An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China
Abstract
:1. Introduction
2. Results
2.1. PST Components in Zhejiang Province
2.2. Factors Affecting the Distribution of PSTs
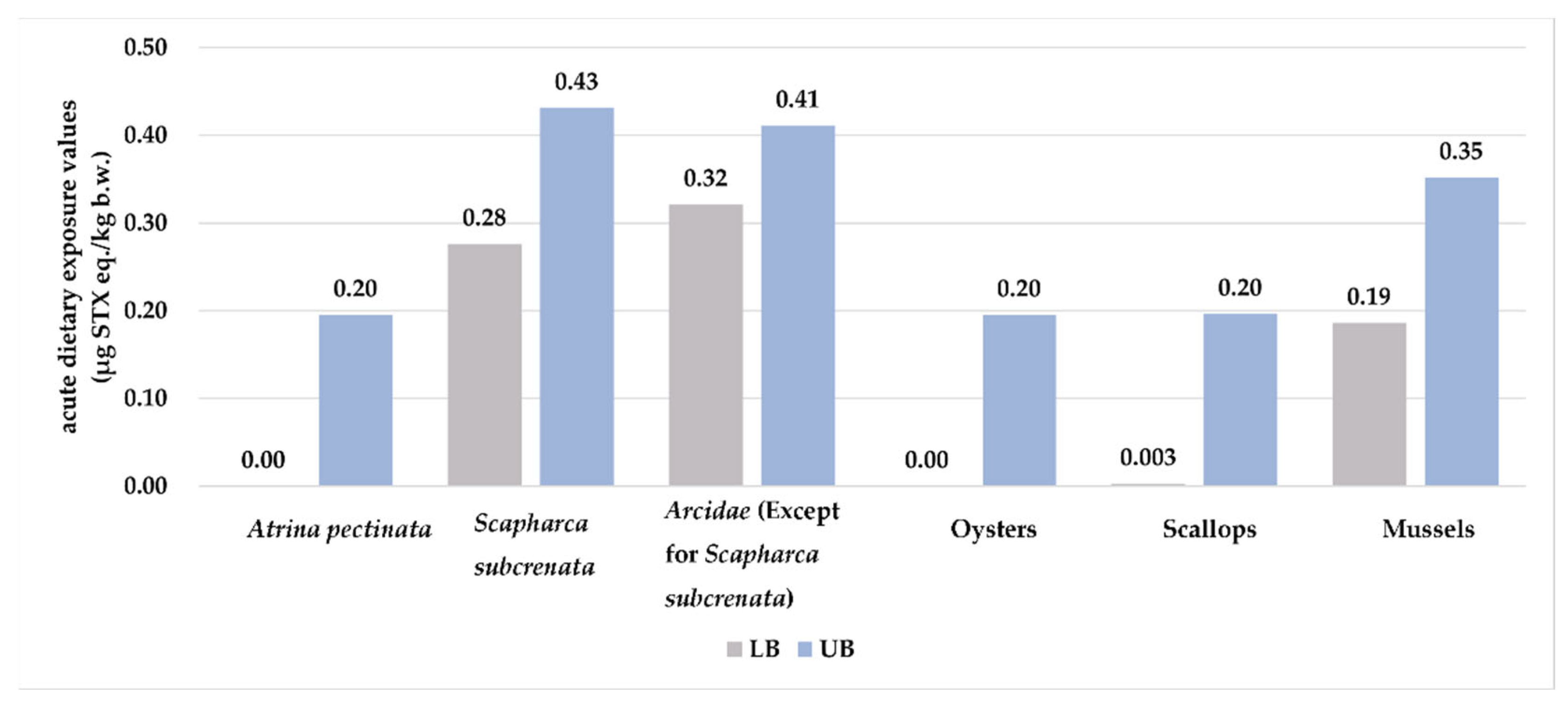
2.3. Dietary Exposure Assessment
2.4. One-Time Safe Consumption of Shellfish for Young Children
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample and Preparation
5.2. Chemical Reagents
5.3. Sample Analysis
5.4. Method Validation and Quality Control
5.5. Contamination Data Processing
5.6. Food Consumption Data
5.7. Assessment Methods
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Farabegoli, F.; Blanco, L.; Rodriguez, L.P.; Vieites, J.M.; Cabado, A.G. Phycotoxins in Marine Shellfish: Origin, Occurrence and Effects on Humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 7, 1019. [Google Scholar] [CrossRef]
- Lewis, A.M.; Dean, K.J.; Hartnell, D.M.; Percy, L.; Turner, A.D.; Lewis, J.M. The value of toxin profiles in the chemotaxonomic analysis of paralytic shellfish toxins in determining the relationship between British Alexandrium spp. and experimentally contaminated Mytilus sp. Harmful Algae 2022, 111, 102131. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, L.; Cao, Y.; Zhao, P.; Zhang, J.; Li, A. Bioaccessibility of paralytic shellfish toxins in different cooked shellfish using the simulated digestive model. Food Chem. 2022, 390, 133094. [Google Scholar] [CrossRef] [PubMed]
- Temple, C.; Hughes, A. A case of fatal paralytic shellfish poisoning in Alaska. Clin. Toxicol. 2022, 60, 414–415. [Google Scholar] [CrossRef]
- Li, A.; Ma, J.; Cao, J.; Wang, Q.; Yu, R.; Thomas, K.; Quilliam, M.A. Analysis of paralytic shellfish toxins and their metabolites in shellfish from the North Yellow Sea of China. Food Addit. Contam. A 2012, 29, 1455–1464. [Google Scholar] [CrossRef]
- Bates, N.; Morrison, C.; Flaig, L.; Turner, A.D. Paralytic shellfish poisoning and palytoxin poisoning in dogs. Vet. Rec. 2020, 187, e46. [Google Scholar] [CrossRef]
- Sobel, J.; Painter, J. Illnesses caused by marine toxins. Clin. Infect. Dis. 2005, 41, 1290–1296. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency (CFIA). Section B—Canadian Shellfish Sanitation Program Manual—Food Guidance by Commodity—Canadian Food Inspection Agency. Available online: https://inspection.canada.ca/food-guidance-by-commodity/fish/canadian-shellfish-sanitation-program/eng/1527251566006/1527251566942?chap=3 (accessed on 21 July 2023).
- Turnbull, A.R.; Harwood, D.T.; Boundy, M.J.; Holland, P.T.; Hallegraeff, G.; Malhi, N.; Quilliam, M.A. Paralytic shellfish toxins—Call for uniform reporting units. Toxicon 2020, 178, 59–60. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. National Shellfish Sanitation Program (NSSP). Guide for the Control of Molluscan Shellfish: 2019 Revision. Available online: https://www.fda.gov/media/143238/download?attachment (accessed on 29 October 2020).
