Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens
Abstract
:1. Introduction
2. Results
2.1. Dietary BA Supplementation Alleviates AFB1-Induced Morphological Changes Induced by AFB1 in the Liver and Gallbladder
2.2. Dietary BA Supplementation Alleviates AFB1-Induced Hepatic Inflammation and Oxidative Stress
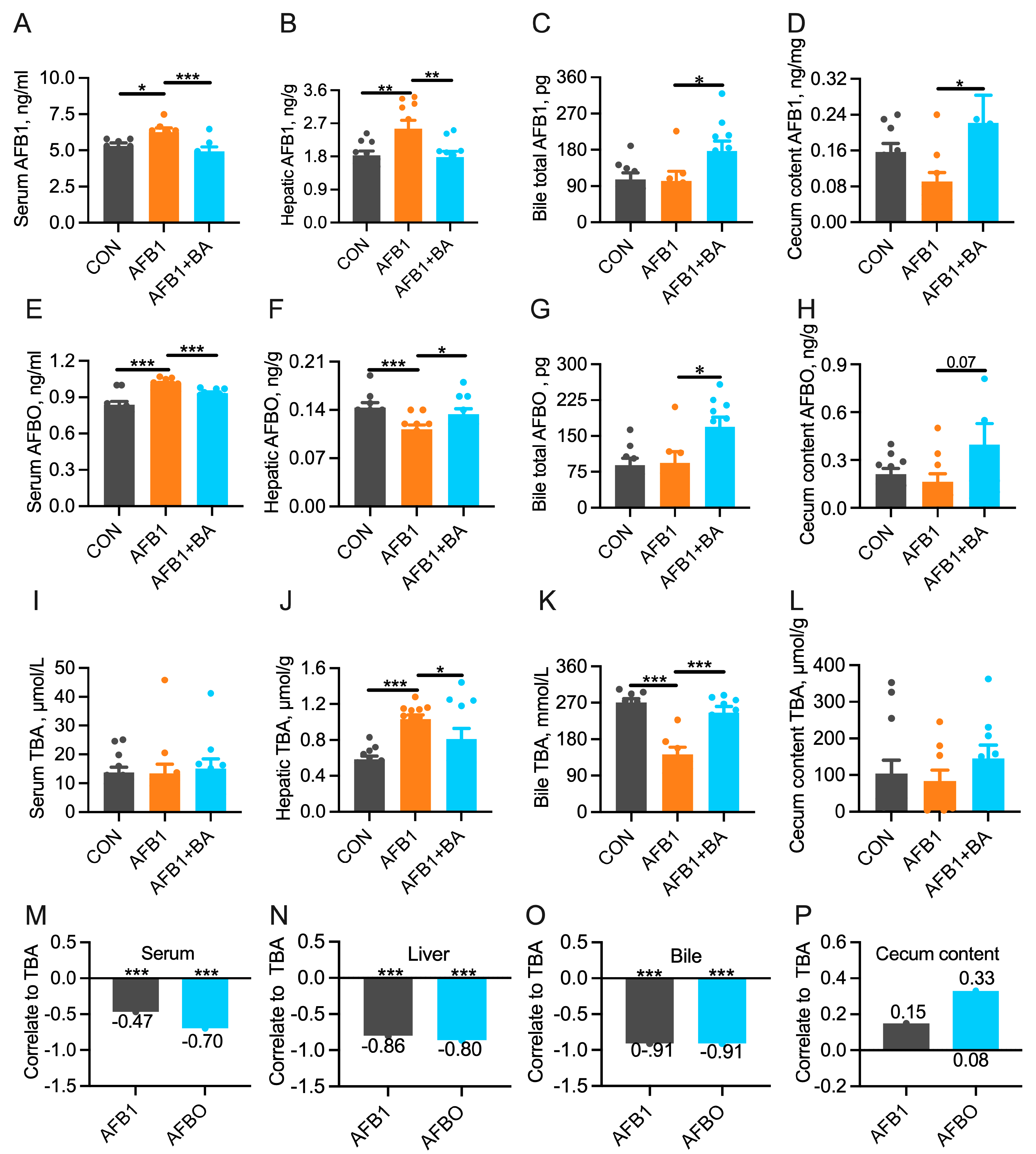
2.3. Dietary BA Supplementation Promotes the Turnover of AFB1/AFBO and Bile Acids
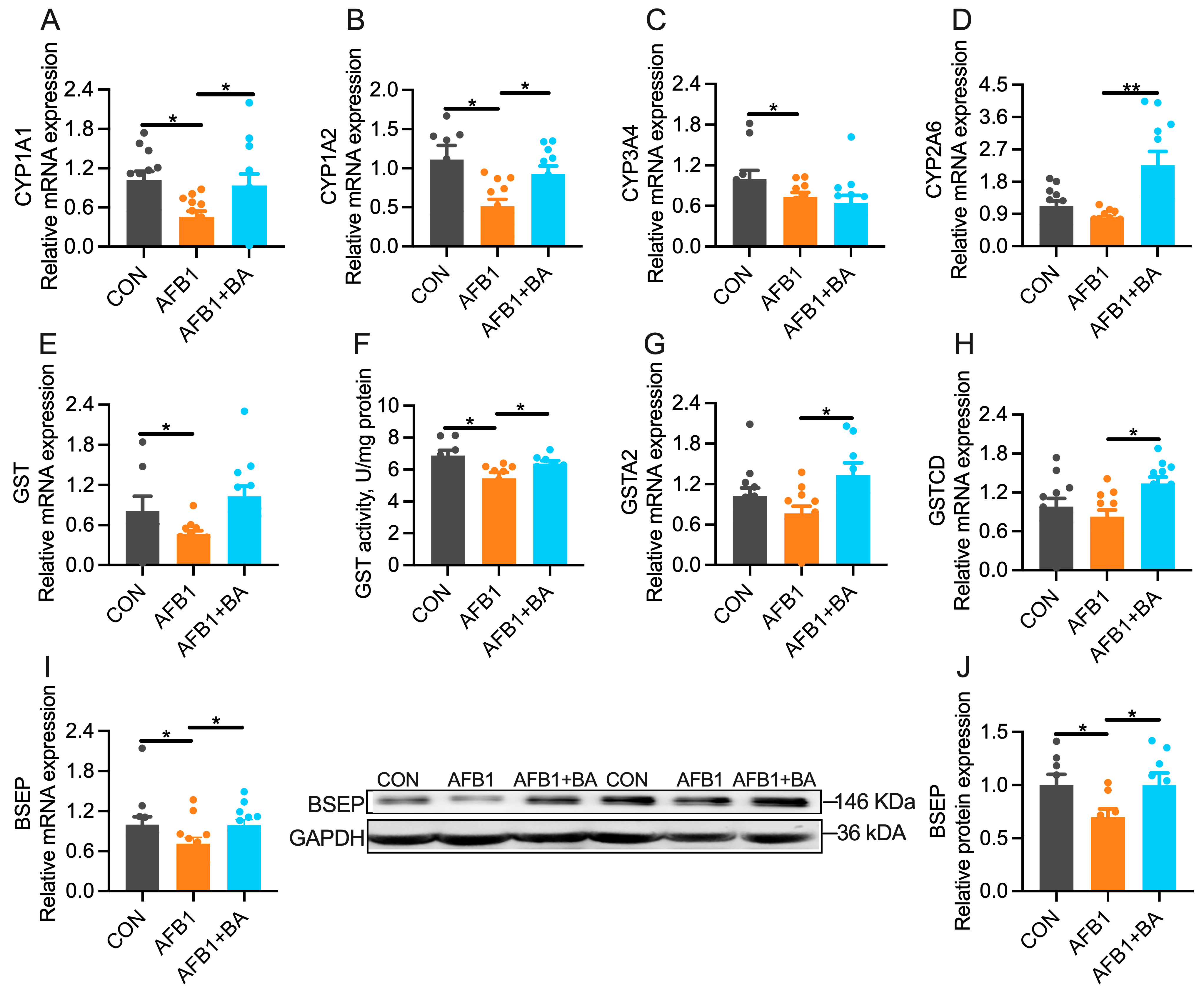
2.4. Dietary BA Supplementation Upregulates Hepatic Expression of Detoxification Enzymes
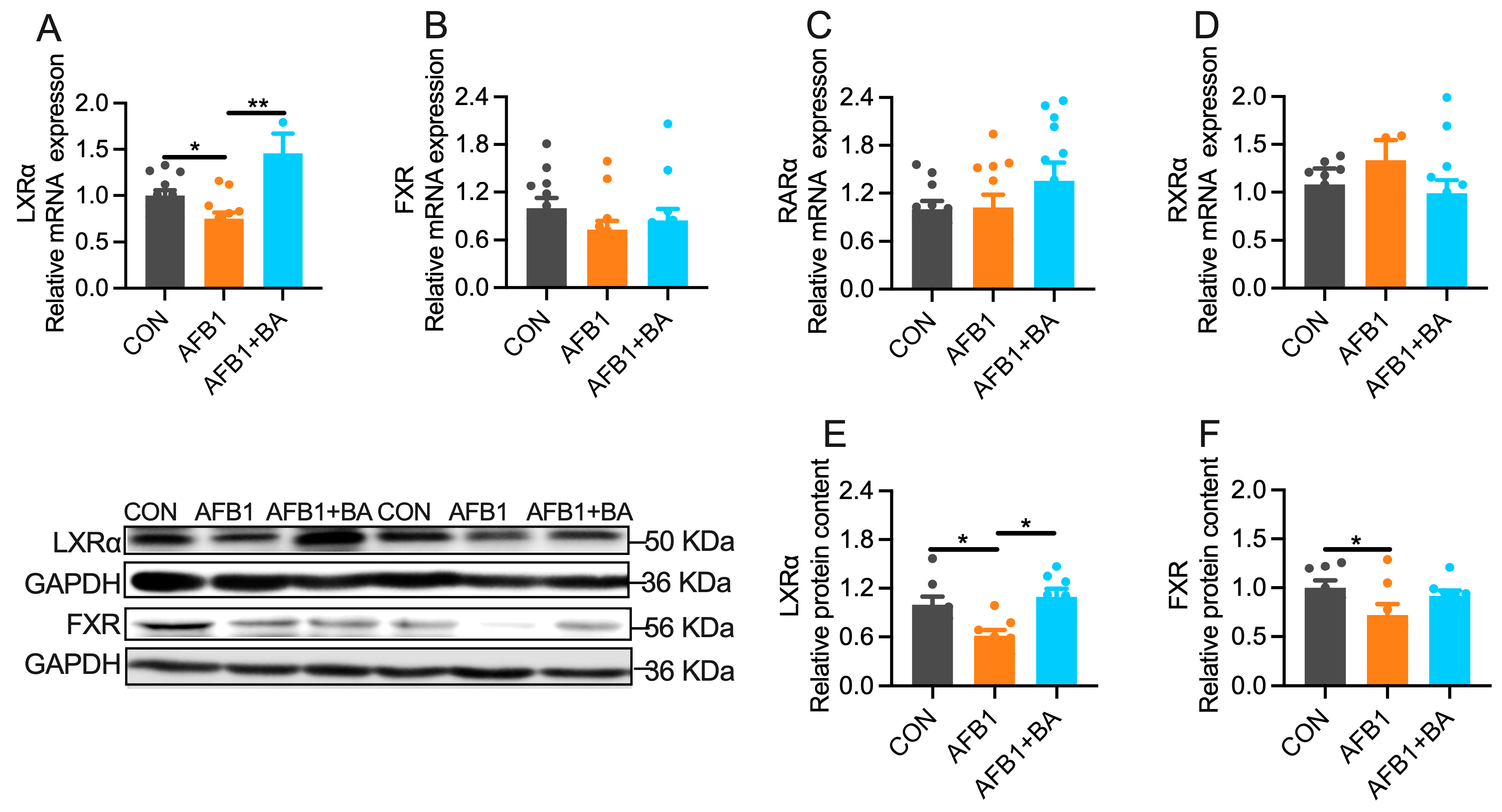
2.5. Dietary BA Supplementation Enhances Hepatic Expression of LXRα
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Bile Acids
5.2. Birds, Diets, and Experimental Design
5.3. Histological Evaluation of Liver
5.4. Determination of Enzymes/Factors Related to Inflammation, Oxidative Stress and Detoxification
5.5. Determination of Total Bile Acids Concentration
5.6. Determination of AFB1 and AFBO Concentrations
5.7. Quantification of mRNA by Real-Time RT-PCR
5.8. Total Protein Extraction and Western Blotting
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin B1 produced by aspergillus flavus and aspergillus parasiticus strains on poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Ghallab, A.; Hassan, R.; Myllys, M.; Albrecht, W.; Friebel, A.; Hoehme, S.; Hofmann, U.; Seddek, A.L.; Braeuning, A.; Kuepfer, L.; et al. Subcellular spatio-temporal intravital kinetics of aflatoxin B1 and ochratoxin A in liver and kidney. Arch. Toxicol. 2021, 95, 2163–2177. [Google Scholar] [CrossRef]
