Cytoprotective, Antiproliferative, and Anti-Oxidant Potential of the Hydroethanolic Extract of Fridericia chica Leaves on Human Cancer Cell Lines Exposed to α- and β-Zearalenol
Abstract
1. Introduction
2. Results
2.1. Viability of HEFc on Cells Lines
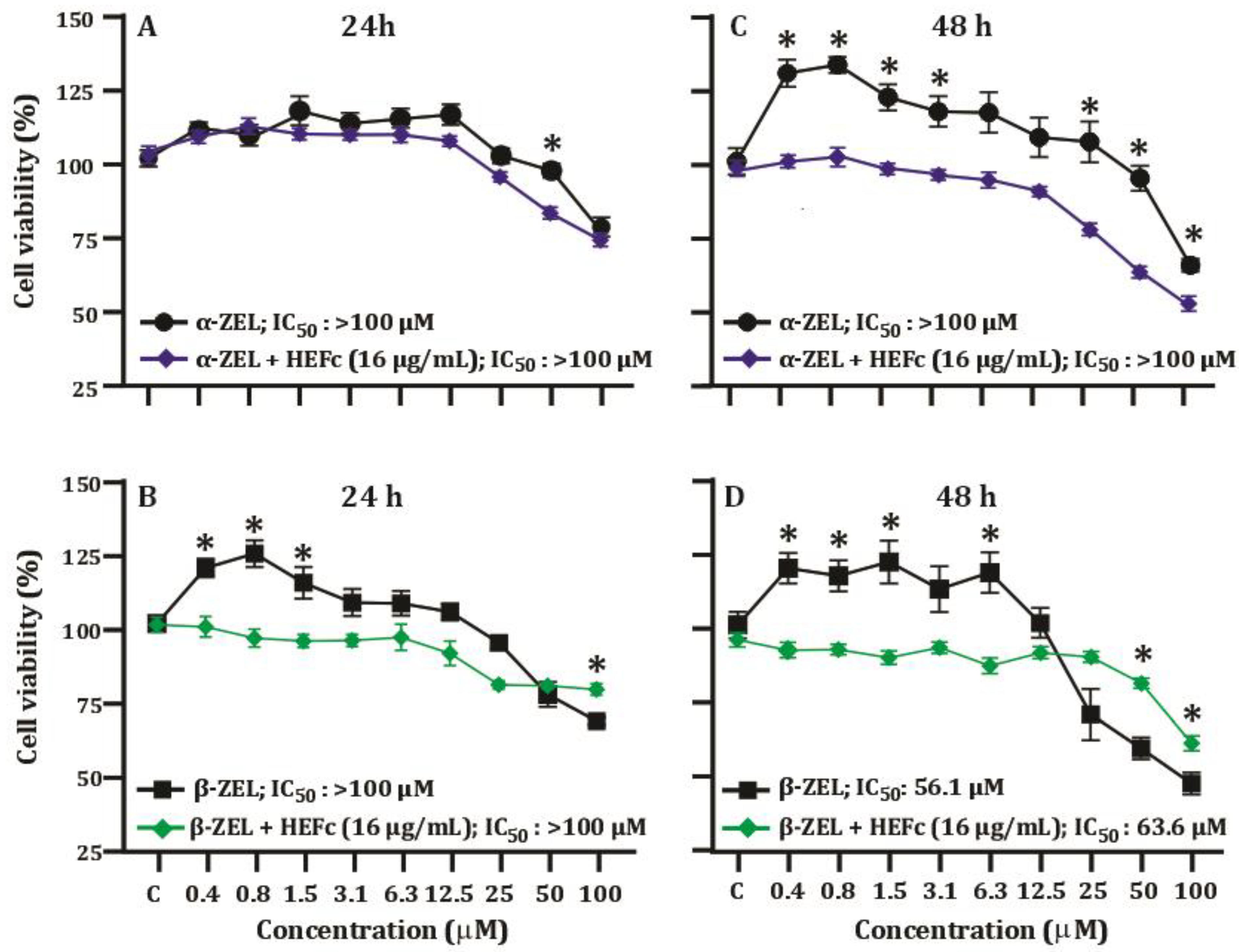
2.2. Viability of Cells Exposed to Mycotoxins
2.3. Antiproliferative Effects of HEFc against α-ZEL and β-ZEL
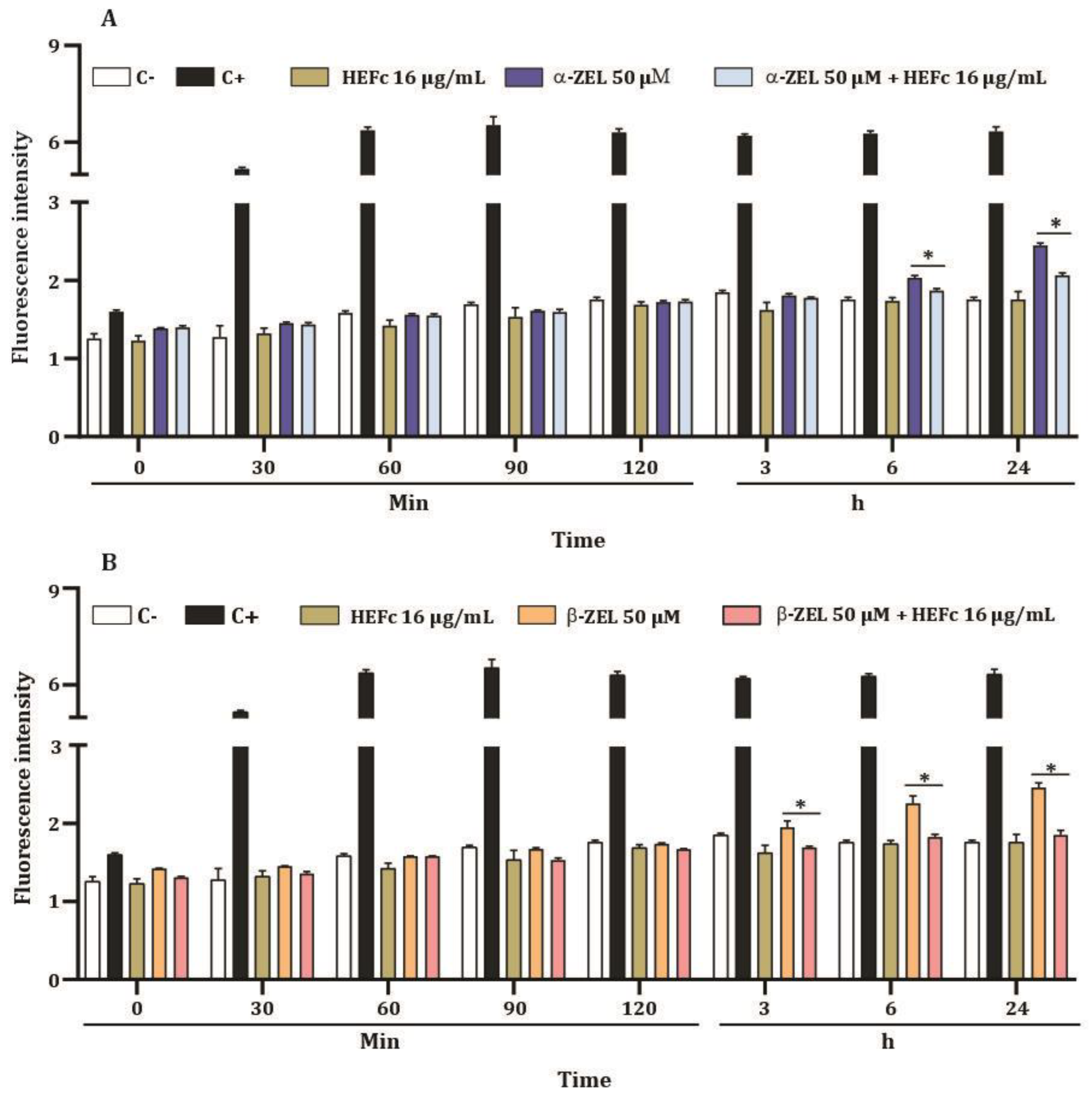
2.4. ROS Levels of HepG2 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Extract Preparation and Characterization
5.3. Maintaining Cell Culture
5.4. Exposure of HepG2, Calu-1, and HEKn Cells to F. chica Extract and Mycotoxins
5.5. Cytoprotective Effects of HEFc against ZEN Metabolites
5.6. ROS Assay
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza, A.S.; Pagadigorria, C.L.S.; Ishii-Iwamoto, E.L.; Bracht, A.; Cortez, D.A.G.; Yamamoto, N.S. Effects of the Arrabidaea chica extract on energy metabolism in the rat liver. Pharm. Biol. 2009, 47, 154–161. [Google Scholar] [CrossRef]
- Vasconcelos, C.C.; Lopes, A.J.O.; Sousa, E.L.F.; Camelo, D.S.; Lima, F.C.V.M.; Rocha, C.Q.D.; Silva, G.E.B.; Garcia, J.B.S.; Cartágenes, M.D.S.D.S. Effects of extract of Arrabidaea chica Verlot on an experimental model of osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4717. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.A.; Pérez Zamora, C.M.; Nuñez, M.B.; Gonzalez, A.M. In vitro antioxidant, antilipoxygenase and antimicrobial activities of extracts from seven climbing plants belonging to the Bignoniaceae. J. Integr. Med. 2018, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.S.; de Oliveira, R.G.; Silva, V.C.; de Sousa, P.T., Jr.; Violante, I.M.P.; Macho, A.; Martins, D.T.O. Anti-inflammatory activity of 