Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study
Abstract
1. Introduction
2. Results
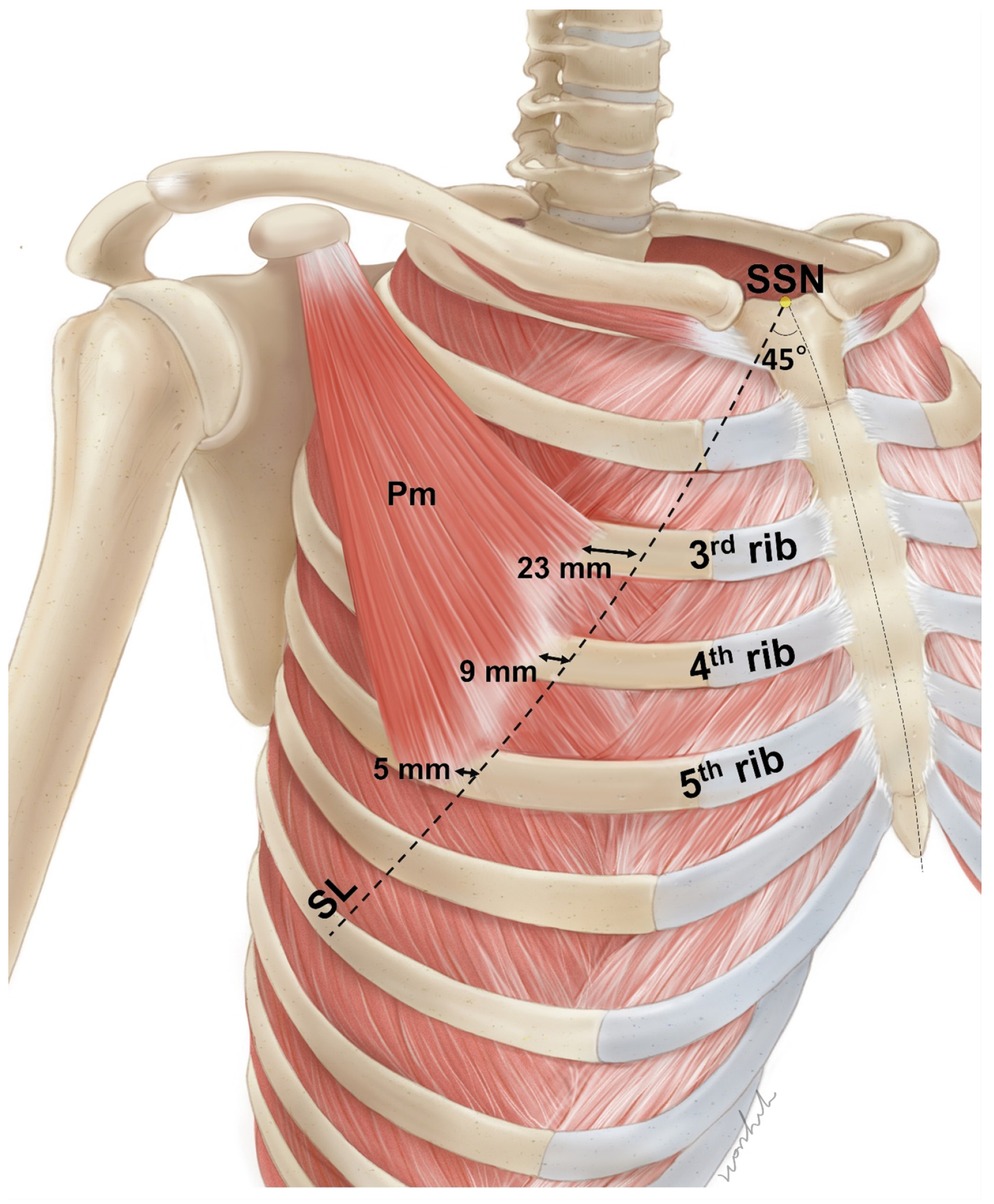
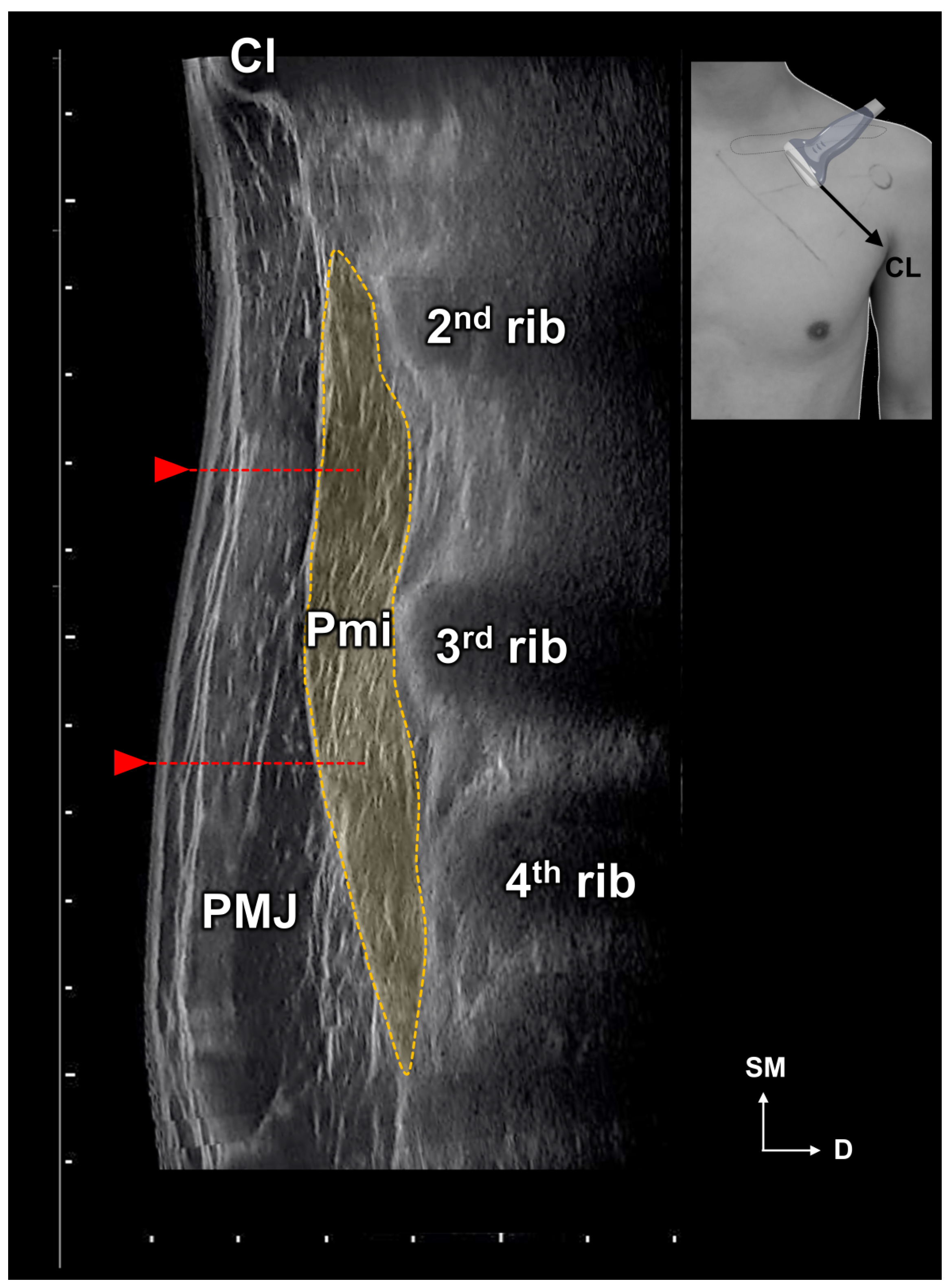
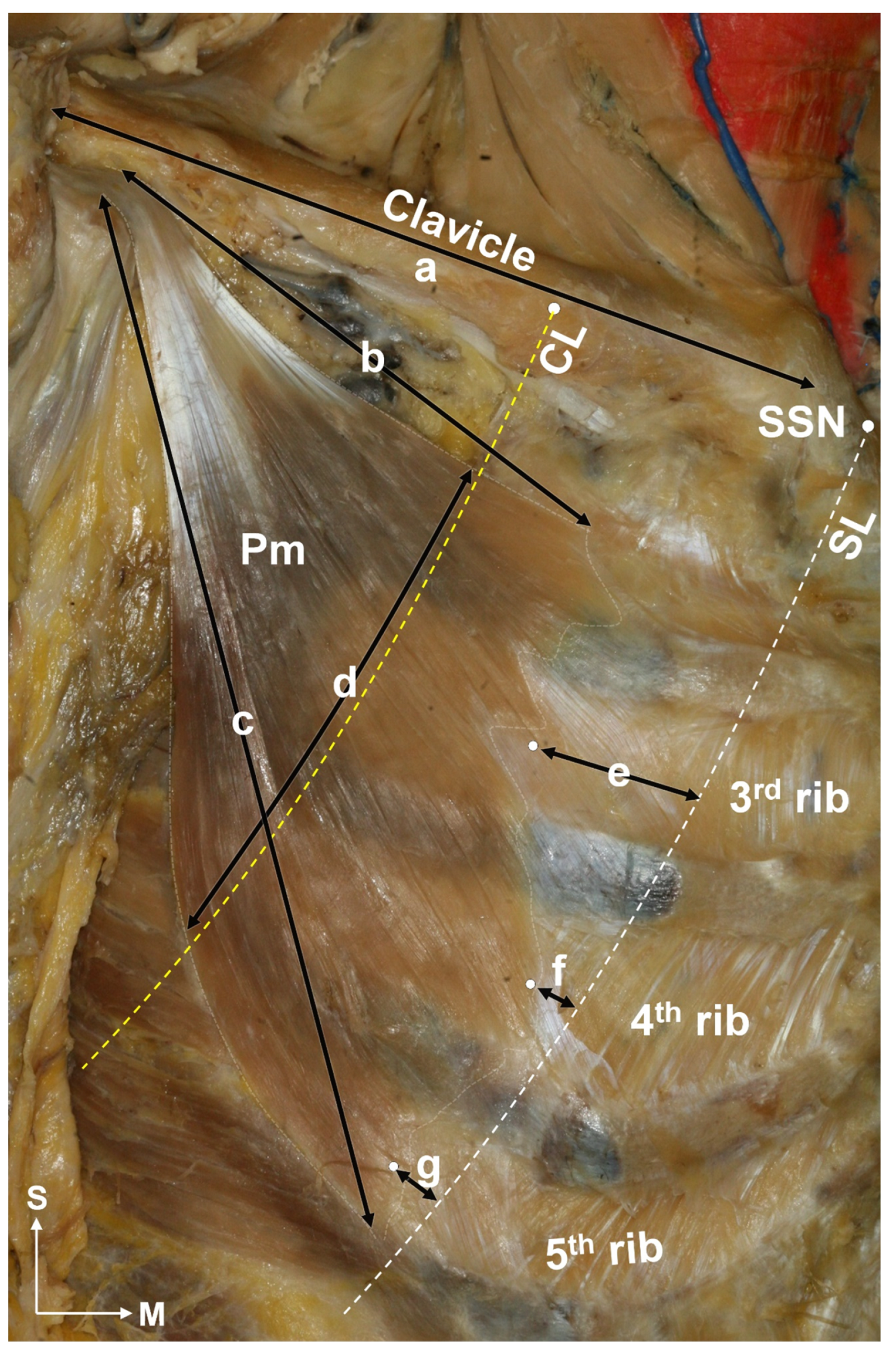
2.1. Locations of the Pm Muscle: Origin and Nerve Entry Points
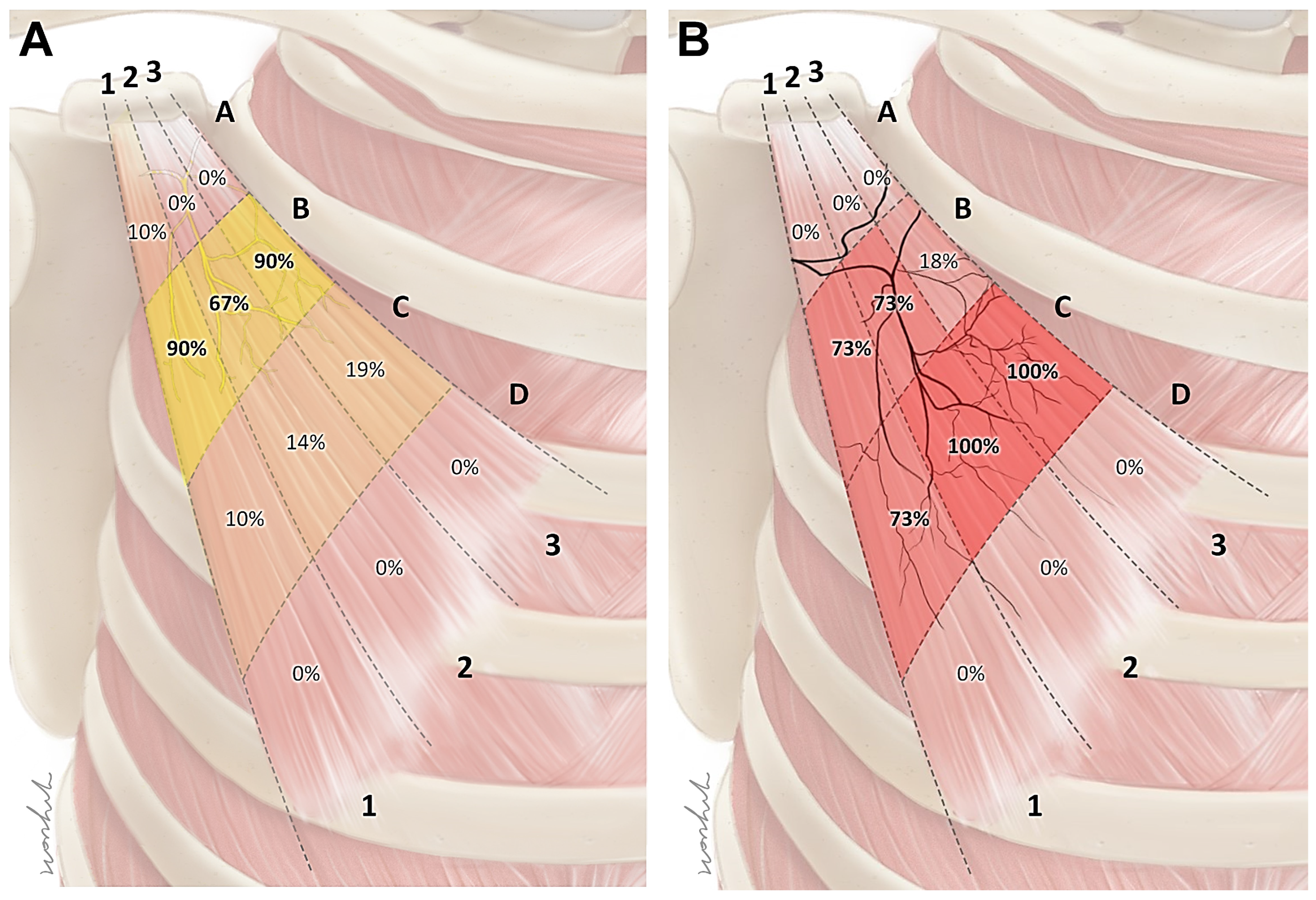
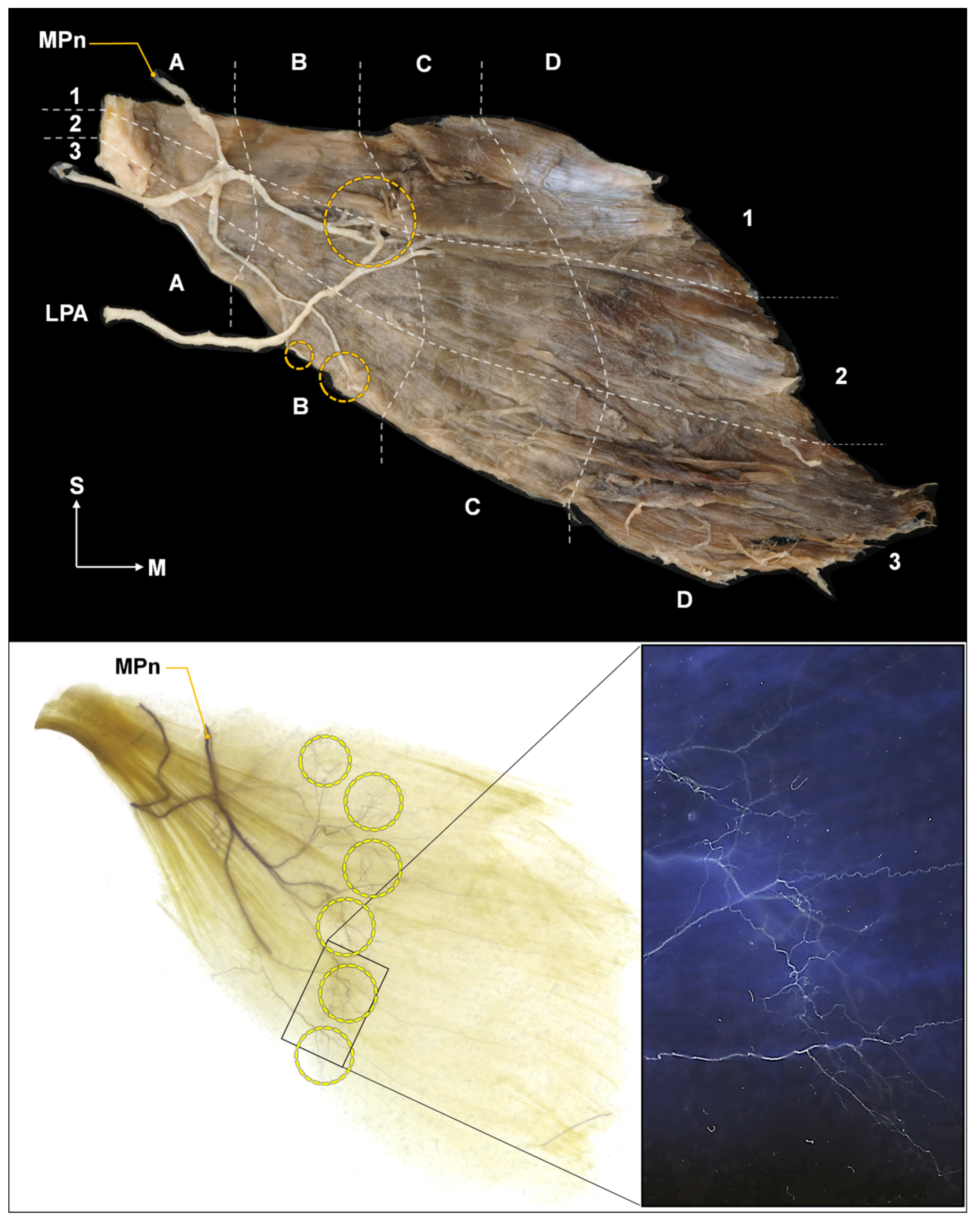
2.2. Intramuscular Arborization Patterns of the Pm Muscle
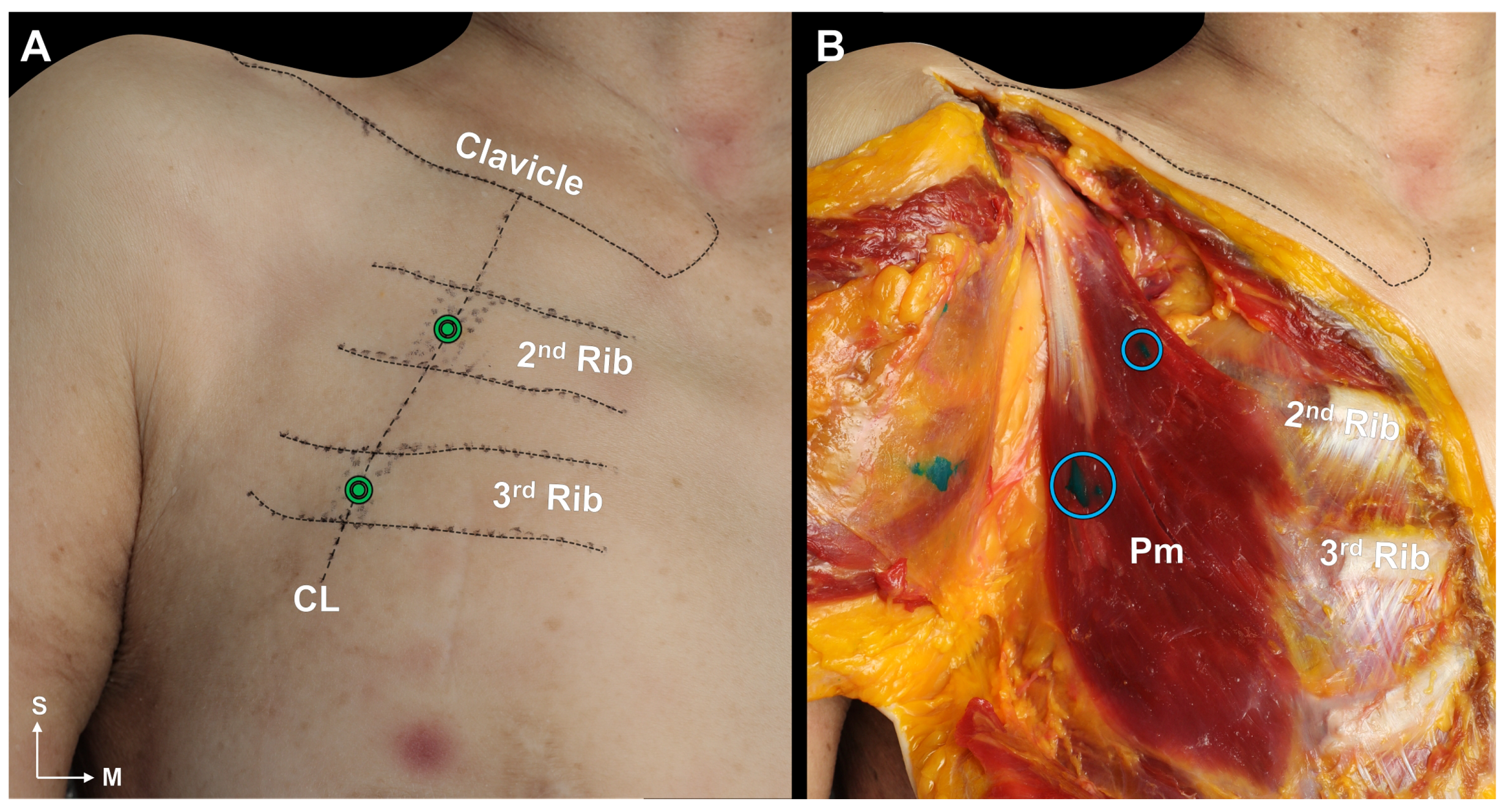
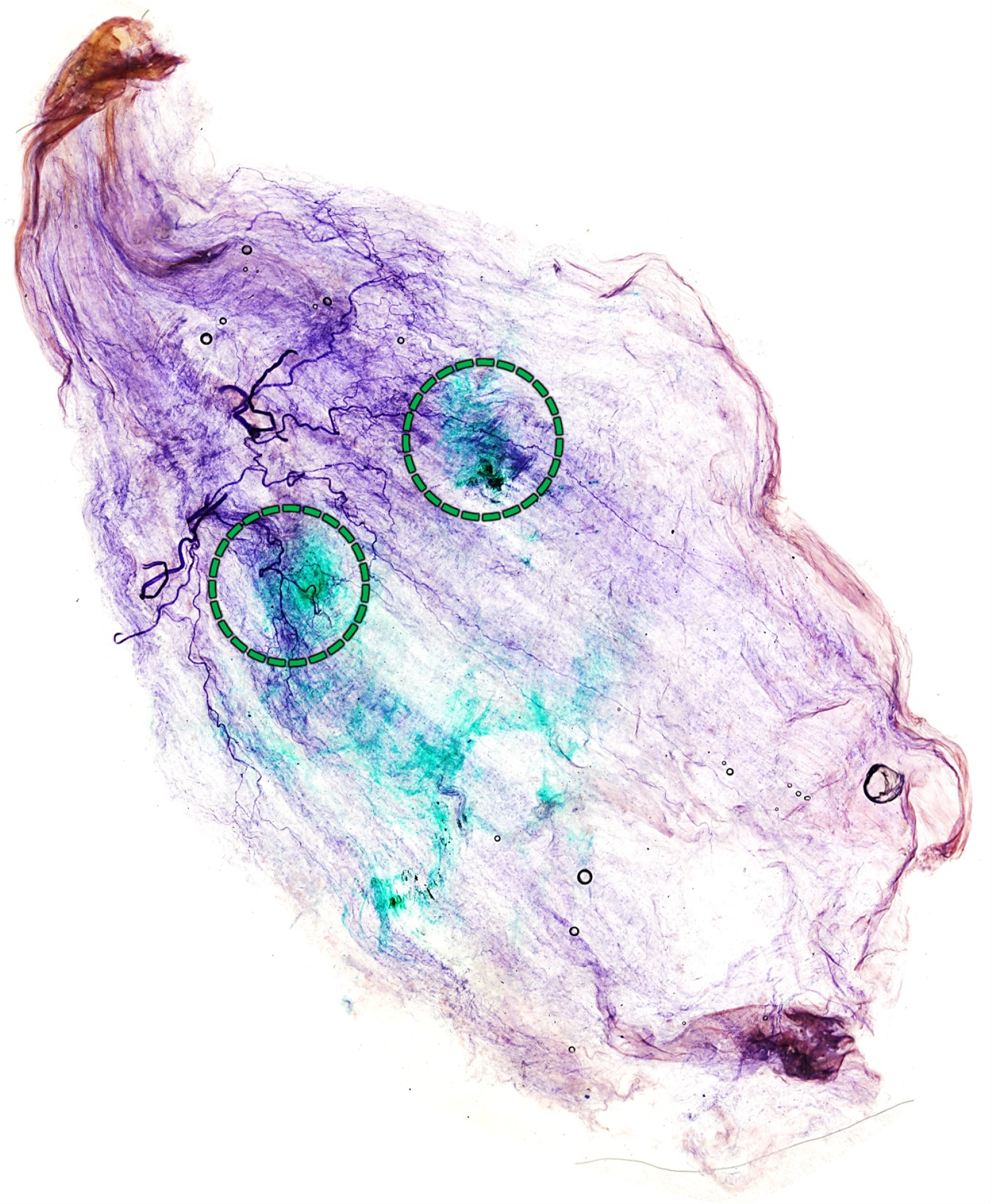
2.3. Blind Dye Injection of the Pm Muscle of Fresh Cadavers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Measurements of the Location of the Pm
5.1.1. Identification of the Location of the Origin of the Pectoralis Minor Muscle Based on the Surface Landmarks
5.1.2. Nerve Entry Point of the Pm Muscle
5.2. Intramuscular Arborization of the Pm Muscle
Modified Sihler’s Staining
5.3. Cadaveric Evaluation of Blind Injection of the Pm Muscle
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BoNT | Botulinum neurotoxins |
| PM | Pectoralis minor muscle |
| PMS | Pectoralis minor syndrome |
| TOS | Thoracic outlet syndrome |