- Nomura, H.; Ogiso, M.; Yamashita, M.; Takaku, H.; Kimura, A.; Chikasou, M.; Nakamura, Y.; Fujii, S.; Watai, M.; Yamada, H. Uptake by dietary exposure and elimination of aflatoxins in muscle and liver of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2011, 59, 5150. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar] [CrossRef]
- Murcia, H.W.; Díaz, G.J.; Cepeda, S.M. Enzymatic Activity in Turkey, Duck, Quail and Chicken Liver Microsomes against Four Human Cytochrome P450 Prototype Substrates and Aflatoxin B1. J. Xenobiotics 2011, 1, e4. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Tanaka, Y.; Nakano, T.; Adachi, T.; Tanaka, H.; Kaminuma, T.; Ishikawa, T. Nuclear receptor-mediated transcriptional regulation in Phase I, II, and III xenobiotic metabolizing systems. Drug Metab. Pharmacokinet. 2006, 21, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Watanabe, K.; Yamazoe, Y.; Yoshinari, K. Liver X receptor alpha bidirectionally transactivates human CYP1A1 and CYP1A2 through two cis-elements common to both genes. Toxicol. Lett. 2012, 215, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chisaki, I.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Iseki, K. Liver X receptor regulates expression of MRP2 but not that of MDR1 and BCRP in the liver. Biochim. Biophys. Acta 2009, 1788, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Sarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.L. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Vue, P.M.; Anderson, A.L.; Devereaux, M.W.; Ghosh, S.; Balasubramaniyan, N.; Fillon, S.A.; Dahrenmoeller, C.; Allawzi, A.; Woods, C.; et al. Macrophage-derived IL-1beta/NF-kappaB signaling mediates parenteral nutrition-associated cholestasis. Nat. Commun. 2018, 9, 1393. [Google Scholar] [CrossRef]