4’,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat. Prod. Res. 2020, 34, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.A.; Freitas, K.M.; Foglio, M.A.; Carvalho, J.E.; Dolder, H.; Gomes, L.; Vidal, B.C.; Pimentel, E.R. Effect of the Arrabidaea chica extract on collagen fiber organization during healing of partially transected tendon. Life Sci. 2013, 92, 799–807. [Google Scholar] [CrossRef]
- Moragas-Tellis, C.J.; Almeida-Souza, F.; Chagas, M.; Souza, P.V.R.; Silva-Silva, J.V.; Ramos, Y.J.; Moreira, D.L.; Calabrese, K.D.S.; Behrens, M.D. The Influence of anthocyanidin profile on antileishmanial activity of Arrabidaea chica Morphotypes. Molecules 2020, 25, 3547. [Google Scholar] [CrossRef]
- dos Santos, V.C.; Longo, T.B.; Garcia, A.L.; Richter, M.F.; Guecheva, T.N.; Henriques, J.A.; Ferraz Ade, B.; Picada, J.N. Evaluation of the mutagenicity and genotoxicity of Arrabidaea chica Verlot (Bignoneaceae), an Amazon plant with medicinal properties. J. Toxicol. Environ. Health Part A 2013, 76, 381–390. [Google Scholar] [CrossRef]
- Juan-García, A.J.T. Introduction to the toxins special issue on toxicological effects of mycotoxin on target cells. Toxins 2020, 12, 446. [Google Scholar] [CrossRef]
- FAO. FAO Cereal Supply and Demand Brief; FAO: Rome, Italy, 2022. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Dong, M.; Tulayakul, P.; Li, J.-Y.; Dong, K.-S.; Manabe, N.; Kumagai, S. Metabolic Conversion of Zearalenone to α-Zearalenol by Goat Tissues. J. Vet. Med. Sci. 2010, 72, 307–312. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Rahimifard, N.; Shoeibi, S.; Hamedani, M.P.; Hosseini, M.-J. Effects of zearalenone and α-Zearalenol in comparison with Raloxifene on T47D cells. Toxicol. Mech. Methods 2009, 19, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kuciel-Lisieska, G.; Obremski, K.; Stelmachów, J.; Gajecka, M.; Zielonka, Ł.; Jakimiuk, E.; Gajecki, M. Presence of zearalenone in blood plasma in women with neoplastic lesions in the mammary gland. Bull. Vet. Inst. Pulawy 2008, 52, 671–674. [Google Scholar]
