Potential Toxicity and Mechanisms of T-2 and HT-2 Individually or in Combination on the Intestinal Barrier Function of Porcine Small Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Results
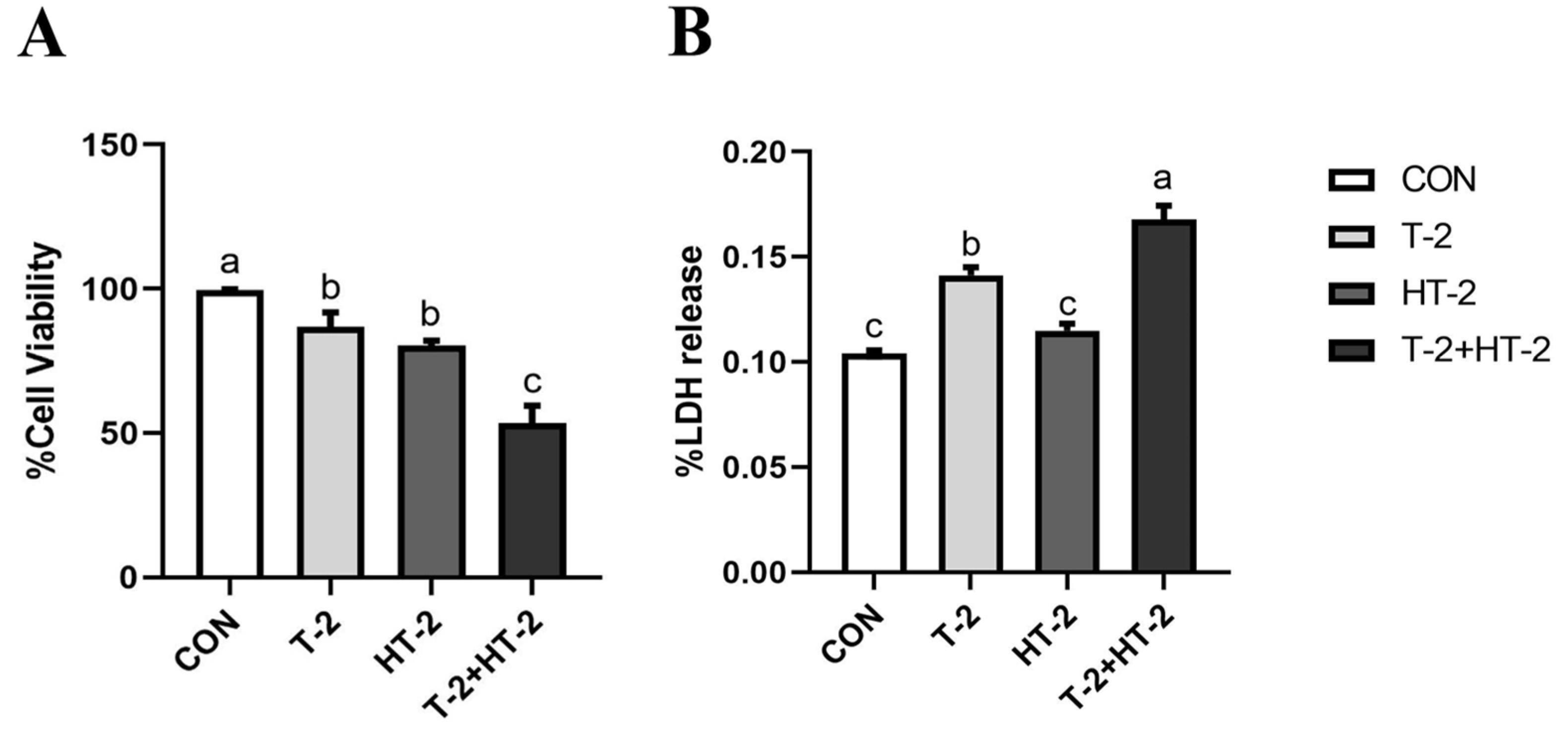
2.1. Effect of T-2 and HT-2 on Cell Viability and Lactate Dehydrogenase (LDH) Activity
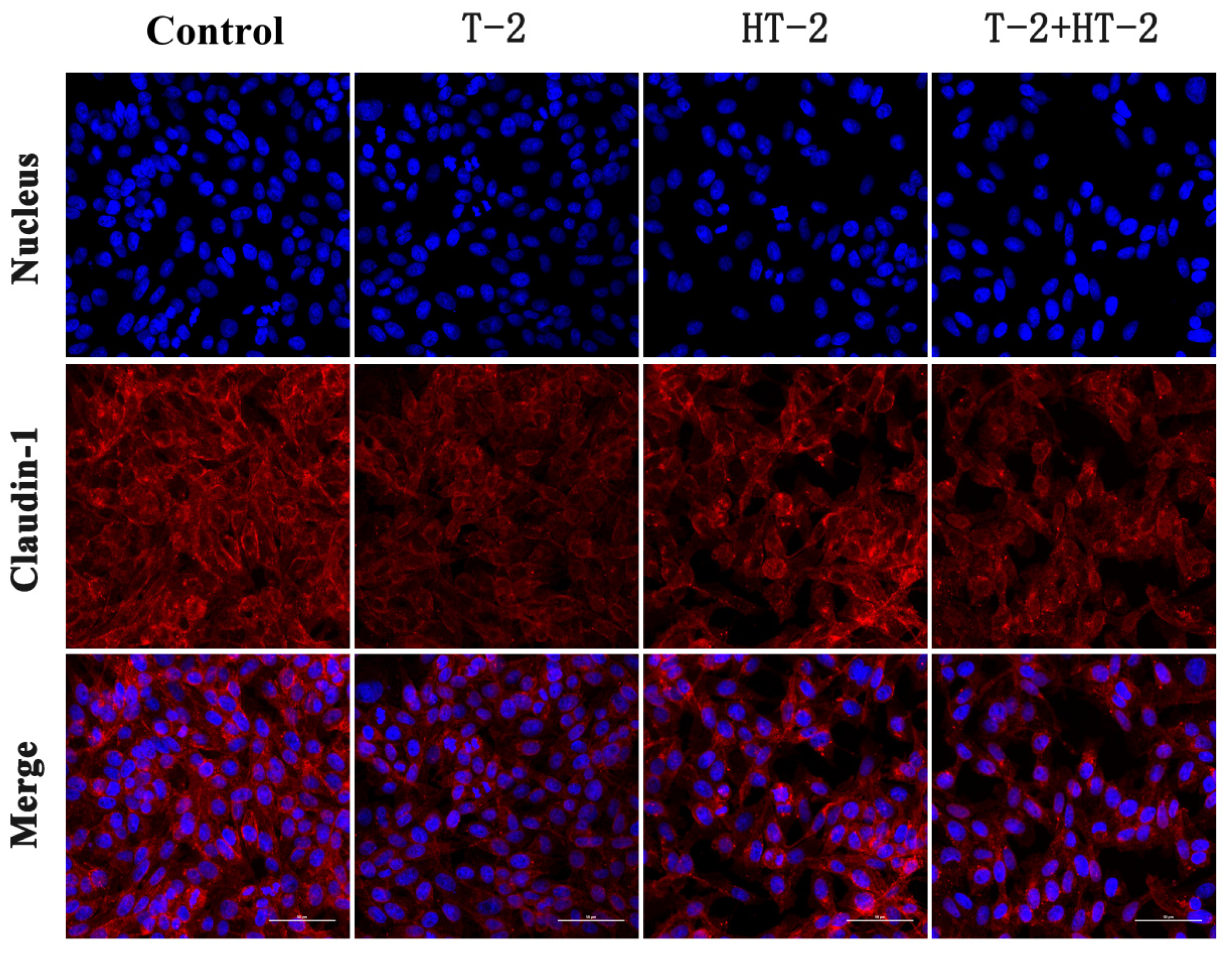
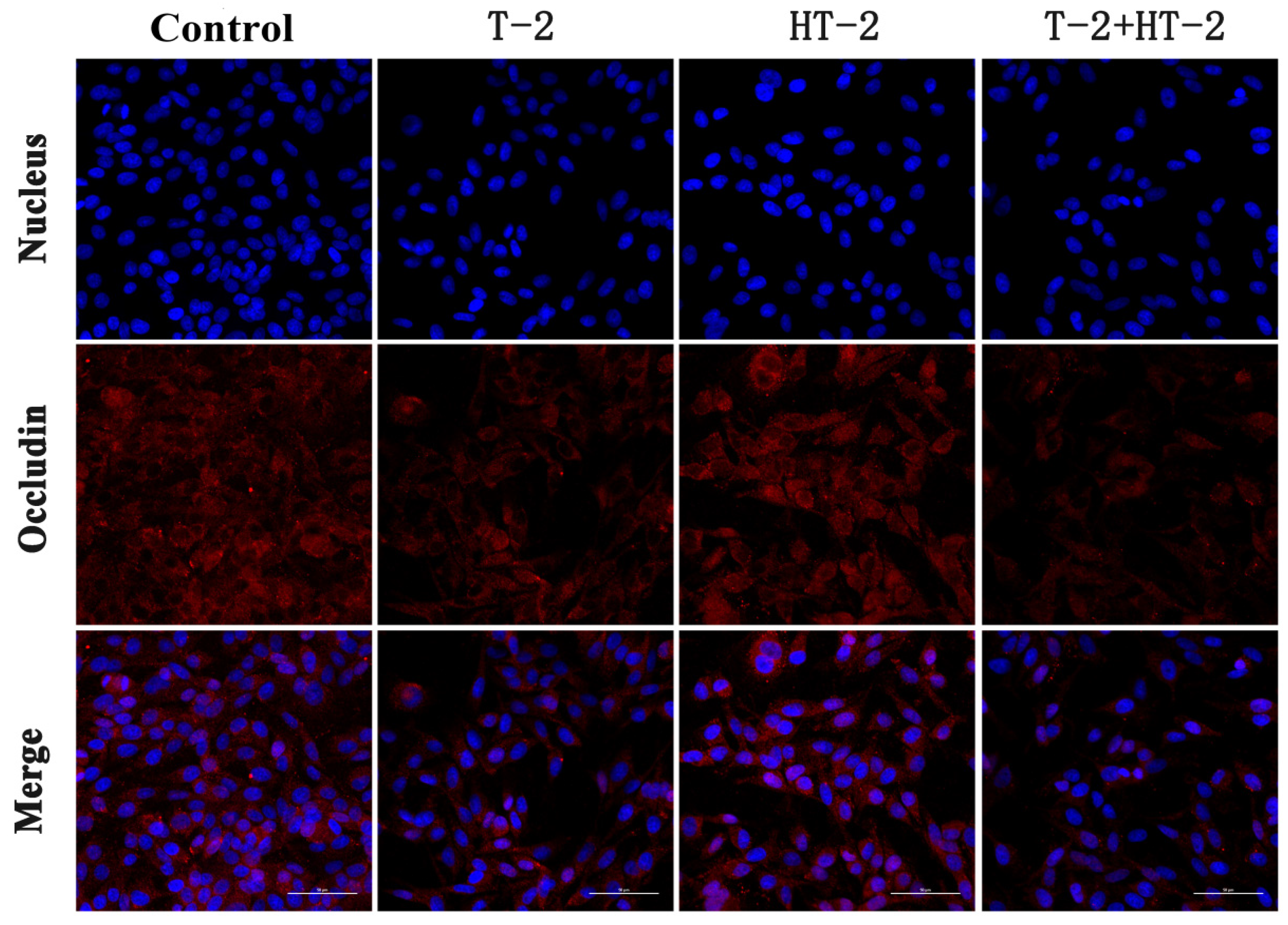
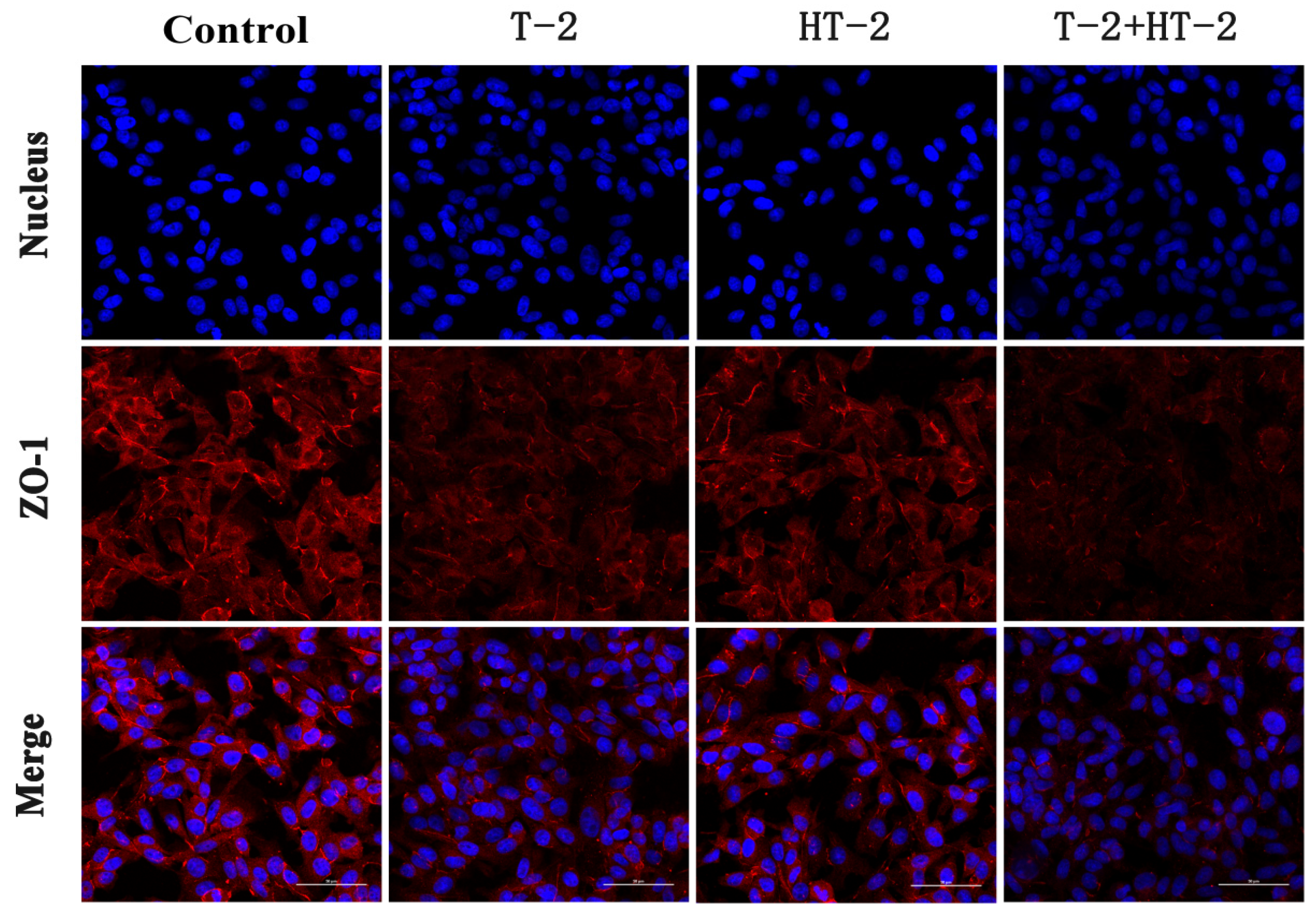
2.2. The Expression of Tight Junction Protein by Immunofluorescence Assay
2.3. Expression of Tight Junction-Related Protein mRNA by Real-Time PCR
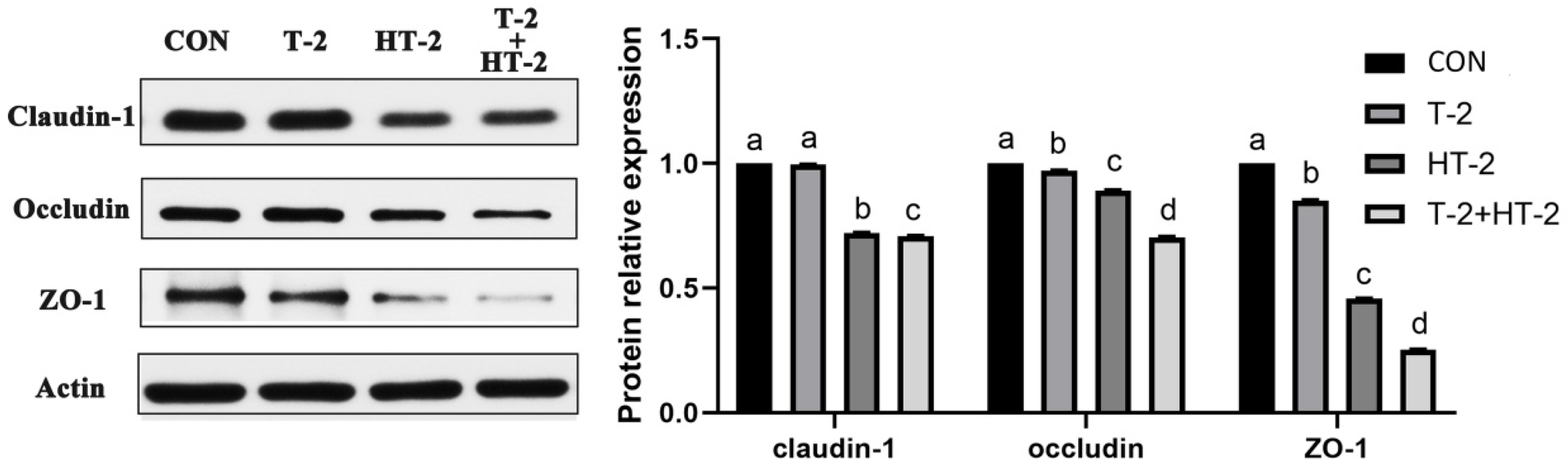
2.4. Expression of Tight Junction-Related Proteins by Western-Blot
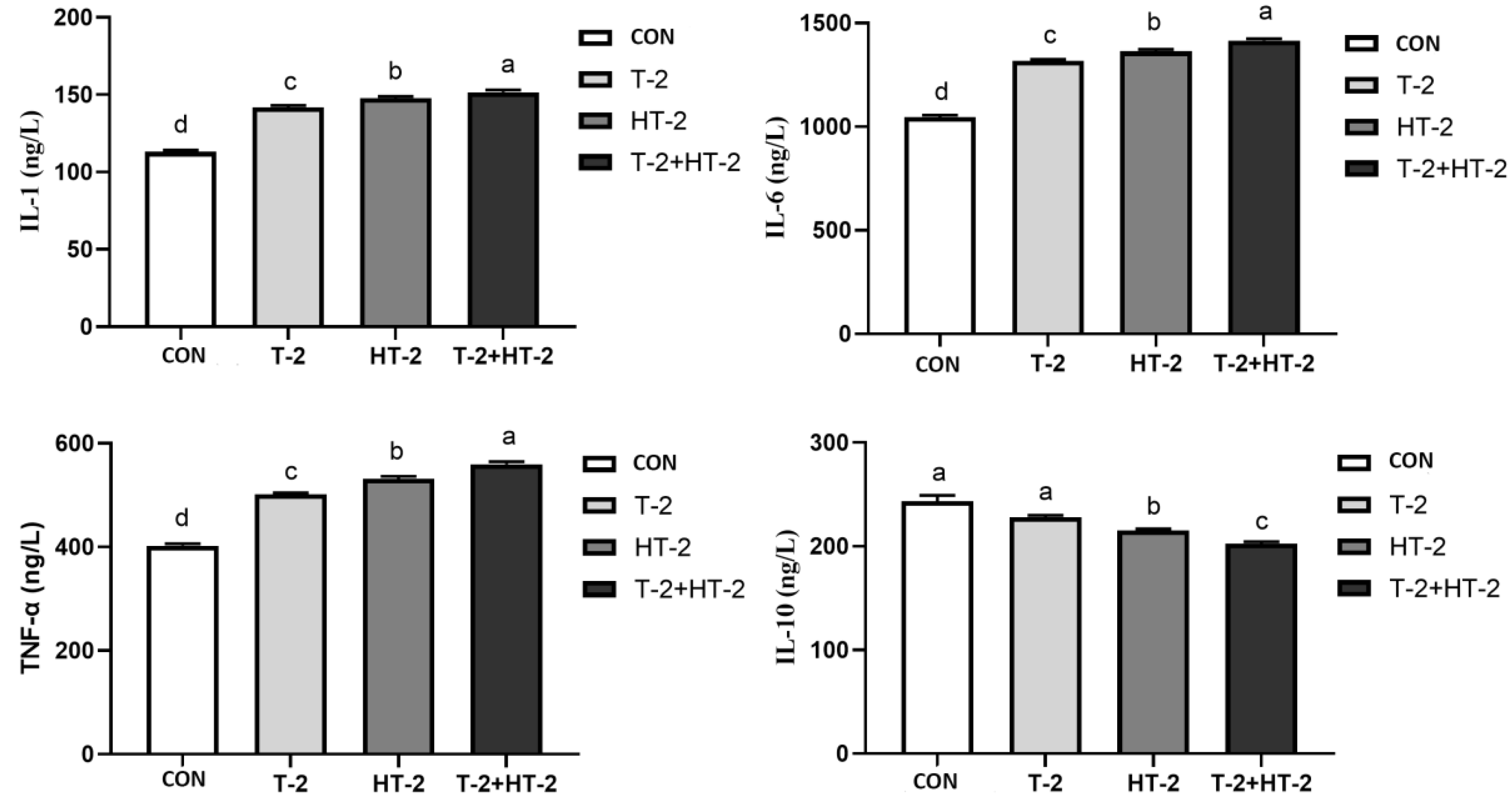
2.5. Effect of T-2 and HT-2 on the Content of Inflammatory Factors
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Cell Culture
5.3. Determination of Cell Viability
5.4. Determination of Lactate Dehydrogenase (LDH) Activity
5.5. Detection of ZO-1, Occludin, and Claudin-1 by Immunofluorescence Staining
5.6. RNA Extraction and Real-Time Quantitative PCR
5.7. Detection of Tight Junction-Associated Proteins
