Abstract
Patients bitten by Protobothrops mucrosquamatus, Viridovipera stejnegeri, and Naja atra develop different degrees of wound infection. This study validated BITE and Cobra BITE scoring systems that we established previously. Bacteriological studies of patients with wound infection were conducted. The operating characteristic curves and area under the curve (AUC) and wound infection rates were compared between the derivation set (our previous study patient population) and the validation set (new patient cohorts enrolled between June 2017 and May 2021). No significant differences in the AUC for both the BITE (0.84 vs. 0.78, p = 0.27) and Cobra BITE (0.88 vs. 0.75, p = 0.21) scoring systems were observed between the derivation and validation sets. Morganella morganii and Enterococcus faecalis were the two most commonly detected bacteria in the microbiological study. More bacterial species were cultured from N. atra-infected wounds. Antibiotics such as amoxicillin with clavulanic acid, oxacillin, and ampicillin may not be suitable for treating patients with P. mucrosquamatus, V. stejnegeri, and N. atra bites in Taiwan. Carbapenem, third-generation cephalosporins, and fluoroquinolone may be superior alternatives.
Keywords:
wound infections; snakebites; Protobothrops mucrosquamatus; Viridovipera stejnegeri; Naja atra Key Contribution:
In this study, BITE and Cobra BITE scoring systems were successfully validated. In addition, we developed two flowcharts for clinicians to estimate infection risk and select antibiotics for the treatment of patients bitten by Protobothrops mucrosquamatus, Viridovipera stejnegeri, and Naja atra in Taiwan.
1. Introduction
Six clinically important venomous snake species are found in Taiwan, namely Protobothrops mucrosquamatus (Taiwan habu), Viridovipera stejnegeri (green bamboo viper), Naja atra (Taiwan or Chinese cobra), Bungarus multicinctus (Taiwan banded krait), Deinagkistrodon acutus (hundred pacer viper), and Daboia siamensis. Two-thirds of all venomous snakebites in Taiwan are caused by P. mucrosquamatus and V. stejnegeri [1]. Patients with venomous snakebites present with various symptoms, including swelling, tenderness, and local heat in the affected limb tissue; cellulitis; and wound infection. N. atra accounts for approximately one-fifth of poisonous snakebites in Taiwan [1]. Instead of neurological complications, patients bitten by N. atra experience tissue damage, wound necrosis, and necrotizing fasciitis because of cytotoxins present in the venom and, these wounds have a high risk of infection [1,2,3,4].
The available treatments for these snakebites are toxoid vaccine injection, antibiotics, symptomatic relief agents, and antivenins [5]. Physicians prescribe freeze-dried hemorrhagic (FH) antivenom to treat bite wounds caused by P. mucrosquamatus or V. stejnegeri and freeze-dried neurotoxic (FN) antivenom for wounds caused by N. atra [6]. Except for wound necrosis caused by N. atra bites that require antibiotic treatment, determining when and how to administer antibiotics is challenging for clinicians treating patients with snakebites; this problem therefore warrants investigation. We have previously developed scoring systems for wounds in the BITE [7] and Cobra BITE studies [8]; however, they have not been validated yet. This study validated our previous two scoring systems for wound infections caused by the bites of P. mucrosquamatus, V. stejnegeri, and N. atra. Moreover, we compared bacteriological results between patients bitten by P. mucrosquamatus or V. stejnegeri and those bitten by N. atra bite. Finally, we provide practical antibiotic treatment suggestions to clinical physicians on the basis of bacteriological wound culture results, including antibiotic sensitivity tests.
2. Results
2.1. Patient Characteristics of the Validation Cohort of the BITE Study
A total of 95 patients received at least one vial of FH antivenom. A median of two vials (interquartile range [IQR] 2–4) of FH antivenom was used for these patients. Among the patients who received FH antivenom treatment, 35 were diagnosed as having wound infections during the treatment course. Lymphocyte count was the only laboratory variable that differed significantly between the patients with and without wound infection (Table S1). Furthermore, 51 of the 95 patients required hospitalization, and the length of hospitalization was 3.86 ± 3.76 days. The wound infection group had more patients requiring hospital admission compared with the no wound infection group (30/35 vs. 21/60, p < 0.01). The length of hospitalization in the wound infection group was significantly longer than that in the no wound infection group (5.31 ± 4.40 vs. 1.79 ± 2.85, p < 0.01; Table S1). Among the hospitalized patients, five received debridement, five received fasciotomy, and two received graft treatment. Two patients received debridement with fasciotomy and graft treatment, and two received debridement and fasciotomy. No patients died, but one patient was admitted to the intensive care unit (ICU) because of the development of rhabdomyolysis and acute kidney injury.