- van der Schoor, L.W.E.; Verkade, H.J.; Bertolini, A.; de Wit, S.; Mennillo, E.; Rettenmeier, E.; Weber, A.A.; Havinga, R.; Valaskova, P.; Jasprova, J.; et al. Potential of therapeutic bile acids in the treatment of neonatal Hyperbilirubinemia. Sci. Rep. 2021, 11, 11107. [Google Scholar] [CrossRef]
- Razori, M.V.; Maidagan, P.M.; Ciriaci, N. Anticholestatic mechanisms of ursodeoxycholic acid in lipopolysaccharide-induced cholestasis. Biochem. Pharmacol. 2019, 168, 48–56. [Google Scholar] [CrossRef]
- Dai, D.; Pan, Y.; Zeng, C.; Liu, S.; Yan, Y.; Wu, X.; Xu, Z.; Zhang, L. Activated FXR promotes xenobiotic metabolism of T-2 toxin and attenuates oxidative stress in broiler chicken liver. Chem. Biol. Interact. 2020, 316, 108912. [Google Scholar] [CrossRef]
- Solcan, C.; Gogu, M.; Floristean, V.; Oprisan, B.; Solcan, G. The hepatoprotective effect of sea buckthorn (Hippophae rhamnoides) berries on induced aflatoxin B1 poisoning in chickens. Poult. Sci. 2013, 92, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Horn, N.; Applegate, T.J. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult. Sci. 2014, 93, 2037–2047. [Google Scholar] [CrossRef]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo evaluation of AFB1 and OTA-toxicity through immunofluorescence and flow cytometry techniques: A systematic review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.G.; Zhao, L.H.; Wei, H.; Duan, G.X.; Zhang, J.Y.; Ji, C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int. J. Mol. Sci. 2014, 15, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Ishfaq, M.; Yu, H.; Yang, Y.; Li, S.; Li, X.; Fazlani, S.A.; Guo, W.; Zhang, X. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult. Sci. 2020, 99, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, W.R.; Tang, J.K.; Jin, X.; Xue, H.H.; Wang, T.; Zhang, L.W.; Sun, Q.Y.; Liang, Z.X. Dietary phillygenin supplementation ameliorates aflatoxin B1-induced oxidative stress, inflammation, and apoptosis in chicken liver. Ecotoxicol. Environ. Saf. 2022, 236, 113481. [Google Scholar] [CrossRef]
- Yin, C.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. Effects of bile acids on growth performance and lipid metabolism during chronic heat stress in broiler chickens. Animals 2021, 11, 630. [Google Scholar] [CrossRef]
- Kusaczuk, M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef]
- Guo, J.L.; Kuang, W.M.; Zhong, Y.F.; Zhou, Y.L.; Chen, Y.J.; Lin, S.M. Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish. Immunol. 2020, 97, 602–607. [Google Scholar] [CrossRef]
- Mitsuyoshi, H.; Nakashima, T.; Sumida, Y.; Yoh, T.; Nakajima, Y.; Ishikawa, H.; Inaba, K.; Sakamoto, Y.; Okanoue, T.; Kashima, K. Ursodeoxycholic acid protects hepatocytes against oxidative injury via induction of antioxidants. Biochem. Biophys. Res. Commun. 1999, 263, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, D.; Wang, C.; Li, N.; Liu, D.; Wang, C.; Yan, G.; Zhang, S.; Jiang, Y.; Shen, M.; et al. Comparison study of protective effects of porcine bile acids and sheep bile acids against heat stress in chickens. J. Sci. Food Agric. 2023, 103, 5687–5696. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Ohyanagi, H. Protective effect of the intravenous administration of ursodeoxycholic acid against endotoxemia in rats with obstructive jaundice. Surg. Today 1997, 27, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lu, Y.; Li, J.; Wang, Y.; Pan, L.; Zhang, M. Effects of bile acids on aflatoxin B1 bioaccumulation, detoxification system, and growth performance of Pacific white shrimp. Food Chem. 2022, 371, 131169. [Google Scholar] [CrossRef]
- Prakash, C.; Zuniga, B.; Song, C.S.; Jiang, S.; Cropper, J.; Park, S.; Chatterjee, B. Nuclear Receptors in Drug Metabolism, Drug Response and Drug Interactions. Nucl. Recept. Res. 2015, 2, 101178. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin Successfully Inhibited the Computationally Identified CYP2A6 Enzyme-Mediated Bioactivation of Aflatoxin B1 in Arbor Acres broiler. Front. Pharmacol. 2017, 21, 143. [Google Scholar] [CrossRef]
- Ates, M.B.; Ortatatli, M. Phase-1 bioactivation mechanisms of aflatoxin through AhR, CAR and PXR nuclear receptors and the interactions with Nigella sativa seeds and thymoquinone in broilers. Ecotoxicol. Environ. Saf. 2021, 208, 111774. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Muhammad, I.; Sun, X.; Cui, X.; Cheng, P.; Qayum, A.; Zhang, X. Biochemical basis for the age-related sensitivity of broilers to aflatoxin B1. Toxicol. Mech. Methods 2018, 28, 361–368. [Google Scholar] [CrossRef]
- Emerole, G.O. Excretion of aflatoxin B1 as a glutathione conjugate. Eur. J. Drug Metab. Pharmacokinet. 1981, 6, 265–268. [Google Scholar] [CrossRef]
- Wallace, B.D.; Redinbo, M.R. Xenobiotic-sensing nuclear receptors involved in drug metabolism: A structural perspective. Drug Metab. Rev. 2013, 45, 79–100. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P.; Cheng, Y.; Wang, P.; Ma, X.; Liu, M.; Wang, X.; Xu, F. Diet-induced obese alters the expression and function of hepatic drug-metabolizing enzymes and transporters in rats. Biochem. Pharmacol. 