| CL | The line passing the medial one-third of the clavicle at a 45-degree angle |
| SL | The line passing through the suprasternal notch at a 45-degree angle |
References
- Sanders, R.J.; Hammond, S.L.; Rao, N.M. Thoracic outlet syndrome: A review. Neurologist 2008, 14, 365–373. [Google Scholar] [CrossRef]
- Sanders, R.J. Recurrent neurogenic thoracic outlet syndrome stressing the importance of pectoralis minor syndrome. Vasc. Endovasc. Surg. 2011, 45, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.J.; Annest, S.J. Pectoralis Minor Syndrome: Subclavicular Brachial Plexus Compression. Diagnostics 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.J.; Rao, N.M. The forgotten pectoralis minor syndrome: 100 operations for pectoralis minor syndrome alone or accompanied by neurogenic thoracic outlet syndrome. Ann. Vasc. Surg. 2010, 24, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Aktas, I.; Kaya, E.; Akpinar, P.; Atici, A.; Unlu Ozkan, F.; Palamar, D.; Akgun, K. Spasticity-induced Pectoralis minor syndrome: A case-report. Top. Stroke Rehabil. 2020, 27, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Donahue, D.M.; Godoy, I.R.B.; Gupta, R.; Donahue, J.A.; Torriani, M. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: Correlation with surgical outcomes. Skelet. Radiol. 2020, 49, 715–722. [Google Scholar] [CrossRef]
- Martinez Del Carmen, D.T.; Marti Mestre, F.X.; Tripodi, P.; Macia Vidueira, I.; Ramos Izquierdo, R.; Romera Villegas, A. Role of Botulinum Toxin in Pectoralis Minor Syndrome. Ann. Vasc. Surg. 2022, 81, 225–231. [Google Scholar] [CrossRef]
- Haeni, D.; Martinez-Catalan, N.; Esper, R.N.; Wagner, E.R.; El Hassan, B.T.; Sanchez-Sotelo, J. Arthroscopic release of the pectoralis minor tendon from the coracoid for pectoralis minor syndrome. J. Exp. Orthop. 2022, 9, 57. [Google Scholar] [CrossRef]
- Singla, D.; Veqar, Z. Association Between Forward Head, Rounded Shoulders, and Increased Thoracic Kyphosis: A Review of the Literature. J. Chiropr. Med. 2017, 16, 220–229. [Google Scholar] [CrossRef]
- Foley, J.M.; Finlayson, H.; Travlos, A. A review of thoracic outlet syndrome and the possible role of botulinum toxin in the treatment of this syndrome. Toxins 2012, 4, 1223–1235. [Google Scholar] [CrossRef]