5.8. Effect of T-2 and HT-2 on Inflammation Factors
5.9. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 toxin–the most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Liu, J.; Chen, J.; Huang, C.; Wang, J.; Liang, Z.; Wen, L.; Yi, J.; Yuan, Z. Betulinic acid attenuates T-2 toxin-induced cytotoxicity in porcine kidney cells by blocking oxidative stress and endoplasmic reticulum stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 249, 109124. [Google Scholar] [CrossRef]
- Kuchenbuch, H.; Becker, S.; Schulz, M.; Cramer, B.; Humpf, H. Thermal stability of T-2 and HT-2 toxins during biscuit- and crunchy muesli-making and roasting. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, U.; Packa, D.; Wiwart, M. Microbial inhibition of fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins 2017, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Pernica, M.; Kyralová, B.; Svoboda, Z.; Boško, R.; Brožková, I.; Česlová, L.; Benešová, K.; Červenka, L.; Běláková, S. Levels of T-2 toxin and its metabolites, and the occurrence of Fusarium fungi in spring barley in the Czech Republic. Food Microbiol. 2022, 102, 103875. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Pascale, M.; Visconti, A. Current analytical methods for trichothecene mycotoxins in cereals. Trends Analyt. Chem. 2009, 28, 758–768. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Liu, J.; Wang, G.; Wan, K. Contamination level of T-2 and HT-2 toxin in cereal crops from Aba area in Sichuan Province, China. Bull. Environ. Contam. Toxicol. 2012, 88, 396–400. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Kleintjens, T.; Sajic, N.; Haasnoot, W.; Campbell, K.; Elliott, C.; Salden, M. T-2 toxin/HT-2 toxin and Ochratoxin A ELISAs development and in-house validation in food in accordance with commission regulation (EU) No 519/2014. Toxins 2017, 9, 388. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.; Kaushik, N.; Choi, E. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef]
- Busman, M.; Poling, S.; Maragos, C. Observation of T-2 Toxin and HT-2 Toxin glucosides from fusarium sporotrichioides by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Toxins 2011, 3, 1554–1568. [Google Scholar] [CrossRef]
- Sobral, M.; Faria, M.; Cunha, S.; Ferreira, I. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef]