- Cao, Y.; Qiu, J.; Li, A.; Zhang, L.; Yan, G.; Ji, Y.; Zhang, J.; Zhao, P.; Wu, X. Occurrence and spatial distribution of paralytic shellfish toxins in seawater and marine organisms in the coastal waters of Qinhuangdao, China. Chemosphere 2023, 315, 137746. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2021/1374 of 12 April 2021 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council on specific hygiene requirements for food of animal origin (Text with EEA relevance). Off. J. Eur. Union 2021, 297, 1–15. [Google Scholar]
- Goya, A.B.; Baqer, D.; Alexander, R.P.; Stubbs, P.; Dean, K.; Lewis, A.M.; Coates, L.; Maskrey, B.H.; Turner, A.D. Marine Biotoxins in Whole and Processed Scallops from the Argentine Sea. Mar. Drugs 2022, 20, 634. [Google Scholar] [CrossRef]
- Kibler, S.R.; Litaker, R.W.; Matweyou, J.A.; Hardison, D.R.; Wright, B.A.; Tester, P.A. Paralytic shellfish poisoning toxins in butter clams (Saxidomus gigantea) from the Kodiak Archipelago, Alaska. Harmful Algae 2022, 111, 102165. [Google Scholar] [CrossRef]
- Rourke, W.A.; Justason, A.; Martin, J.L.; Murphy, C.J. Shellfish Toxin Uptake and Depuration in Multiple Atlantic Canadian Molluscan Species: Application to Selection of Sentinel Species in Monitoring Programs. Toxins 2021, 13, 168. [Google Scholar] [CrossRef]
- Zheng, R.; Yang, Y.; Zhang, W.; Hua, Y. Contamination status of paralytic shellfish toxins in shellfish from Southeastern China in 2017–2021. Environ. Sci. Pollut. Res. 2023, 30, 34728–34740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, S.; Zhang, J.; Zhang, J.; Wang, Z.; Pan, L.; Huang, B.; Huang, K.; Chen, X.; Zhao, Q.; et al. Dietary exposure assessment of paralytic shellfish toxins through shellfish consumption in Shenzhen population, China. Environ. Sci. Pollut. Res. 2022, 29, 10222–10234. [Google Scholar] [CrossRef]
- Hajime, T. Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report). Mar. Pollut. Bull. 2006, 52, 1735–1745. [Google Scholar] [CrossRef]
- Suleiman, M.; Jelip, J.; Rundi, C.; Chua, T.H. Case Report: Paralytic Shellfish Poisoning in Sabah, Malaysia. Am. J. Trop. Med. Hyg. 2017, 97, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Wilson, K.; Veitch, M.G. An Outbreak of Paralytic Shellfish Poisoning in Tasmania. Commun. Dis. Intell. 2018, 42, S2209–S6051. [Google Scholar]
- Turner, A.D.; Dhanji-Rapkova, M.; Rowland-Pilgrim, S.; Turner, L.M.; Rai, A.; Venugopal, M.N.; Karunasagar, I.; Godhe, A. Assessing the presence of marine toxins in bivalve molluscs from southwest India. Toxicon 2017, 140, 147–156. [Google Scholar] [CrossRef] [PubMed]
- La Barbera-Sánchez, A.; Franco, S.J.; Rojas, D.A.L.; Chang-Yen, I. Paralytic shellfish poisoning (PSP) in Margarita Island, Venezuela. Rev. Biol. Trop. 2004, 52 (Suppl. S1), 89–98. [Google Scholar]
- Liu, Y.; Yu, R.; Kong, F.; Chen, Z.; Dai, L.; Gao, Y.; Zhang, Q.; Wang, Y.; Yan, T.; Zhou, M. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Mar. Pollut. Bull. 2017, 115, 324–331. [Google Scholar] [CrossRef]
- Andres, J.K.; Yñiguez, A.T.; Maister, J.M.; Turner, A.D.; Olano, D.; Mendoza, J.; Salvador-Reyes, L.; Azanza, R.V. Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis). Toxins 2019, 11, 468. [Google Scholar] [CrossRef]