- Qerama, E.; Fuglsang-Frederiksen, A.; Jensen, T.S. The role of botulinum toxin in management of pain: An evidence-based review. Curr. Opin. Anaesthesiol. 2010, 23, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Fugate, M.W.; Rotellini-Coltvet, L.; Freischlag, J.A. Current management of thoracic outlet syndrome. Curr. Treat. Options Cardiovasc. Med. 2009, 11, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ramcharitar, R.K.; Elghawy, O.; Man, L.; Sharma, A.M.; Peruri, A.; Park, A.W.; Norton, P.; Khaja, M.; Tripathi, R. Images in Vascular Medicine Pectoralis minor syndrome—A forgotten vascular compression syndrome. Vasc. Med. 2022, 27, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Jones, M.; Clarkson, R.; Donaldson, O. Pectoralis minor syndrome: Diagnosis with Botulinum injection and treatment with tenotomy—A prospective case series. Shoulder Elbow 2022, 14, 157–161. [Google Scholar] [CrossRef]
- Lu, D.W.; Lippitz, J. Complications of Botulinum Neurotoxin. Disease-a-Month 2009, 55, 198–211. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Hentzen, C.; Cuvillon, P.; Allart, E.; Bonan, I.V.; Boyer, F.C.; Coroian, F.; Genet, F.; Honore, T.; Jousse, M.; et al. French clinical guidelines for peripheral motor nerve blocks in a PRM setting. Ann. Phys. Rehabil. Med. 2019, 62, 252–264. [Google Scholar] [CrossRef]
- Winston, P.; Reebye, R.; Picelli, A.; David, R.; Boissonnault, E. Recommendations for Ultrasound Guidance for Diagnostic Nerve Blocks for Spasticity. What Are the Benefits? Arch. Phys. Med. Rehabil. 2023, 104, 1539–1548. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Choi, Y.J.; Lee, J.H.; Hu, K.S.; Kim, H.J. Intramuscular Neural Distribution of Rhomboid Muscles: Evaluation for Botulinum Toxin Injection Using Modified Sihler’s Method. Toxins 2020, 12, 289. [Google Scholar] [CrossRef]
- Park, M.Y.; Ahn, K.Y. Scientific review of the aesthetic uses of botulinum toxin type A. Arch. Craniofac. Surg. 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Odderson, I.R.; Chun, E.S.; Kolokythas, O.; Zierler, R.E. Use of sonography in thoracic outlet syndrome due to a dystonic pectoralis minor. J. Ultrasound Med. 2009, 28, 