2.2. Patient Characteristics of the Validation Cohort of the Cobra BITE Study
Thirty-one patients bitten by N. atra received at least one FN treatment vial. Six patients developed wound necrosis and were not enrolled in the Cobra BITE score validation cohort. Eleven of the remaining 25 patients with N. atra bites without wound necrosis had wound infections. No significant differences in age, sex, and laboratory variables were noted between the wound infection and no wound infection groups (Table S2). The median doses of FN were 6 (IQR 2–10) and 4 (IQR 2–6) in the wound infection and no wound infection groups (p = 0.23), respectively. The wound infection group had more patients requiring admission compared with the no wound infection group (n = 10/11 vs. 7/14, p = 0.04). The length of hospitalization in the wound infection group was significantly longer than that in the no wound infection group (6.27 ± 4.38 vs. 1.64 ± 1.86, p = 0.01; Table S2). Among the patients bitten by N. atra, six received debridement, four received fasciotomy, and three received graft treatment. Two patients received debridement with fasciotomy and graft treatment, and one received debridement and fasciotomy. One patient received debridement with graft treatment. No patient was admitted to the ICU or died.
2.3. Comparison of Patient Characteristics between the Derivation and Validation Cohorts for BITE and Cobra BITE Studies
The samples of the patients in the derivation and validation groups were similar, except that both validation sets had higher infection and wound infection rates (Table 1).

Table 1.
Patient characteristics of the validation cohorts and variables used in the validation sets of the BITE and Cobra BITE studies.
2.4. Validation of the BITE and Cobra BITE Studies
The BITE and Cobra BITE scoring systems maintained their predictive ability when applied to their validation sets. The BITE and Cobra BITE scoring systems successfully stratified patients into groups with an increased risk of wound infection (Figure 1 and Figure 2).
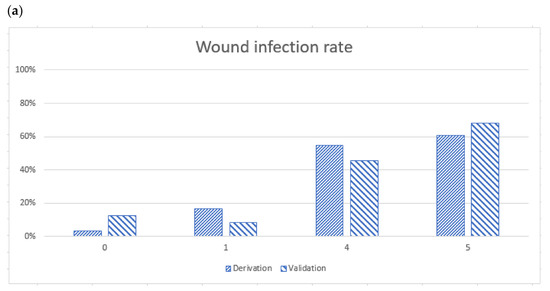
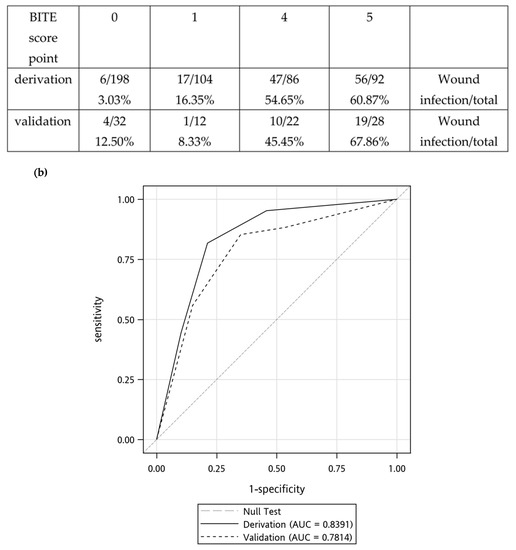
Figure 1.
(a) Wound infection rates determined using the BITE scores, and the X axe meant the BITE score points (b) Receiver operator characteristic curves for derivation and validation sets. The derivation set was the previous BITE study, and the area under the curve (AUC) was 0.8391. The AUC of the validation cohort was 0.7814. Both the derivation and validation AUC were >0.5. We suggest the administration of antibiotics to patients with a BITE score of 4 or 5 points.
Figure 2.
(a) Wound infection rates determined using the Cobra BITE scoring system and the X axe meant the Cobra BITE score points (b) Receiver operator characteristic curves for derivation and validation sets. The derivation set was the previous Cobra BITE study, and the area under the curve (AUC) was 0.8780. The AUC of the validation cohort was 0.7500. Both the derivation and validation AUC were >0.5. Antibiotics should be given if the Cobra BITE score is ≥5.