- Qiu, J.; Meng, F.; Ding, L.; Che, Y.; McCarron, P.; Beach, D.G.; Li, A. Dynamics of paralytic shellfish toxins and their metabolites during timecourse exposure of scallops Chlamys farreri and mussels Mytilus galloprovincialis to Alexandrium pacificum. Aquat. Toxicol. 2018, 200, 233–240. [Google Scholar] [CrossRef]
- Cabado, A.G.; Lago, J.; Gonzalez, V.; Blanco, L.; Paz, B.; Diogene, J.; Ferreres, L.; Rambla-Alegre, M. Detoxification of paralytic shellfish poisoning toxins in naturally contaminated mussels, clams and scallops by an industrial procedure. Food Chem. Toxicol. 2020, 141, 111386. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.C.; Shaw, G.R.; Morrall, J.; Senogles, P.J.; Woods, T.A.; Papageorgiou, J.; Kapralos, C.; Wickramasinghe, W.; Davis, B.C.; Eaglesham, G.K.; et al. Chlorination for degrading saxitoxins (paralytic shellfish poisons) in water. Environ. Technol. 2003, 24, 1341–1348. [Google Scholar] [CrossRef]
- Zamyadi, A.; Ho, L.; Newcombe, G.; Bustamante, H.; Prevost, M. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Res. 2012, 46, 1524–1535. [Google Scholar] [CrossRef]
- Vasama, M.; Kumar, H.; Salminen, S.; Haskard, C.A. Removal of paralytic shellfish toxins by probiotic lactic acid bacteria. Toxins 2014, 6, 2127–2136. [Google Scholar] [CrossRef]
- Zheng, X. Precise Monitoring and Edible Risk Assessment of Cultured Shellfish Toxins in Bohai Sea, China. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Shin, C.; Jo, H.; Kim, S.; Kang, G. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Res. Int. 2018, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, J.; Yao, X.; Yao, S.; Guo, Y.; Zhao, Y.; Jin, M. Contamination status and the evaluation of dietary exposure of marine biotoxins in seafood in Ningbo City in 2017–2019. J. Hyg. Res. 2021, 50, 296–300. [Google Scholar] [CrossRef]
- Lu, J. Study on Detection Technology, Pollution Level and Dietary Exposure Assessment of Marine Biotoxin in Bivalve Shellfish of Fujian Province, China. Master’s Thesis, Fujian Medical University, Fujian, China, 2018. [Google Scholar]
- Shi, W.; Chen, Y.; Xianqin, H.; Wang, Y.; Chunqing, L.; Gao, L. Dietary exposure assessment of 13 paralytic shellfish toxins in commercially available shellfish products in Tianjin, China. Jiangxi Fish. Sci. Technol. 2021, 6, 48–50, 52. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs of the People’s Republic of China, National Fisheries Technology Extension Center (NFTEC), China Society of Fisheries (CSF). China Fishery Statistical Yearbook—2019; China Agriculture Press: Beijing, China, 2018; p. 27. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United States. Dietary exposure assessment of chemicals in food, Chapter 6. In Principles and Methods for the Risk Assessment of Chemicals in Food; Environmental Health Criteria 240; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Authority, E.F.S. Management of left-censored data in dietary exposure assessment of chemical substances. Efsa J. 2010, 8, 1557. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Cheng, Y.; Wen, H.; Liu, R.; Wang, L.; Shan, C.; Chai, C. Multi-year assessment of paralytic shellfish toxins in hard clam species along the coastline of Jiangsu Province, China. Acta Oceanol. Sin. 2019, 38, 24–33. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Y.; Wang, R.; Zhao, X.; Wang, T. Investigation on the paralytic shellfish poisons in bivalves collected from different coastal areas in Hainan Island, China. J. Food Saf. Qual. 2021, 12, 9605–9611. [Google Scholar] [CrossRef]