1235–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oddy, M.J.; Brown, C.; Mistry, R.; Eastwood, D.M. Botulinum toxin injection site localization for the tibialis posterior muscle. J. Pediatr. Orthop. B 2006, 15, 414–417. [Google Scholar] [CrossRef]
- Kim, H.; Nam, K.; Kang, D.; Oh, S.; Kho, D. Anatomical Analysis of Clavicles in Korean Adults and Compatibility of Pre-Contoured Anatomical Plates. J. Korean Orthop. Assoc. 2013, 48, 350–358. [Google Scholar] [CrossRef]
- Anson, B.J.; Beaton, L.E.; McDonald, J.J. The Origin of the M. Pectoralis Minor. J. Anat. 1938, 72, 629–630. [Google Scholar] [PubMed]
- Dede, B.T.; Temel, M.H.; Bağcıer, F. Dry needling with blinded technique in pectoralis minor syndrome. Turk. J. Phys. Med. Rehabil. 2023, 4, 257–258. [Google Scholar] [CrossRef]
- Aktaş, İ.; Ünlü Özkan, F. Pectoralis minor syndrome. Turk. J. Phys. Med. Rehabil. 2022, 22, 447–455. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Molenaers, G. Localization of the motor endplate zone in human skeletal muscles of the lower limb: Anatomical guidelines for injection with botulinum toxin. Dev. Med. Child Neurol. 2011, 53, 108–119. [Google Scholar] [CrossRef]