According to the BITE scoring system, the wound infection rates in the derivation cohort were 3.03%, 16.35%, 54.65%, and 60.87% for scores of 0, 1, 4, and 5, respectively. The wound infection rates in the validation cohort were 12.50%, 8.33%, 45.45%, and 67.86% for scores of 0, 1, 4, and 5, respectively (Figure 1a). The area under the receiver operating characteristic (ROC) curve of the derivation set was 0.84 (95% confidence interval [CI], 0.80 to 0.88). The area under the ROC curve of the validation set in the BITE study was 0.78 (95% CI, 0.69 to 0.88, Figure 1b). The DeLong test did not indicate significant differences in the AUC between the validation and derivation cohorts (p = 0.27).
According to the Cobra BITE scoring system, the wound infection rates in the derivation cohort were 1.92%, 11.11%, 0.00%, 36.00%, and 50% for scores of 0, 1, 3, 4, and 5, respectively. The infection rates were 100% for scores 7 and 8, respectively. The wound infection rates in the validation cohort were 0.00%, 33.33%, 0.00%, 75.00%, and 50% for scores of 0, 1, 3, 4, and 5, respectively. The infection rate was 100% for a score of 8. (Figure 2a). However, the wound infection rate for a score of 4 differed between the derivation and validation cohorts in which the validation cohort had a higher risk of wound infection. This difference might be due to the small number of patients (n = 7) with a score of 4 in the validation cohort. The area under the curve (AUC) of the derivation cohort in the Cobra BITE study was 0.88 (95% CI, 0.81 to 0.95), and the AUC of the validation set was 0.75 (95% CI, 0.56 to 0.98; Figure 2b). We compared the AUC between the derivation and validation sets by using the DeLong test and determined that the AUC did not significantly differ between the two sets (p = 0.21).
2.5. Bacteriology and Antibiotic Susceptibility Testing
The bacteriology and culture reports of wound swabs from P. mucrosquamatus, V. stejnegeri, and N. atra for the derivation and validation sets are presented in Table 2. Morganella morganii and Enterococcus faecalis were the most common bacteria in wound cultures regardless of the snake species. These two bacteria were also the most prevalent in the wounds of the patients with infection caused by N. atra bites.

Table 2.
Microorganisms found in the wound cultures of patients with Protobothrops mucrosquamatus, Viridovipera stejnegeri, and Naja atra bites %.
Generally, more bacteria were isolated from wounds caused by N. atra bites. Multiple aerobic Gram-positive bacteria, such as Staphylococcus species, Viridans streptococcus, and Corynebacterium species, were found in the patients with P. mucrosquamatus and V. stejnegeri bites. Considerably higher numbers of aerobic Gram-negative bacteria, such as Acinetobacter species, Shewanella algae, Proteus vulgaris, Citrob freundii, Bacteroides fragilis, Bacteroides thetaiotaomicron, and Serratia marcescens, and anaerobic bacteria were detected in the infected wounds of patients with N. atra bites.
2.6. Use of Empirical Antibiotics and Antibiotic Therapy for Infected Snakebite Wounds in the Validation Set
Twenty-two patients who received FN antivenin therapy received empirical intravascular antibiotic treatment. Before culture results were obtained, amoxicillin with clavulanic acid was the most commonly used antibiotic, followed by cefuroxime and clindamycin (Table 3). However, the wound culture reports demonstrated that these empirical antibiotics were ineffective in controlling wound infection because the wounds were infected by antimicrobial-resistant bacteria.

Table 3.
Use of empirical antibiotics and antibiotic susceptibility.
Among the 95 patients who received FH antivenin treatment, 38 received intravenous antibiotics in the emergency department (ED). Amoxicillin with clavulanic acid was still the most frequently used antibiotic. Cefazolin with gentamicin, clindamycin, vancomycin with ceftriaxone, oxacillin, ampicillin with sulbactam, and cefuroxime were all used to treat the patients with snakebites. Similarly, the empirical use of amoxicillin with clavulanic acid and cefazolin was not sufficient to control wound infection because of the presence of antibiotic-resistant bacteria.
2.7. Validation of BITE and Cobra BITE Studies and the Use of Antibiotics
The findings of the Cobra BITE study indicated that antibiotics and ≥4 doses of antivenin should be administered to patients with N. atra bites but without tissue necrosis (Cobra BITE score ≥5, which indicates a wound infection rate of at least 50%). However, if tissue necrosis occurs, these patients should be admitted to the ward and receive antibiotic treatment (Figure 3).