- Ling, A.; Sun, L.; Guo, W.; Sun, S.; Yang, J.; Zhao, Z. Individual and combined cytotoxic effects of T-2 toxin and its four metabolites on porcine Leydig cells. Food Chem. Toxicol. 2020, 139, 111277. [Google Scholar] [CrossRef]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E.; Haesebrouck, F.; De Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusariummycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.; Raleigh, D.; Yu, D.; Turner, J. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 24, 1995. [Google Scholar] [CrossRef] [PubMed]
- Mariani, V.; Palermo, S.; Fiorentini, S.; Lanubile, A.; Giuffra, E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet. Immunol. Immunopathol. 2009, 131, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Campisi, L.; Barbet, G.; Ding, Y.; Esplugues, E.; Flavell, R.; Blander, J. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat. Immunol. 2016, 17, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Ni, X.; Jin, C.; Ren, W.; Li, J.; Deng, J.; Deng, B.; Yin, Y. Cecropin A modulates tight junction-related protein expression and enhances the barrier function of porcine intestinal epithelial cells by suppressing the MEK/ERK pathway. Int. J. Mol. Sci. 2018, 19, 1941. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Yuan, Z.; Yi, J.; Chen, J.; Wang, N.; Tian, Y. Autophagy and apoptosis interact to modulate T-2 toxin-induced toxicity in liver cells. Toxins 2019, 11, 45. [Google Scholar] [CrossRef]
- Lee, S. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Groschwitz, K.; Hogan, S. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Li, S.; Perveen, A.; Shen, J.; Li, C. Effects of maternal T-2 toxin exposure on microorganisms and intestinal barrier function in young mice. Ecotoxicol. Environ. Saf. 2022, 247, 114252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Qiu, M.; Sun, L.; Wang, X.; Li, C.; Xu, D.; Gooneratne, R. Cytotoxicity of T-2 and modified T-2 toxins: Induction of JAK/STAT pathway in RAW264.7 cells by hepatopancreas and muscle extracts of shrimp fed with T-2 toxin. Toxicol. Res. 2017, 6, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, K.; Long, M.; Yang, S.; Zhang, Y. An update on immunotoxicity and mechanisms of action of six environmental mycotoxins. Food Chem. Toxicol. 