- Lapatki, B.G.; van Dijk, J.P.; van de Warrenburg, B.P.; Zwarts, M.J. Botulinum toxin has an increased effect when targeted toward the muscle’s endplate zone: A high-density surface EMG guided study. Clin. Neurophysiol. 2011, 122, 1611–1616. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Bar-On, L.; Desloovere, K.; Molenaers, G. Role of motor end plate-targeted Botulinum toxin type A injections in children with cerebral palsyitle. Acta Orthop. Belg. 2015, 81, 167–171. [Google Scholar]
- Selander, D.; Dhunér, K.G.; Lundborg, G. Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol. Scand. 1977, 21, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Steinfeldt, T.; Nimphius, W.; Werner, T.; Vassiliou, T.; Kill, C.; Karakas, E.; Wulf, H.; Graf, J. Nerve injury by needle nerve perforation in regional anaesthesia: Does size matter? BJA Br. J. Anaesth. 2009, 104, 245–253. [Google Scholar] [CrossRef] [PubMed]

| Rib Number * | 1.5–3.5 | 2–4 | 2–4.5 | 2–5 | 2.5–4 | 2.5–4.5 | 2.5–5 | 3–5 | 3–5.5 | 3–6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | 1 | 4 | 5 | 2 | 2 | 3 | 3 | 10 | 2 | 1 |