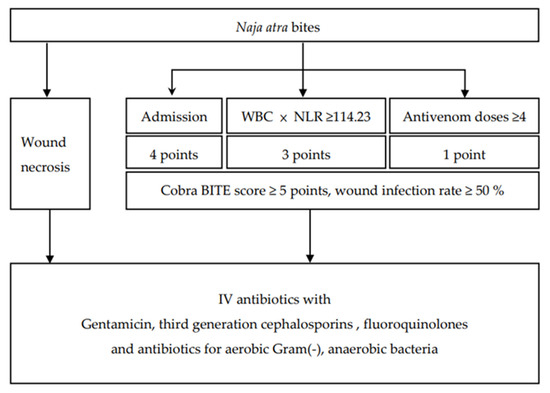
Figure 3.
The Cobra BITE score and the suggested antibiotics for patients bitten by Naja atra.
On the basis of the findings of the BITE study, we suggest the administration of antibiotics to patients with a BITE score of 4 or 5 points [7]. When patients are bitten by P. mucrosquamatus and V. stejnegeri and receive FH antivenin in the ED, antibiotics should be administered if the neutrophil-to-lymphocyte ratio of patients is ≥19.84 (Figure 4).
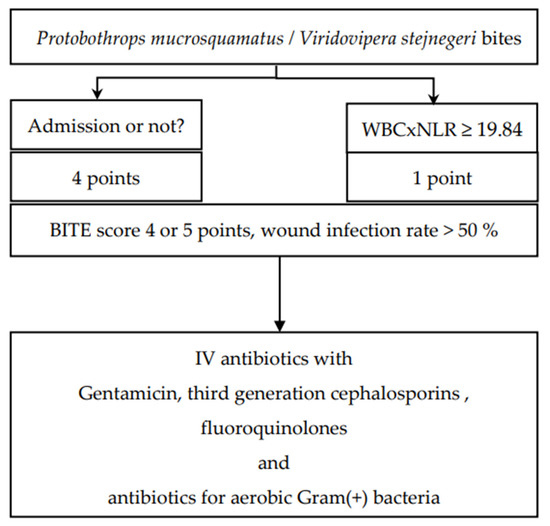
Figure 4.
The BITE score and suggested antibiotics for patients bitten by Protobothrops mucrosquamatus and Viridovipera stejnegeri.
3. Discussion
We validated our two previously reported snakebite-related wound infection prediction scoring systems in this study. In addition, we compared the bacteriology of infected wounds caused by P. mucrosquamatus, V. stejnegeri, and N. atra bites. Although M. morganii and E. faecalis were the most commonly detected bacteria in wound cultures regardless of the snake species, considerable differences in causative bacterial strains were noted among wound infections caused by P. mucrosquamatus, V. stejnegeri, and N. atra bites. The findings of this study can help physicians in promptly treating snakebite wound infections.
3.1. Bacteriology of Snake Oral Cavity and Wound Culture in N. atra Bites
This study examined eight samples from patients’ wound or pus cultures. M. morganii and E. faecalis were the most frequently detected bacteria in wound or pus cultures. This finding is consistent with those of previous studies [7,8,9]. In 2021, Mao et al. [10] reported that M. morganii was the most frequently detected bacterium in both N. atra bite wounds and oropharyngeal swabs. However, a study published in 2022 by Chuang et al. [11] indicated that E. faecalis was the most commonly isolated bacterium from the oral cavity of N. atra and M. morganii was detected in only 1 of the 30 wound culture samples collected from patients with N. atra bites. Pseudomonas species were the most frequently detected aerobic Gram-negative bacteria in N. atra wounds.
Shek et al. [12] reported that M. morganii was detected in the oral cavity of 23 of 32 N. atra in Hong Kong, and Pseudomonas species were detected in the oral cavity of only eight snakes. In 2016, Su et al. [13] reported that M. morganii and E. faecalis were the most commonly detected bacteria in the wound culture of specimens obtained from patients with N. atra bites who underwent surgery; however, they did not determine Pseudomonas species; this finding is consistent with that of our study. Another study conducted in India reported that Pseudomonas and Proteus species instead of M. morganii were the most widely detected Gram-negative bacteria in the oral cavity of N. naja. Bacillus and Staphylococcus species instead of E. faecalis were the most frequently identified Gram-positive bacteria [14]. Further research is warranted because of the discrepancies in these findings and because the literature cannot fully explain the gap in findings between the wound and oral cavity cultures. The immunocompromised state of injuries caused by snakebite wounds might provide a suitable culture medium for these bacteria.
