Monoclonal-Based Antivenomics Reveals Conserved Neutralizing Epitopes in Type I PLA2 Molecules from Coral Snakes
Abstract
1. Introduction
2. Results
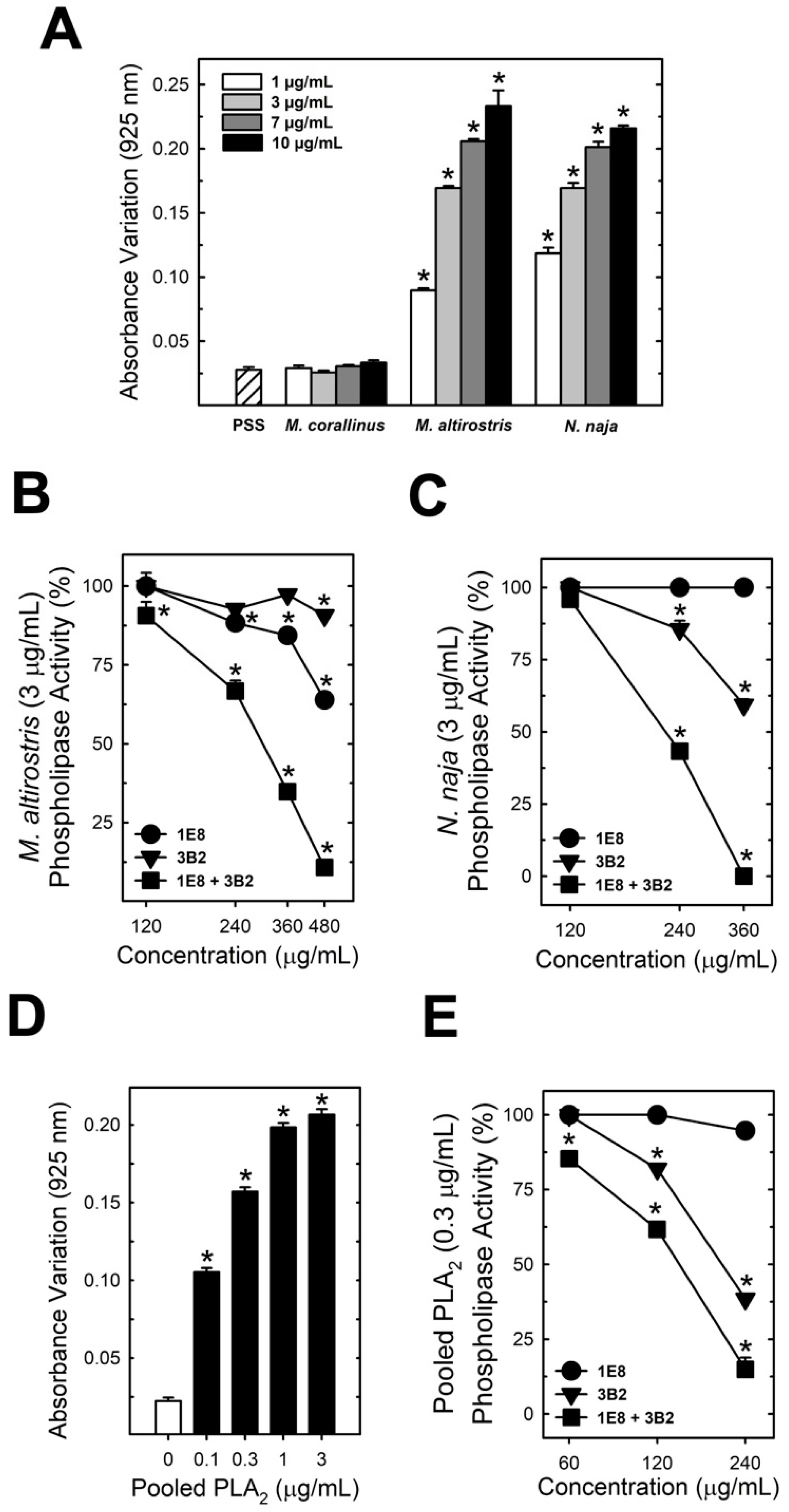
2.1. Identification of the Major Toxic Components of M. altirostris Venom
2.2. Monoclonal-Based Antivenomics Analyses of Antibodies against M. altirostris Venom Toxins
2.3. Neutralization Properties of Monoclonal Antibodies towards PLA2 Toxicity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. ELISA
5.3. M. altirostris Venom Fractionation and In Vivo Toxicity Analysis (LD50)
5.4. Production and Purification of Monoclonal Antibodies (mAbs)
5.5. Monoclonal-Based Antivenomics
5.6. Dot Blot
5.7. Phospholipase Activity In Vitro
5.8. Myotoxic Activity In Vivo
5.9. Inhibition of Lethality by mAbs
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slowinski, J.B.; Keogh, J.S. Phylogenetic relationships of elapid snakes based on cytochrome b mtDNA sequences. Mol. Phylogenet. Evol. 2000, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Slowinski, J.B.; Boundy, J.; Lawson, R. The Phylogenetic Relationships of Asian Coral Snakes (Elapidae: Calliophis and Maticora) Based on Morphological and Molecular Characters. Herpetologica 2001, 57, 233–245. [Google Scholar]
- Castoe, T.A.; Smith, E.N.; Brown, R.M.; Parkinson, C.L. Higher-level phylogeny of Asian and American coralsnakes, their placement within the Elapidae (Squamata), and the systematic affinities of the enigmatic Asian coralsnake Hemibungarus calligaster. Zool J. Linnean Soc. 2007, 15, 809–831. [Google Scholar] [CrossRef][Green Version]
- Campbell, J.A.; Lamar, W.W. The venomous reptiles of the Western Hemisphere; Cornell University Press: Ithaca, NY, USA, 2004; 1032p. [Google Scholar]
- Slowinski, J.B. A Phylogenetic analysis of the New World coral snakes (Elapidae: Leptomicrurus, Micruroides, and Micrurus) based on allozymic and morphological characters. J. Herpetol. 1995, 29, 325–338. [Google Scholar] [CrossRef]
- Roze, J.A. Coral Snakes of the Americas: Biology, Identification and Venoms; Krieger Publishing Company: Malabar, FL, USA, 1996; 328p. [Google Scholar]
- Gutiérrez, J.M.; Lomonte, B.; Portilla, E.; Cerdas, L.; Rojas, E. Local effects induced by coral snake venoms: Evidence of myonecrosis after experimental inoculations of venoms from five species. Toxicon 1983, 21, 777–784. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rojas, G.; da Silva, N.J.; Núñez, J. Experimental myonecrosis induced by the venoms of South American Micrurus (coral snakes). Toxicon 1992, 30, 1299–1302. [Google Scholar] [CrossRef]
- Weis, R.; McIsaac, R.J. Cardiovascular and muscular effects of venom from coral Micrurus fulvius. Toxicon 1971, 9, 219–228. [Google Scholar] [CrossRef]
- Vital-Brazil, O. Coral snake venoms: Mode of action and pathophysiology of experimental envenomation. Rev. Inst. Med. Trop. S. Paulo 1987, 29, 119–126. [Google Scholar] [CrossRef]