2019, 164, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Hiipakka, R.A.; Liao, S. Selective activation of liver X receptor alpha by 6alpha-hydroxy bile acids and analogs. Steroids 2000, 65, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.T.; Hirooka, E.Y.; Alvares, E.; Silva, P.L.; Bracarense, A.P.F.R.L.; Flaiban, K.K.M.D.C.; Akagi, C.Y.; Kawamura, O.; Costa, M.C.D.; Itano, E.N. Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice. Toxins 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xia, B.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. The Effect of Exogenous Bile Acids on Antioxidant Status and Gut Microbiota in Heat-Stressed Broiler Chickens. Front. Nutr. 2021, 8, 747136. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cardoso, V.; Vermelho, A.B.; Ribeiro de Lima, C.A.; Mendes de Oliveira, J.; Freire de Lima, M.E.; Pinto da Silva, L.H.; Direito, G.M.; Miranda Danelli, M.D. Antigenotoxic Effect of Piperine in Broiler Chickens Intoxicated with Aflatoxin B1. Toxins 2016, 8, 316. [Google Scholar] [CrossRef]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K.; Furuse, M. Change in hepatic and plasma bile acid contents and its regulatory gene expression in the chicken embryo. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 344–347. [Google Scholar] [CrossRef]
- Duan., Y.; Fu., W.; Wang, S.; Ni, Y.; Zhao, R. Effects of tonic immobility (TI) and corticosterone (CORT) on energy status and protein metabolism in pectoralis major muscle of broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 169, 90–95. [Google Scholar] [CrossRef] [PubMed]