| Percentage | 3% | 12% | 15% | 6% | 6% | 9% | 9% | 30% | 6% | 3% |
| Rib Number | 2nd and 3rd Rib | 2nd, 3rd, and 4th Rib | 3rd, 4th, and 5th Rib |
|---|---|---|---|
| Cases | 10/33 | 22/33 | 1/33 |
| Percentage | 30% | 67% | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lee, H.-J.; Yi, K.-H.; Lee, K.-W.; Gil, Y.-C.; Kim, H.-J. Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study. Toxins 2023, 15, 603. https://doi.org/10.3390/toxins15100603
Lee J-H, Lee H-J, Yi K-H, Lee K-W, Gil Y-C, Kim H-J. Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study. Toxins. 2023; 15(10):603. https://doi.org/10.3390/toxins15100603
Chicago/Turabian StyleLee, Ji-Hyun, Hyung-Jin Lee, Kyu-Ho Yi, Kang-Woo Lee, Young-Chun Gil, and Hee-Jin Kim. 2023. "Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study" Toxins 15, no. 10: 603. https://doi.org/10.3390/toxins15100603
APA StyleLee, J.-H., Lee, H.-J., Yi, K.-H., Lee, K.-W., Gil, Y.-C., & Kim, H.-J. (2023). Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study. Toxins, 15(10), 603. https://doi.org/10.3390/toxins15100603