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, Clinical Features, Clinical Management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; pp. 709–761. [Google Scholar]
- Bucaretchi, F.; Capitani, E.M.; Vieira, R.J.; Rodrigues, C.K.; Zannin, M.; Da Silva, N.J., Jr.; Casais-e-Silva, L.L.; Hyslop, S. Coral snake bites (Micrurus spp.) in Brazil: A review of literature reports. Clin. Toxicol. 2016, 54, 222–234. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Batista, C.V.; Restano-Cassulini, R.; Pando, V.; Villa-Hernandez, O.; Zavaleta-Martínez-Vargas, A.; Salas-Arruz, M.C.; de la Vega, R.C.; Becerril, B.; Possani, L.D. Proteomic analysis of the venom from the fish eating coral snake Micrurus surinamensis: Novel toxins, their function and phylogeny. Proteomics 2008, 9, 1919. [Google Scholar] [CrossRef]
- Aird, S.D.; da Silva, N.J., Jr.; Qiu, L.; Villar-Briones, A.; Saddi, V.A.; Grau, M.L.; Mikheyev, A.S.; Pires de Campos Telles, M. Coralsnake Venomics: Analyses of Venom Gland Transcriptomes and Proteomes of Six Brazilian Taxa. Toxins 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Leão, L.I.; Ho, P.L.; Junqueira-de-Azevedo, I.L. Transcriptomic basis for an antiserum against Micrurus corallinus (coral snake) venom. BMC Genom. 2009, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Netto, C.; Junqueira-de-Azevedo, I.deL.; Silva, D.A.; Ho, P.L.; Leitão-de-Araújo, M.; Alves, M.L.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J. Proteom. 2011, 74, 1795. [Google Scholar] [CrossRef]
- Fernandez, J.; Alape-Girón, A.; Angulo, Y.; Sanz, L.; Gutiérrez, J.M.; Calvete, J.J.; Lomonte, B. Venomic and antivenomic analyses of the Central American coral snake, Micrurus nigrocinctus (Elapidae). J. Proteome Res. 2011, 10, 1816. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Gutiérrez, J.M.; Lomonte, B. Proteomic and biological characterization of the venom of the redtail coral snake, Micrurus mipartitus (Elapidae), from Colombia and Costa Rica. J. Proteom. 2011, 75, 655. [Google Scholar] [CrossRef]
- Ciscotto, P.H.; Rates, B.; Silva, D.A.; Richardson, M.; Silva, L.P.; Andrade, H.; Donato, M.F.; Cotta, G.A.; Maria, W.S.; Rodrigues, R.J.; et al. Venomic analysis and evaluation of antivenom cross-reactivity of South American Micrurus species. J. Proteom. 2011, 74, 1810. [Google Scholar] [CrossRef]
- Sanz, L.; de Freitas-Lima, L.N.; Quesada-Bernat, S.; Graça-de-Souza, V.K.; Soares, A.M.; Calderón, L.A.; Calvete, J.J.; Caldeira, C.A.S. Comparative venomics of Brazilian coral snakes: Micrurus frontalis, Micrurus spixii spixii, and Micrurus surinamensis. Toxicon 2019, 166, 39. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais-E-Silva, L.L.; Corrêa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New insights into the phylogeographic distribution of the 3FTx/PLA2 venom dichotomy across genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef]