- Ali, N.; Degen, G.H. Biomonitoring of zearalenone and its main metabolites in urines of Bangladeshi adults. Food and chemical toxicology: An international journal published for the British Industrial Biological Research Association. Food Chem. Toxicol. 2019, 130, 276–283. [Google Scholar] [CrossRef]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Viergutz, T.; Jonas, L.; Schneider, F. Influence of the mycotoxins α- and β-zearalenol and deoxynivalenol on the cell cycle of cultured porcine endometrial cells. Reprod. Toxicol. 2003, 17, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.J.; Yang, H.; Jiao, Y.H.; Pang, Q.; Wang, Y.J.; Wang, M.; Shan, A.S.; Feng, X.J. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of human exposure to deoxynivalenol, ochratoxin A, zearalenone and their metabolites biomarker in urine samples using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Agahi, F.; Álvarez-Ortega, N.; Font, G.; Juan-García, A.; Juan, C. Oxidative stress, glutathione, and gene expression as key indicators in SH-SY5Y cells exposed to zearalenone metabolites and beauvericin. Toxicol. Lett. 2020, 334, 44–52. [Google Scholar] [CrossRef]
- Fleck, S.C.; Hildebrand, A.A.; Müller, E.; Pfeiffer, E.; Metzler, M. Genotoxicity and inactivation of catechol metabolites of the mycotoxin zearalenone. Mycotoxin Res. 2012, 28, 267–273. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Car-Cinogenic Risks to Humans, 56, 599. 1993. Available online: https://monographs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-humans-65/ (accessed on 14 November 2022).