Although the species and habitats of snakes differ, Panda et al. [14] reported that nearly half of the bacterial cultures were resistant to amoxicillin with clavulanic acid, and nearly 100% of bacterial culture reports exhibited resistance to penicillin. Therefore, they did not suggest using amoxicillin with clavulanic acid, oxacillin, and penicillin as prophylactic antibiotics [14]. These results are similar to those reported by Lam et al. [15]. They indicated that only 23% of bacterial cultures were sensitive to amoxicillin with clavulanic acid, and approximately 40% were sensitive to cefuroxime. Enterobacter species exhibited only approximately 14% sensitivity to amoxicillin with clavulanic acid. Mao et al. [16] reported that M. morganii should be treated with third-generation cephalosporin or fluoroquinolone with aminopenicillin instead of first- and second-generation cephalosporin because of their resistance. A possible cause of resistance to first-generation cephalosporins can be the presence of the VGH116 genome in M. morganii [17]. Resiere et al. [18] suggested that amoxicillin with clavulanic acid was not suitable for treating snakebites and that third-generation cephalosporin may be administered if required. Ngoc et al. [19] conducted a study in in Vietnam and examined the wound culture reports of Naja species’ snakebites. They observed that E. faecalis exhibited sensitivity to all antibiotics and M. morganii was sensitive to cephalosporin, quinolone, and carbapenem. They also noted that the treatment of wounds caused by Naja species with clindamycin and ciprofloxacin reduced wound necrosis and infection. Considering that M. morganii and E. faecalis were the most commonly detected bacteria in cultures, we suggest using third-generation cephalosporins (such as ceftriaxone and ceftazidime) and fluoroquinolones (ciprofloxacin, levofloxacin).
3.2. Bacteriology of Snake Oral Cavity and Wound Culture in P. mucrosquamatus and V. stejnegeri Bites
P. mucrosquamatus and V. stejnegeri bites are more common than N. atra bites in Taiwan [20]. Unlike N. atra bites, these snakebites cause less bacterial wound infection and cellulitis [4]. Pseudomonas species were the most frequently detected aerobic Gram-negative bacteria in the oral cavity of P. mucrosquamatus and V. stejnegeri [11]. M. morganii and E. faecalis have not been frequently identified in the bacterial culture reports of V. stejnegeri bites [21]. Similarly, another study revealed that bites from P. mucrosquamatus produced similar results—M. morganii and Enterococcus species were not the dominant pathogens in isolated culture reports [22]. These findings are consistent with those of both the derivation and validation cohorts of our study. Thus, third-generation cephalosporin, fluoroquinolone, piperacillin with tazobactam, vancomycin, and teicoplanin may still be adequate treatment options.
3.3. Antibiotic Use
Because M. morganii and E. faecalis were the most commonly identified bacteria in cultures, third-generation cephalosporins (such as ceftriaxone and ceftazidime) and fluoroquinolones (ciprofloxacin and levofloxacin) should be administered. However, the microorganisms may exhibit resistance to first- and second-generation cephalosporins, such as cefazolin, cefuroxime, and ampicillin with sulbactam [11].
A more sophisticated approach for the selection of antibiotics should be adopted. Patients may require multiple surgical procedures because of N. atra bite–related wound necrosis [23,24]; thus, use of antibiotics such as metronidazole, amoxicillin with clavulanic acid, and piperacillin with tazobactam to combat both aerobic and anaerobic microorganisms is recommended [25] (Figure 3). Patients with P. mucrosquamatus or V. stejnegeri bites may require antibiotics that can kill anaerobic Gram-positive bacteria (Figure 4).
Prophylactic antibiotics are still extensively used in Taiwan to treat patients with snakebites [26]. In our study, most patients received oral amoxicillin with clavulanic acid as prophylactic antibiotics or intravascular amoxicillin with clavulanic acid as empirical antibiotics following hospital admission. According to the findings of antibiotic susceptibility testing, some patients who underwent FN treatment were not suitable for treatment with cefuroxime, cefazolin, and ampicillin with sulbactam for cellulitis. Resistance may be exhibited by M. morganii, P. vulgaris, and E. faecalis.
3.4. Limitations
This study validated the BITE and Cobra BITE scoring systems. However, this study had some limitations. The number of samples collected in this study was limited, particularly for patients with N. atra bites, although this study was conducted in the largest hospital in Taiwan.
We calculated the required power by comparing the admission rate between the patients with and without wound infection in the BITE and Cobra BITE validation sets (the power was 0.95 and 0.96, respectively). By using an independent χ² test, we calculated a presumed effect size of 0.9 under a two-tailed comparison with a preset α level of 0.05.
Another limitation was that our central laboratory only tested the antibiotics sensitivity test but lacking the antibiotics concentrations.
