperine derivatives as multichitinase inhibitors. J. Agric. Food Chem. 2022, 70, 10326–10336. [Google Scholar] [CrossRef]
- Zhong, F.; Yu, L.; Jiang, X.; Chen, Y.; Wang, S.; Chao, L.; Jiang, Z.; He, B.; Xu, C.; Wang, S.; et al. Potential inhibitory effects of compounds ZK-PI-5 and ZK-PI-9 on trehalose and chitin metabolism in Spodoptera frugiperda (J. E. Smith). Front. Physiol. 2023, 14, 1178996. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Velmathi, S. Synthesis and characterization of piperine amide analogues: Their in-silico and invitro analysis as potential antibacterial agents. Results Chem. 2022, 4, 100369. [Google Scholar] [CrossRef]
- Das, S.; Paul, P.; Dastidar, D.G.; Chakraborty, P.; Chatterjee, S.; Sarkar, S.; Maiti, D.; Tribedi, P. Piperine exhibits potential antibiofilm activity against Pseudomonas aeruginosa by accumulating reactive oxygen species, affecting cell surface hydrophobicity and quorum sensing. Appl. Biochem. Biotech. 2023, 195, 3229–3256. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, G.B.; Awasthi, S.P.; Zahid, M.S.H.; Hatanaka, N.; Hinenoya, A.; Iwaoka, E.; Aoki, S.; Ramamurthy, T.; Yamasaki, S. Piperine, an active ingredient of white pepper, suppresses the growth of multidrug-resistant toxigenic Vibrio cholerae and other pathogenic bacteria. Lett. Appl. Microbiol. 2022, 74, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.S., Jr.; Martins, E.P.S.; Souza, H.D.S.; de Oliveira, R.F.; Alves, F.S.; Lima, E.O.; Cordeiro, L.V.; Trindade, E.O.; Lira, B.F.; Rocha, G.B.; et al. Synthesis, spectroscopic characterization, DFT calculations and preliminary antifungal activity of new piperine derivatives. J. Braz. Chem. Soc. 2021, 32, 490–502. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Y.; Liu, J.; Wu, L.; Liu, T.; Cui, J.; Lee, D.Y.W. Synthesis and biological activity of piperine derivatives as potential PPARγ agonists. Drug Des. Dev. Ther. 2020, 14, 2069–2078. [Google Scholar] [CrossRef]
- Silva, W.L.; de Andrade, F.H.D.; Lins, T.B.; da Silva, A.L.; da Cruz Amorim, C.A.; dos Santos Lima, M.J.; da Silva, P.C.D.; Vilela, W.T.; Nascimento, P.H.d.B.; de Oliveira, J.F.; et al. Synthesis, thermal behavior and biological evaluation of benzodioxole derivatives as potential cytotoxic and antiparasitic agents. Med. Chem. Res. 2023, 32, 944–956. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Chang, Y.; Wang, S.J. Piperine provides neuroprotection against kainic acid-induced neurotoxicity via maintaining NGF signalling pathway. Molecules 2022, 27, 2638. [Google Scholar] [CrossRef]
- Alsareii, S.A.; Ahmad, J.; Umar, A.; Ahmad, M.Z.; Shaikh, I.A. Enhanced in vivo wound healing efficacy of a novel piperine-containing bioactive hydrogel in excision wound rat model. Molecules 2023, 28, 545. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Wang, Y.; Thapa, S.; Zhang, S.; Xu, H. Synthesis of piperine-based ester derivatives with diverse aromatic rings and their agricultural bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann. Insects 2023, 14, 40. [Google Scholar] [CrossRef]
- Gu, Y.; Norton, J.R.; Salahi, F.; Lisnyak, V.G.; Zhou, Z.; Snyder, S.A. Highly selective hydrogenation of C=C bonds catalyzed by a Rhodium hydride. J. Am. Chem. Soc. 2021, 143, 9657–9663. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xiang, J.Y.; Yuan, H.Y.; Wu, J.Q.; Li, H.Z.; Shen, Y.H.; Xu, M. Isolation, synthesis and absolute configuration of 4,5-dihydroxypiperines improving behavioral disorder in AlCl3-induced dementia. Bioorg. Med. Chem. Lett. 2021, 42, 128057. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.X.; Pham, P.H.; Nguyen, T.T.; Yang, C.H.; Pham, H.T.B.; Nguyen, T.T.; Wang, H.; Phan, N.T.S. Trisulfur-radical-anion-triggered C(sp2)-H amination of electron-deficient alkenes. Org. Lett. 2020, 22, 9751–9756. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz-Rajchel, H.; Kaźmierczak, M. Chemo-, regio-, and stereoselectivity in 1,3-dipolar cycloaddition of piperine with nitrones. A cycloadditive route to aminoalcohols. New J. Chem. 2020, 44, 6015–6025. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Farag, S.M.; Hegazy, L.; Jiang, H.; Wang, M.Q. The larvicidal activity of natural inspired piperine-based dienehydrazides against Culex pipiens. Bioorg. Chem. 2020, 94, 103464. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, M.; Ning, J.; Deng, X.; Feng, L.; Huang, H.; Yao, D.; Ma, X. The development of novel cytochrome P450 2J2 (CYP2J2) inhibitor and the underlying interaction between inhibitor and CYP2J2. J. Enzym. Inhib. Med. Ch. 2021, 36, 737–748. [Google Scholar] [CrossRef]
- Han, Q.; Wu, N.; Li, H.L.; Zhang, J.Y.; Li, X.; Deng, M.F.; Zhu, K.; Wang, J.E.; Duan, H.X.; Yang, Q. A piperine-based scaffold as a novel starting point to develop inhibitors against the potent molecular target OfChtI. J. Agric. Food Chem. 2021, 69, 7534–7544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).