- Juan-García, A.; Agahi, F.; Drakonaki, M.; Tedeschi, P.; Font, G.; Juan, C. Cytoprotection assessment against mycotoxins on HepG2 cells by extracts from Allium sativum L. Food Chem. Toxicol. 2021, 151, 112129. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) Muscle: Purification, identification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lan, Y.; Miao, J.; Chen, X.; Chen, B.; Liu, G.; Wu, X.; Zhu, X.; Cao, Y. Phytochemicals, antioxidant capacity and cytoprotective effects of jackfruit (Artocarpus heterophyllus Lam.) axis extracts on HepG2 cells. Food Biosci. 2021, 41, 100933. [Google Scholar] [CrossRef]
- Pires, F.C.S.; Oliveira, J.C.D.; Menezes, E.G.O.; Ferreira, M.C.R.; Siqueira, L.M.M.; Almada-Vilhena, A.O.; Pieczarka, J.C.; Nagamachi, C.Y.; Carvalho Junior, R.N.D. Bioactive compounds and evaluation of antioxidant, cytotoxic and cytoprotective effects of murici pulp extracts (Byrsonima crassifolia) obtained by supercritical extraction in HepG2 cells treated with H2O2. Foods 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef]
- Scolastici, C.; de Lima, R.A.; Barbisan, L.F.; Ferreira, A.L.D.A.; Ribeiro, D.A.; Salvadori, D.M.F. Antigenotoxicity and antimutagenicity of lycopene in HepG2 cell line evaluated by the comet assay and micronucleus test. Toxicol. Vitr. 2008, 22, 510–514. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Murin, R.; Zubor, P.; Bujnak, J.; Kwon, T.K.; Büsselberg, D.; Prosecky, R.; et al. The role of plant-derived natural substances as immunomodulatory agents in carcinogenesis. J. Cancer Res. Clin. Oncol. 2020, 146, 3137–3154. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Silver Nanoparticles: An instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Moolgavkar, S.H.; Luebeck, E.G.J.G. Multistage carcinogenesis and the incidence of human cancer. Genes Chromosom. Cancer 2003, 38, 302–306. [Google Scholar] [CrossRef]
- Abbas, Z.; Rehman, S. An overview of cancer treatment modalities. Neoplasm 2018, 1, 139–157. [Google Scholar]
- Ribeiro, A.F.C.; Telles, T.C.; Ferraz, V.P.; Souza-Fagundes, E.M.; Cassali, G.D.; Carvalho, A.T.; Melo, M.M. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Rev. Bras. Farmacogn. 2012, 22, 364–373. [Google Scholar] [CrossRef]
- Michel, A.F.R.M.; Melo, M.M.; Campos, P.P.; Oliveira, M.S.; Oliveira, F.A.S.; Cassali, G.D.; Ferraz, V.P.; Cota, B.B.; Andrade, S.P.; Souza-Fagundes, E.M. Evaluation of anti-inflammatory, antiangiogenic and antiproliferative activities of Arrabidaea chica crude extracts. J. Ethnopharmacol. 2015, 165, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Taboada-Alquerque, M.; Franco, J.; Stashenko, E.E.; Juan, C.; Juan-García, A.; Olivero-Verbel, J.J.T. Protective effects of the hydroethanolic extract of Fridericia chica on undifferentiated human neuroblastoma cells exposed to α-Zearalenol (α-ZEL) and β-Zearalenol (β-ZEL). Toxins 2021, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Agahi, F.; Penalva-Olcina, R.; Font, G.; Juan-García, A.; Juan, C. Effects of Voghiera garlic extracts in neuronal human cell line against zearalenone’s derivates and beauvericin. Food Chem. Toxicol. 2022, 162, 112905. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Espín, S.; García-Fernández, A.J.; Ruiz, M.J. Estrogenic activity of zearalenone, α-zearalenol and β-zearalenol assessed using the E-screen assay in MCF-7 cells. Toxicol. Mech. Methods 2018, 28, 239–242. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, D.; Li, Y. Effects of zearalenone on mRNA expression and activity of cytochrome P450 1A1 and 1B1 in MCF-7 cells. Ecotoxicol. Environ. Saf. 2004, 58, 187–193. [Google Scholar] [CrossRef]
- Molina-Molina, J.-M.; Real, M.; Jimenez-Diaz, I.; Belhassen, H.; Hedhili, A.; Torné, P.; Fernández, M.F.; Olea, N. Assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. Food Chem. Toxicol. 2014, 74, 233–239. [Google Scholar] [CrossRef]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef]