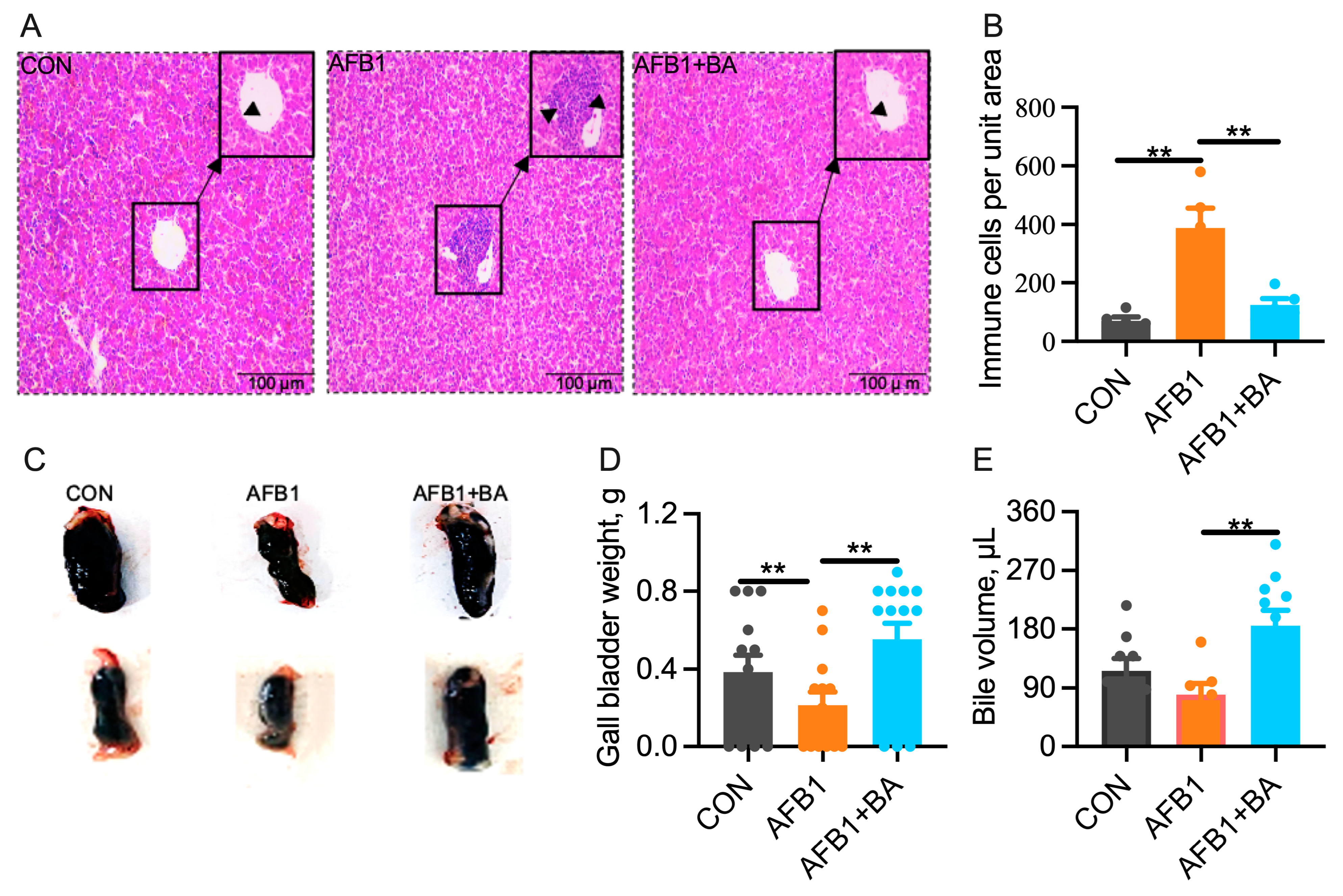


| Items | CON | AFB1 | AFB1 + BA |
|---|---|---|---|
| Ingredients, % | |||
| Wheat | 25 | 25 | 25 |
| Corn powder | 20 | 20 | 20 |
| Corn meal | 14 | 14 | 14 |
| Soybean meal | 12 | 12 | 12 |
| Rice powder | 10 | 10 | 10 |
| Corn gluten powder | 4 | 4 | 4 |
| Feather powder | 2 | 2 | 2 |
| DDGS | 2 | 2 | 2 |
| Candy meal | 1 | 1 | 1 |
| Rapeseed meal | 3 | 3 | 3 |
| Corn | 2 | 2 | 2 |
| Soybean oil | 0.5 | 0.5 | 0.5 |
| Limestone | 1.2 | 1.2 | 1.2 |
| Calcium hydrogen phosphate | 1.4 | 1.4 | 1.4 |
| Sodium chloride | 0.29 | 0.29 | 0.29 |
| Lysine | 0.81 | 0.81 | 0.81 |
| Methionine | 0.13 | 0.13 | 0.13 |
| Tryptophan | 0.01 | 0.01 | 0.01 |
| Choline | 0.1 | 0.1 | 0.1 |
| Premix | 0.56 | 0.56 | 0.56 |
| Bile acids, mg/kg | 0 | 0 | 250 |
| Calculated nutrient composition, % | |||
| Metabolizable energy, kcal/kg | 3100 | 3100 | 3100 |
| Crude protein | 21 | 21 | 21 |
| Calcium | 1.30 | 1.30 | 1.30 |
| Available phospholipid | 0.80 | 0.80 | 0.80 |
| Sodium chloride | 0.80 | 0.80 | 0.80 |
| Methionine | 0.90 | 0.90 | 0.90 |
| Lysine | 0.60 | 0.60 | 0.60 |
| Target Genes | GenBank Accession | Primer Sequences (5′ to 3′) | PCR Product (bp) |
|---|---|---|---|
| CYP1A1 | NM_205147.2 | F: TCGTCAACGACCTCTTTGGG R: CAAGGCAGCGTACATCATGC | 77 |
| CYP1A2 | NM_205146.3 | F: GAGGATCCTGTGTGGTTCCG R: AACTAAGGGGAAGCGTGGTG | 132 |
| CYP3A4 | NM_001329508.2 | F: CCCTGCAAAGACACTCCGAT R: CTGGGGCCAAGGAATTGTCA | 299 |
| CYP2A6 | PMID: 28377720 | F: CTGCAGAGAATGGCATGAAG R: CCTGCAAGACTGCAAGGAA | 110 |
| TNFα | NM_204267.2 | F: CTGAGGCATTTGGAAGCAGC R: GACAGGGTAGGGGTGAGGAT | 208 |
| IL-1β | NM_000576.3 | F: AACCTCTTCGAGGCACAAGG R: AGCCATCATTTCACTGGCGA | 122 |