- Lin, Z.; Geng, H.; Tang, W.; Yu, R. Transformation process of paralytic shellfish toxins in Scapharca subcrenata. Oceanol. Limnol. Sin. 2022, 53, 1131–1142. [Google Scholar] [CrossRef]
- Chung, P.H.; Chuang, S.K.; Tsang, T. Consumption of viscera as the most important risk factor in the largest outbreak of shellfish poisoning in Hong Kong, 2005. Southeast Asian J. Trop. Med. Public Health 2006, 37, 120–125. [Google Scholar]
- Department of Natural Resources of Zhejiang Province. Marine Disaster Bulletin of Zhejiang Province. 2019. Available online: https://zrzyt.zj.gov.cn/art/2020/5/27/art_1289933_43538689.html (accessed on 27 May 2020).
- Department of Natural Resources of Zhejiang Province. Marine Disaster Bulletin of Zhejiang Province. 2018. Available online: https://zrzyt.zj.gov.cn/art/2019/5/12/art_1289933_43537795.html (accessed on 12 May 2019).
- Lin, Z.R.; Geng, H.X.; Zhang, Q.C.; Chen, Z.F.; Dai, L.; Yu, R.C. Toxin production of dinoflagellate Gymnodinium catenatum isolated from the East China Sea. Harmful Algae 2022, 113, 102188. [Google Scholar] [CrossRef]
- Wang, P.; Mao, K.; Meng, F.; Yuan, Z. Spatiotemporal evolution of sea surface temperature in the East China Sea. Remote Sens. Land Resour. 2020, 32, 227–235. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Liu, B.; Yang, H.; Qin, W.; Li, L.; Chang, F. Investigation and analysis of paralytic shellfish poison pollution in retail shellfish in Hebei province from 2018 to 2020. J. Food Saf. Qual. 2021, 12, 1746–1752. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Picot, C.; Tran, T.L.; Carpentier, F.G.; Roudot, A.C.; Parent-Massin, D.M. Evaluation of shellfish consumption in Nha Trang City, Southern coastal Vietnam. Malays. J. Nutr. 2012, 18, 37–45. [Google Scholar] [PubMed]
- Picot, C.; Nguyen, T.A.; Carpentier, F.G.; Roudot, A.C.; Parent-Massin, D. Relevant shellfish consumption data for dietary exposure assessment among high shellfish consumers, Western Brittany, France. Int. J. Environ. Health Res. 2011, 21, 86–105. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Rossignoli, A.E.; Riobo, P.; Rodriguez, F. First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain. Toxins 2020, 12, 723. [Google Scholar] [CrossRef]
- Arya, R.; Antonisamy, B.; Kumar, S. Sample size estimation in prevalence studies. Indian. J. Pediatr. 2012, 79, 1482–1488. [Google Scholar] [CrossRef]
- GB 5009.213-2016; National Standard of Food Safety: Determination of Paralytic Shellfish Toxins in Shellfish. National Health and Family Planning Commission of the People’s Republic of China. China Food and Drug Administration: Beijing, China, 2016.