2022, 163, 112895. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium mycotoxins T-2 toxin and HT-2 toxin in wheat. J. Agric. Food Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Kim, M.; Kim, K.; Ji, S.; Baek, Y.; Chun, J.; Jung, H.; Choe, C.; Lee, H.; Kim, M.; et al. Effect of commercially purified deoxynivalenol and zearalenone mycotoxins on microbial diversity of pig cecum contents. Anim. Biosci. 2021, 34, 243–255. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Gene | Accession Number | Sequences (5′→3′) | Product |
|---|---|---|---|
| Claudin-1 | NC_010455.5 | F: GGCAGATCCAGTGCAAAGTC R: CCCAGCAGGATGCCAATTAC | 94 bp |
| Occludin | NC_010458.4 | F: CATTATGCACCCAGCAACGA R: GCACATCACGATAACGAGCA | 168 bp |
| ZO-1 | NC_010443.5 | F: GGGCTCTTGGCTTGCTATTC R: AAGGCCTCGGAATCTCCAAA | 160 bp |
| β-actin | 424396 | F: CTGGACTTCGAGCAGGAGATGG R: TTCGTGGATGCCGCAGGATTC | 168 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Wang, J.; Han, M.; Wang, L.; Li, L.; Zhang, J.; Chen, S.; Guo, J.; Zhai, X.; Yang, J. Potential Toxicity and Mechanisms of T-2 and HT-2 Individually or in Combination on the Intestinal Barrier Function of Porcine Small Intestinal Epithelial Cells. Toxins 2023, 15, 682. https://doi.org/10.3390/toxins15120682