- Bénard-Valle, M.; Carbajal-Saucedo, A.; de Roodt, A.; López-Vera, E.; Alagón, A. Biochemical characterization of the venom of the coral snake Micrurus tener and comparative biological activities in the mouse and a reptile model. Toxicon 2013, 77, 6. [Google Scholar] [CrossRef]
- Carbajal-Saucedo, A.; López-Vera, E.; Bénard-Valle, M.; Smith, E.; Zamudio, F.; de Roodt, A.; Olvera-Rodríguez, A. Isolation, characterization, cloning and expression of an alpha-neurotoxin from the venom of the Mexican coral snake Micrurus laticollaris (squamata: Elapidae). Toxicon 2013, 66, 64. [Google Scholar] [CrossRef]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The venom-gland transcriptome of the eastern coral snake (Micrurus fulvius) reveals high venom complexity in the intragenomic evolution of venoms. BMC Genom. 2013, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Vergara, I.; Pedraza-Escalona, M.; Paniagua, D.; Restano-Cassulini, R.; Zamudio, F.; Batista, C.V.; Possani, L.D.; Alagón, A. Eastern coral snake Micrurus fulvius venom toxicity in mice is mainly determined by neurotoxic phospholipases A2. J. Proteom. 2014, 105, 295. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Vargas-Vargas, N.; Pla, D.; Sasa, M.; Rey-Suárez, P.; Sanz, L.; Gutiérrez, J.M.; Calvete, J.J.; Lomonte, B. Snake venomics of Micrurus alleni and Micrurus mosquitensis from the Caribbean region of Costa Rica reveals two divergent compositional patterns in New World elapids. Toxicon 2015, 107, 217. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suárez, P.; Núñez, V.; Fernández, J.; Lomonte, B. Integrative characterization of the venom of the coral snake Micrurus dumerilii (Elapidae) from Colombia: Proteome, toxicity, and cross-neutralization by antivenom. J. Proteom. 2016, 136, 262. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Pla, D.; Pérez, A.; Rodríguez, Y.; Zavaleta, A.; Salas, M.; Lomonte, B.; Calvete, J.J. Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus tschudii tschudii, Supports the 3FTx/PLA2 Dichotomy across Micrurus Venoms. Toxins 2016, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Rey-Suárez, P.; Fernández, J.; Sasa, M.; Pla, D.; Vargas, N.; Bénard-Valle, M.; Sanz, L.; Corrêa-Netto, C.; Núñez, V.; et al. Venoms of Micrurus coral snakes: Evolutionary trends in compositional patterns emerging from proteomic analyses. Toxicon 2016, 122, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Olamendi-Portugal, T.; Batista, C.V.F.; Pedraza-Escalona, M.; Restano-Cassulini, R.; Zamudio, F.Z.; Benard-Valle, M.; de Roodt, A.R.; Possani, L.D. New insights into the proteomic characterization of the coral snake Micrurus pyrrhocryptus venom. Toxicon 2018, 153, 23–31. [Google Scholar] [CrossRef]
- Lippa, E.; Török, F.; Gómez, A.; Corrales, G.; Chacón, D.; Sasa, M.; Gutiérrez, J.M.; Lomonte, B.; Fernández, J.J. First look into the venom of Roatan Island’s critically endangered coral snake Micrurus ruatanus: Proteomic characterization, toxicity, immunorecognition and neutralization by an antivenom. Proteomics 2019, 30, 198. [Google Scholar] [CrossRef]