- Chen, X.; Huang, B.; Zhao, Q.; Wang, Z.; Liu, W.; Zhang, J.; Zhou, Y.; Sun, Q.; Huang, H.; Huang, X.; et al. Shellfish contamination with lipophilic toxins and dietary exposure assessments from consumption of shellfish products in Shenzhen, China. Ecotoxicol. Environ. Saf. 2021, 221, 112446. [Google Scholar] [CrossRef]
- Chinese Center for Disease Control and Prevention. Chinese Food Composition Table Standard Edition, 6th ed.; Peking University Medical Press: Beijing, China, 2019; Volume 2, pp. 134–138. [Google Scholar]
- Fang, L.; Zhao, B.; Zhang, R.; Wu, P.; Zhao, D.; Chen, J.; Pan, X.; Wang, J.; Wu, X.; Zhang, H.; et al. Occurrence and exposure assessment of aflatoxins in Zhejiang province, China. Environ. Toxicol. Pharmacol. 2022, 92, 103847. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Silipunyo, T.; Huang, H.; Yin, Q.; Han, B.; Wang, M. Risk Assessment of Triflumezopyrim and Imidacloprid in Rice through an Evaluation of Residual Data. Molecules 2022, 27, 5685. [Google Scholar] [CrossRef] [PubMed]
| Individual PSTs | N (%) 1 | Concentration Range |
|---|---|---|
| (<LOD 2-Max, µg/kg) | ||
| STX | 13 (2.4) | <20–168 |
| neoSTX | 4 (0.7) | <20–101 |
| GTX1 | 0 (0) | ND 3 |
| GTX2 | 8 (1.5) | <20–128 |
| GTX3 | 4 (0.7) | <20–60.2 |
| GTX4 | 0 (0) | ND |
| GTX5 | 23 (4.2) | <20–242 |
| dcSTX | 1 (0.2) | <20–26 |
| dcGTX2 | 13 (2.4) | <20–58.4 |
| dcGTX3 | 3 (0.5) | <20–23.1 |
| Sample Size | Detected N (%) 1 | Mean 2 | Median 3 | Max 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| (µg STX eq./kg) | (µg STX eq./kg) | (µg STX eq./kg) | |||||||
| LB 5 | UB 6 | LB | UB | LB | UB | ||||
| Total species | 546 | 38 (7.0) | 4.1 | 135.1 | 0 | 132.2 | 186.9 | 291.9 | |
| Species | |||||||||
| Atrina pectinata | 2 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 | |
| Scapharca subcrenata | 24 | 10 (41.7) | 53.7 | 174.2 | 0 | 132.2 | 186.9 | 291.9 | |
| Arcidae (Except for Scapharca subcrenata) | 53 | 3 (5.7) | 8.5 | 137.3 | 0 | 132.2 | 217.4 | 278.2 | |
| Oysters | 77 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 | |
| Scallops | 36 | 1 (2.8) | 0.1 | 132.2 | 0 | 132.2 | 1.9 | 132.9 | |
| Mussels | 354 | 24 (6.8) | 1.4 | 133.0 | 0 | 132.2 | 126.0 | 238.2 | |
| Sampling time | |||||||||
| May | 2018 | 30 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 |
| 2019 | 70 | 8 (11.4) | 4.7 | 135.0 | 0 | 132.2 | 126.0 | 238.2 | |
| June | 2018 | 123 | 3 (2.4) | 0.1 | 132.3 | 0 | 132.2 | 5.4 | 136.4 |
| 2019 | 134 | 13 (9.7) | 1.3 | 132.8 | 0 | 132.2 | 35.6 | 153.6 | |
| July | 2018 | 40 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 |
| 2019 | 28 | 6 (21.4) | 32.2 | 159.3 | 0 | 132.2 | 186.9 | 291.9 | |
| August | 2018 | 40 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 |
| 2019 | 10 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 | |
| September | 2018 | 48 | 2 (4.2) | 3.7 | 135.0 | 0 | 132.2 | 176.3 | 267.3 |