4. Conclusions
Both our previously published BITE and Cobra BITE scoring systems were successfully validated in this study. These two practical tools may aid physicians in treating patients in Taiwan bitten by N. atra, P. mucrosquamatus, and V. stejnegeri. In addition, some antibiotics such as amoxicillin with clavulanic acid, oxacillin, and ampicillin may no longer be reliable and useful for snakebite wound treatment. Third-generation cephalosporins, carbapenem, and fluoroquinolone can be considered as alternatives.
5. Materials and Methods
5.1. Patient Selection, Data Resource Setting, and Collected Variables
This study adopted a retrospective design. We searched the electronic medical records from the ED of the Chang Gung Memorial Hospital (CGMH) network, which contains two medical centers (Linkou and Kaohsiung CGMH) and five regional hospitals (Taoyuan, Taipei, Keelung, Yunlin, and Chiayi CGMH). The CGMH network is the largest private medical network in Taiwan. This medical system contains approximately 10,000 beds and receives approximately 2.4 million admissions annually. The CGMH network also receives approximately 8.2 million outpatients every year. Thus, nearly a third of the Taiwanese population has received treatment in the CGMH network hospitals [27].
The patient cohorts in the BITE and Cobra BITE study were considered the derivation set. We used a derivation set to figure out the possible predictive factors and established the scoring systems. After we developed the two scoring models (the BITE and Cobra BITE study) for the evaluation of snakebite wound infection risk, we still needed to confirm the validity of our previous achievement. Therefore, we collected another separate and independent group of patients bitten by P. mucrosquamatus, V. stejnegeri, or N. atra. These groups were regarded as the validation set. We used the validation set to verify the two scoring models.
We identified adult patients who were bitten by venomous snakes, namely N. atra, P. mucrosquamatus, and V. stejnegeri, and subsequently sought treatment at our ED between 1 June 2017, and 31 May 2021. These patients received one vial of FH antivenom (the designated antivenom for P. mucrosquamatus and V. stejnegeri snakebites) or FN antivenom (the designated antivenom for N. atra bites) at least during their visit to EDs. Patients who received both FH and FN simultaneously during their ED visits or received other antivenoms were excluded. Patients who did not receive any antivenom treatment were also excluded. These patients were included in the validation cohort.
The characteristics of patients who comprised the derivation cohort have been described in our previous studies. Two emergency physicians carefully reviewed these patients’ medical records (C.-C.L. and H.Y). Collected variables were abstracted from the electronic medical record database. Demographic characteristics such as age and sex were recorded. Laboratory variables measured in the ED included white blood cell count with differential count, neutrophil-to-lymphocyte ratio, hemoglobin, red blood cell distribution width, platelet count, prothrombin time, activated partial thromboplastin time, blood creatinine, blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase, myoglobin, potassium, sodium, and blood glucose. Additionally, other details regarding treatment modalities such as antivenom dose, method of antibiotic administration, type of surgical procedure, and hospitalization duration were obtained. The bacteriology data of wound and pus cultures from snakebite victims were retrieved.
5.2. Current Treatment Protocol for Patients with Snakebites and Definitions of Wound Infection, Polymicrobial Infection, and Wound Necrosis
Our routine management protocols are based on World Health Organization’s guidelines [28] and have been described in previous articles. The selection of antivenom was based on the venomous snake species. Thus, clinicians would ask patients in the ED to identify the snake by using a pictorial chart available in our EDs, and clinicians would confirm this identification based on the patient’s symptoms and signs. The patients would be monitored for at least 24 h after the administration of appropriate antivenoms either in ED or the ordinary ward. If clinical symptoms, such as limb swelling, improved, the patients could return home and follow-up was conducted at an outpatient clinic. This protocol was the same as that described in previous articles.
To get the culture samples from patients’ pus or wounds, we used sterile syringes to aspirate the pus and discharge from abscesses or wounds with pus formation. The culture samples were collected during the procedure if a patient accepted debridement.
If the aspiration procedure was difficult, our staff would use sterile cotton swabs to stick the wound discharge. The aerobic culture and anaerobic culture would both be collected.
Before these procedures, our staff wound sterilize the wound surface and skin around the bite wounds with 75% rubbing alcohol solution. After all procedures were completed, the culture samples would be sent to our central laboratory as soon as possible.
Wounds were defined as infected snakebite wounds if the clinical conditions were consistent with the following criteria: (1) observation of microorganisms in wound or pus culture reports, (2) diagnosis of cellulitis, abscess, or necrotizing fasciitis in medical records, and (3) experience of wound debridement by a surgeon.
Patients with N. atra bites who developed wound necrosis were defined as having wound infection. However, these patients’ clinical variables were not used to validate the BITE and Cobra BITE scores. We only used their bacteriology results for the selection of antibiotics. To describe bacteriology findings in the culture report, we defined polymicrobial infection as the discovery of two or more microbial species in one infected wound.