- Shen, M.; Shi, H. Estradiol and estrogen receptor agonists oppose oncogenic actions of leptin in HepG2 cells. PLoS ONE 2016, 11, e0151455. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, W.; Feng, N.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. The Role of miRNAs in Zearalenone-Promotion of TM3 Cell Proliferation. Int. J. Environ. Res. Public Health 2019, 16, 1517. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Spandidos, D.A. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J. Nutr. Biochem. 2013, 24, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Dev. Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, S.; Zhang, Y.; Zhou, X.; Chen, W. Vicenin-2 is a novel inhibitor of STAT3 signaling pathway in human hepatocellular carcinoma. J. Funct. Foods 2020, 69, 103921. [Google Scholar] [CrossRef]
- Mafioleti, L.; da Silva Junior, I.F.; Colodel, E.M.; Flach, A.; de Oliveira Martins, D.T. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013, 150, 576–582. [Google Scholar]
- de Medeiros, B.J.L.; dos Santos Costa, K.; Ribeiro, J.F.A.; Silva Jr, J.O.C.; Barbosa, W.L.R.; Carvalho, J.C.T. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl.(pariri). Pharmacogn. Res. 2011, 3, 79–84. [Google Scholar]
- Takenaka, I.K.T.M.; Amorim, J.M.; de Barros, P.A.V.; Brandão, G.C.; Contarini, S.M.L.; de Sales Souza e Melo, É.; Fernandes, S.O.A. Chemical characterization and anti-inflammatory assessment of the hydroethanolic extract of Fridericia chica. Rev. Bras. Farmacogn. 2020, 30, 559–567. [Google Scholar] [CrossRef]
- Siraichi, J.T.G.; Felipe, D.F.; Brambilla, L.Z.S.; Gatto, M.J.; Terra, V.N.A.; Cecchini, A.L.; Cortez, L.E.R.; Rodrigues-Filho, E.; Cortez, D.A. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in Southern Brazil. PLoS ONE 2013, 8, e72733. [Google Scholar] [CrossRef]
- Tirado-Ballestas, I.; Alvarez-Ortega, N.; Maldonado-Rojas, W.; Olivero-Verbel, J.; Caballero-Gallardo, K. Oxidative stress and alterations in the expression of genes related to inflammation, DNA damage, and metal exposure in lung cells exposed to a hydroethanolic coal dust extract. Mol. Biol. Rep. 2022, 49, 4861–4871. [Google Scholar] [CrossRef]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A.J.T. Individual and combined effect of zearalenone derivates and beauvericin mycotoxins on SH-SY5Y cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef]



| IC50 (48 h) | SI | |
|---|---|---|
| HepG2 | 168 | 3.5 |
| Calu-1 | 249 | 2.4 |
| HEKn | 602.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Ortega, N.; Caballero-Gallardo, K.; Juan, C.; Juan-Garcia, A.; Olivero-Verbel, J. Cytoprotective, Antiproliferative, and Anti-Oxidant Potential of the Hydroethanolic Extract of Fridericia chica Leaves on Human Cancer Cell Lines Exposed to α- and β-Zearalenol. Toxins 2023, 15, 36. https://doi.org/10.3390/toxins15010036
Alvarez-Ortega N, Caballero-Gallardo K, Juan C, Juan-Garcia A, Olivero-Verbel J. Cytoprotective, Antiproliferative, and Anti-Oxidant Potential of the Hydroethanolic Extract of Fridericia chica Leaves on Human Cancer Cell Lines Exposed to α- and β-Zearalenol. Toxins. 2023; 15(1):36. https://doi.org/10.3390/toxins15010036
Chicago/Turabian StyleAlvarez-Ortega, Neda, Karina Caballero-Gallardo, Cristina Juan, Ana Juan-Garcia, and Jesus Olivero-Verbel. 2023. "Cytoprotective, Antiproliferative, and Anti-Oxidant Potential of the Hydroethanolic Extract of Fridericia chica Leaves on Human Cancer Cell Lines Exposed to α- and β-Zearalenol" Toxins 15, no. 1: 36. https://doi.org/10.3390/toxins15010036
APA StyleAlvarez-Ortega, N., Caballero-Gallardo, K., Juan, C., Juan-Garcia, A., & Olivero-Verbel, J. (2023). Cytoprotective, Antiproliferative, and Anti-Oxidant Potential of the Hydroethanolic Extract of Fridericia chica Leaves on Human Cancer Cell Lines Exposed to α- and β-Zearalenol. Toxins, 15(1), 36. https://doi.org/10.3390/toxins15010036