| 2019 | 23 | 6 (26.1) | 28.8 | 148.8 | 0 | 132.2 | 217.4 | 278.2 | |
| Sampling site | |||||||||
| Hangzhou | 29 | 12 (41.4) | 5.7 | 134.8 | 0 | 132.2 | 35.6 | 153.6 | |
| Ningbo | 124 | 3 (2.4) | 0.1 | 132.3 | 0 | 132.2 | 5.4 | 136.4 | |
| Taizhou | 124 | 16 (12.9) | 8.5 | 137.1 | 0 | 132.2 | 217.4 | 278.2 | |
| Wenzhou | 145 | 0 (0) | 0 | 132.2 | 0 | 132.2 | 0 | 132.2 | |
| Zhoushan | 124 | 7 (5.6) | 8.3 | 139.2 | 0 | 132.2 | 186.9 | 291.9 | |
| Age (Years) | N | Body Weight (kg) | Consumption(g/d) | Dietary Exposure LB 1-UB 2 (µg STX eq./kg b.w.) | %ARfD 3 LB-UB (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | P95 | A 4 | B 5 | C 6 | D 7 | A | B | C | D | |||
| All | 1075 | 57.9 | 30.0 | 85.5 | 0–0.07 | 0.10–0.15 | 0–0.20 | 0.28–0.43 | 0–14 (0–10) | 20–30 (14–21) | 0–40 (0–29) | 56–86 (40–61) |
| ≤6 | 50 | 19.2 | 19.5 | 64.0 | 0–0.13 | 0.19–0.30 | 0–0.44 | 0.62–0.97 | 0–26 (0–19) | 38–60 (27–43) | 0–88 (0–63) | 124–194 (89–139) |
| 7–13 | 83 | 35.4 | 23.4 | 58.5 | 0–0.09 | 0.12–0.19 | 0–0.22 | 0.31–0.48 | 0–18 (0–13) | 24–38 (17–27) | 0–44 (0–31) | 62–96 (44–69) |
| 14–17 | 22 | 57.7 | 31.2 | 78.9 | 0–0.07 | 0.10–0.16 | 0–0.18 | 0.26–0.40 | 0–14 (0–10) | 20–32 (14–23) | 0–36 (0–26) | 52–80 (37–57) |
| 18–59 | 817 | 62.0 | 30.0 | 92.0 | 0–0.06 | 0.09–0.14 | 0–0.20 | 0.28–0.43 | 0–12 (0–9) | 18–28 (13–20) | 0–40 (0–29) | 56–86 (40–61) |
| ≥60 | 103 | 62.2 | 27.6 | 71.0 | 0–0.06 | 0.08–0.13 | 0–0.15 | 0.21–0.33 | 0–12 (0–9) | 16–26 (11–19) | 0–30 (0–21) | 42–66 (30–47) |
| Species | Contamination Level 1 (µg STX eq./kg) | Serving Size 1 2(g/d) | Serving Size 2 3(g/d) |
|---|---|---|---|
| Atrina pectinata | 132.2 | 72.8 | 101.9 |
| Scapharca subcrenata | 291.9 | 33.0 | 46.1 |
| Arcidae (Except for Scapharca subcrenata) | 278.2 | 34.6 | 48.4 |
| Oysters | 132.2 | 72.8 | 101.9 |
| Scallops | 132.9 | 72.4 | 101.3 |
| Mussels | 238.2 | 40.4 | 56.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Q.; Zhang, R.; Wu, P.; Chen, J.; Pan, X.; Zhao, D.; Wang, J.; Zhang, H.; Qi, X.; Wu, X.; et al. An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China. Toxins 2023, 15, 624. https://doi.org/10.3390/toxins15110624
Weng Q, Zhang R, Wu P, Chen J, Pan X, Zhao D, Wang J, Zhang H, Qi X, Wu X, et al. An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China. Toxins. 2023; 15(11):624. https://doi.org/10.3390/toxins15110624
Chicago/Turabian StyleWeng, Qin, Ronghua Zhang, Pinggu Wu, Jiang Chen, Xiaodong Pan, Dong Zhao, Jikai Wang, Hexiang Zhang, Xiaojuan Qi, Xiaoli Wu, and et al. 2023. "An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China" Toxins 15, no. 11: 624. https://doi.org/10.3390/toxins15110624
APA StyleWeng, Q., Zhang, R., Wu, P., Chen, J., Pan, X., Zhao, D., Wang, J., Zhang, H., Qi, X., Wu, X., Han, J., & Zhou, B. (2023). An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China. Toxins, 15(11), 624. https://doi.org/10.3390/toxins15110624