He W, Wang J, Han M, Wang L, Li L, Zhang J, Chen S, Guo J, Zhai X, Yang J. Potential Toxicity and Mechanisms of T-2 and HT-2 Individually or in Combination on the Intestinal Barrier Function of Porcine Small Intestinal Epithelial Cells. Toxins. 2023; 15(12):682. https://doi.org/10.3390/toxins15120682
Chicago/Turabian StyleHe, Weihua, Jianhua Wang, Mengyi Han, Lihua Wang, Ling Li, Jiahui Zhang, Siqi Chen, Jiayi Guo, Xiaohu Zhai, and Junhua Yang. 2023. "Potential Toxicity and Mechanisms of T-2 and HT-2 Individually or in Combination on the Intestinal Barrier Function of Porcine Small Intestinal Epithelial Cells" Toxins 15, no. 12: 682. https://doi.org/10.3390/toxins15120682
APA StyleHe, W., Wang, J., Han, M., Wang, L., Li, L., Zhang, J., Chen, S., Guo, J., Zhai, X., & Yang, J. (2023). Potential Toxicity and Mechanisms of T-2 and HT-2 Individually or in Combination on the Intestinal Barrier Function of Porcine Small Intestinal Epithelial Cells. Toxins, 15(12), 682. https://doi.org/10.3390/toxins15120682