5.3. Statistical Analysis and Validation of the BITE and Cobra BITE Scores
Categorical variables are described as the frequency and percentage, and continuous variables are described as the mean and standard error of the mean. Univariate analysis was performed using Student’s t test (for numerical variables) and the chi-square test (for categorical variables). A p value of <0.1 was considered statistically significant. We used scoring systems described in the BITE and Cobra BITE studies to evaluate infection risk. Then, we calculated ROC curves and AUC for the validation cohort. Finally, we compared the AUC of the derivation set (BITE and Cobra BITE studies) and the validation set using the DeLong test. A p value of <0.05 was regarded as statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins15010078/s1, Table S1: The characteristics of the validation cohort of the BITE study; Table S2: The characteristics of the validation set of the Cobra BITE study; Table S3: bacteriology culture reports and antibiotic susceptibility; Table S4: Abbreviation table.
Author Contributions
Conceptualization, C.-C.L.; methodology, C.-C.L.; validation, C.-C.L., H.Y. and S.-Y.G.; formal analysis, S.-Y.G.; data curation, C.-C.L. and H.Y.; writing—original draft preparation, C.-C.L. and H.Y.; writing—review and editing, C.-C.L.; supervision, C.-C.L.; project administration, C.-C.L. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB no. 202101796B0, date of approval: 18 October 2021). The study complied with the Declaration of Helsinki. The consent of the study participants was waived because this was a retrospective study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Hsin-Yu Wang for her excellent secretarial work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, C.-K.; Lin, C.-C.; Shih, F.-Y.; Chaou, C.-H.; Lin, J.C.-C.; Lai, T.-I.; Tseng, C.-Y.; Fang, C.-C. Population-based study of venomous snakebite in Taiwan. J. Acute Med. 2015, 5, 38–42. [Google Scholar] [CrossRef]
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Lai, C.S.; Lai, K.L.; Ho, C.H.; Wang, T.H.; Yang, C.C. Naja atra snakebite in Taiwan. Clin. Toxicol. 2018, 56, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Chou, Y.-S.; Chen, C.-Y.; Liu, K.-L.; Huang, G.-J.; Yu, J.-S.; Wu, C.-J.; Liaw, G.-W.; Hsieh, C.-H.; Chen, C.-K. Pathogenesis of local necrosis induced by Naja atra venom: Assessment of the neutralization ability of Taiwanese freeze-dried neurotoxic antivenom in animal models. PLoS Negl. Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef]
- Huang, L.-W.; Wang, J.-D.; Huang, J.-A.; Hu, S.-Y.; Wang, L.-M.; Tsan, Y.-T. Wound infections secondary to snakebite in central Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef]
- Seifert, S.A.; Armitage, J.O.; Sanchez, E.E. Snake Envenomation. N. Engl. J. Med. 2022, 386, 68–78. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chaou, C.-H.; Tseng, C.-Y. An investigation of snakebite antivenom usage in Taiwan. J. Formos. Med Assoc. 2016, 115, 672–677. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.Y.; Shi-Ying, G.; Seak, C.J.; Spot Investigators. Wound Infections of Snakebites from the Venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan: Bacteriology, Antibiotic Susceptibility, and Predicting the Need for Antibiotics-A BITE Study. Toxins 2020, 12, 575. [Google Scholar] [CrossRef]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound Infections from Taiwan Cobra (Naja atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Wu, K.G.; Chen, C.J.; Wang, C.M. Bacterial infection in association with snakebite: A 10-year experience in a northern Taiwan medical center. J. Microbiol. Immunol. Infect. 2011, 44, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Chuang, H.N.; Shih, C.H.; Hsieh, H.H.; Jiang, Y.H.; Chiang, L.C.; Lin, W.L.; Hsiao, T.H.; Liu, P.Y. An investigation of conventional microbial culture for the Naja atra bite wound, and the comparison between culture-based 16S Sanger sequencing and 16S metagenomics of the snake oropharyngeal bacterial microbiota. PLoS Negl. Trop. Dis. 2021, 15, e0009331. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Lin, W.-H.; Chen, Y.-C.; Chien, C.-C.; Chiu, I.-M.; Tsai, T.-S. Oral Bacteria and Their Antibiotic Susceptibilities in Taiwanese Venomous Snakes. Microorganisms 2022, 10, 951. [Google Scholar] [CrossRef]