| INF-γ | NM_205149.2 | F: CACTGACAAGTCAAAGCCGC R: GCATCTCCTCTGAGACTGGC | 232 |
| iNOS | NM_204961.2 | F: CCAGCTGATTGGGTGTGGAT R: CCTACGGGTCTCATCATGCC | 150 |
| GST | NM_001001777.2 | F: AGGAAACCACGCCTAGAGGA R: TTTCATCCAGTGTACCGCCT | 213 |
| GSTA2 | NM_001001776.2 | F: GGCGCTGCAGTCAAGCTC R: TCCTCGAATTCAACCCCAGC | 282 |
| GSTCD | XM_015276567.4 | F: TCTTGATCGAGCATGGGCTG R: TAAACTGGGGTGCCCACAAA | 111 |
| BSEP | XM_052674968.1 | F: ACCCAGGTGCAAATAGCCAAT R: GCACCCAAACACTTCCCATC | 77 |
| FXR | NM_001396910.1 | F: AAAAGCCTAGACTGGGCCAC R: GCCAACATGCCCATTTGCTT | 253 |
| LXRα | NM_204542.3 | F: CTTCCGCTGGTACTTCCTTTC R: CAGGAGGCCTTCTTCAAGGT | 70 |
| RXRα | XM_003642291.5 | F: CCCTCTGAACACCAGGTGAC R: ACAGAATCCAGCAGGAGCAC | 280 |
| RARα | NM_204536.1 | F: TCACCCCCTACGCCTTTTTC R: AGGATTTGTCCTGGCAGACG | 225 |
| PPIA | NM_001166326.2 | F: GTGACTTTACGCGCCACAAC R: TTGCTCGTCTTGCCGTCTTT | 268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wen, T.; Cao, A.; Wang, J.; Pan, H.; Zhao, R. Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens. Toxins 2023, 15, 694. https://doi.org/10.3390/toxins15120694
Chen L, Wen T, Cao A, Wang J, Pan H, Zhao R. Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens. Toxins. 2023; 15(12):694. https://doi.org/10.3390/toxins15120694
Chicago/Turabian StyleChen, Liang, Tian Wen, Aizhi Cao, Jianmin Wang, Hua Pan, and Ruqian Zhao. 2023. "Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens" Toxins 15, no. 12: 694. https://doi.org/10.3390/toxins15120694
APA StyleChen, L., Wen, T., Cao, A., Wang, J., Pan, H., & Zhao, R. (2023). Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens. Toxins, 15(12), 694. https://doi.org/10.3390/toxins15120694