- Shek, K.C.; Tsui, K.L.; Lam, K.K.; Crow, P.; Ng, K.H.L.; Ades, G.; Yip, K.T.; Grioni, A.; Tan, K.S.; Lung, D.C.; et al. Oral bacterial flora of the Chinese cobra (Naja atra) and bamboo pit viper (Trimeresurus albolabris) in Hong Kong SAR, China. Hong Kong Med. J. 2009, 15, 183–190. [Google Scholar]
- Su, H.Y.; Wang, M.J.; Li, Y.H.; Tang, C.N.; Tsai, M.J. Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be predicted in the emergency department? Hong Kong Med. J. 2016, 22, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Padhi, L.; Sahoo, G. Oral bacterial flora of Indian cobra (Naja naja) and their antibiotic susceptibilities. Heliyon 2018, 4, e01008. [Google Scholar] [CrossRef]
- Lam, K.K.; Crow, P.; Ng, K.H.L.; Shek, K.C.; Fung, H.T.; Ades, G.; Grioni, A.; Tan, K.S.; Yip, K.T.; Lung, D.C.; et al. A cross-sectional survey of snake oral bacterial flora from Hong Kong, SAR, China. Emerg. Med. J. 2010, 28, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Liu, P.Y.; Hung, D.Z.; Lai, W.C.; Huang, S.T.; Hung, Y.M.; Yang, C.C. Bacteriology of Naja atra Snakebite Wound and Its Implications for Antibiotic Therapy. Am. J. Trop. Med. Hyg. 2016, 94, 1129–1135. [Google Scholar] [CrossRef]
- Huang, W.-H.; Kao, C.-C.; Mao, Y.-C.; Lai, C.-S.; Lai, K.-L.; Lai, C.-H.; Tseng, C.-H.; Huang, Y.-T.; Liu, P.-Y. Shewanella algae and Morganella morganii Coinfection in Cobra-Bite Wounds: A Genomic Analysis. Life 2021, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Gutiérrez, J.M.; Neviere, R.; Cabié, A.; Hossein, M.; Kallel, H. Antibiotic therapy for snakebite envenoming. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190098. [Google Scholar] [CrossRef]
- Ngo, N.D.; Le, Q.X.; Pham, A.Q.; Nguyen, N.T.; Ha, H.T.; Dinh, M.M.Q.; Le, T.Q. Clinical Features, Bacteriology, and Antibiotic Treatment Among Patients with Presumed Naja Bites in Vietnam. Wilderness Environ. Med. 2020, 31, 151–156. [Google Scholar] [CrossRef]
- Hung, D.-Z. Taiwan’s Venomous Snakebite: Epidemiological, Evolution and Geographic Differences. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 96–101. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Tsai, W.-J.; Liu, P.-Y.; Ho, C.-H.; Su, H.-Y.; Lai, C.-S.; Lai, K.-L.; Lin, W.-L.; Lee, C.-H.; Yang, Y.-Y.; et al. Envenomation by Trimeresurus stejnegeri stejnegeri: Clinical manifestations, treatment and associated factors for wound necrosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200043. [Google Scholar] [CrossRef]
- Mao, Y.-C.; Liu, P.-Y.; Chiang, L.-C.; Lee, C.-H.; Lai, C.-S.; Lai, K.-L.; Lin, W.-L.; Su, H.-Y.; Ho, C.-H.; Doan, U.V.; et al. Clinical manifestations and treatments of Protobothrops mucrosquamatus bite and associated factors for wound necrosis and subsequent debridement and finger or toe amputation surgery. Clin. Toxicol. 2020, 59, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.-Z.; Liau, M.-Y.; Lin-Shiau, S.-Y. The clinical significance of venom detection in patients of cobra snakebite. Toxicon 2003, 41, 409–415. [Google Scholar] [CrossRef]
- Blokhuis-Arkes, M.H.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; Guebitz, G.; Beuk, R. Rapid enzyme analysis as a diagnostic tool for wound infection: Comparison between clinical judgment, microbiological analysis, and enzyme analysis. Wound Repair Regen. 2015, 23, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Treatment of anaerobic infection. Expert Rev. Anti-Infect. Ther. 2007, 5, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-C.; Hung, D.-Z. Management of Snake Envenomation in Taiwan. In Clinical Toxinology in Asia Pacific and Africa; Springer: Dordrecht, The Netherlands, 2015; pp. 23–52. [Google Scholar]
- Chang Gung Memorial Hospital. 2022. Available online: http://www.chang-gung.com/en/m/about.aspx?id=11&bid=1 (accessed on 2 August 2022).
- World Health Organization. Guidelines for the Management of Snakebites, 2nd ed.; WHO: Geneva, Switzerland, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).