- Bénard-Valle, M.; Neri-Castro, E.; Yañez-Mendoza, M.F.; Lomonte, B.; Olvera, A.; Zamudio, F.; Restano-Cassulini, R.; Possani, L.D.; Jiménez-Ferrer, E.; Alagón, A. Functional, proteomic and transcriptomic characterization of the venom from Micrurus browni browni: Identification of the first lethal multimeric neurotoxin in coral snake venom. J. Proteom. 2020, 225, 103863. [Google Scholar] [CrossRef]
- Mena, G.; Chaves-Araya, S.; Chacón, J.; Török, E.; Török, F.; Bonilla, F.; Sasa, M.; Gutiérrez, J.M.; Lomonte, B.; Fernández, J. Proteomic and toxicological analysis of the venom of Micrurus yatesi and its neutralization by an antivenom. Toxicon 2022, 13, 100097. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rossetto, O. How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem Sci. 2000, 25, 266. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Zhou, X.; Kini, R.M. Snake Venom Phospholipase A2 Enzymes. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CCR Press: Boca Raton, FL, USA, 2009; pp. 173–205. [Google Scholar]
- Vulfius, C.A.; Kasheverov, I.E.; Starkov, V.G.; Osipov, A.V.; Andreeva, T.V.; Filkin, S.Y.; Gorbacheva, E.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS ONE 2014, 9, e115428. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Calvete, J.J.; Fernández, J.; Pla, D.; Rey-Suárez, P.; Sanz, L.; Gutiérrez, J.M.; Sasa, M. Venomic analyses of coralsnakes. In Advances in Coralsnake Biology: With an Emphasis on South America; da Silva, N., Jr., Porras, L.W., Aird, S.D., da Costa Prudente, A.L., Eds.; Eagle Mountain Publishing, LC: Eagle Mountain, UT, USA, 2021; pp. 483–516. ISBN 9780972015462. [Google Scholar]
- Rey-Suárez, P.; Floriano, R.S.; Rostelato-Ferreira, S.; Saldarriaga-Córdoba, M.; Núñez, V.; Rodrigues-Simioni, L.; Lomonte, B. Mipartoxin-I, a novel three-finger toxin, is the major neurotoxic component in the venom of the redtail coral snake Micrurus mipartitus (Elapidae). Toxicon 2012, 60, 851. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suárez, P.; Núñez, V.; Saldarriaga-Córdoba, M.; Lomonte, B. Primary structures and partial toxicological characterization of two phospholipases A2 from Micrurus mipartitus and Micrurus dumerilii coral snake venoms. Biochimie 2017, 137, 88. [Google Scholar] [CrossRef] [PubMed]
- Raw, I.; G.uidolin, R.; Higashi, K.; K.elen, E.M.A. Antivenins in Brazil. In Handbook of Natural Toxins; Tu, A.T., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; Volume 5, pp. 557–581. [Google Scholar]
- Moraes, F.V.; Sousa-e-Silva, M.C.C.; Barbaro, K.C.; Leitão, M.A.; Furtado, M.F.D. Biological and immunochemical characterization of Micrurus altirostris venom and serum neutralization of its toxic activities. Toxicon 2003, 41, 71. [Google Scholar] [CrossRef] [PubMed]
- Abreu, V.A.; Leite, G.B.; Oliveira, C.B.; Hyslop, S.; Furtado, M.F.; Simioni, L.R. Neurotoxicity of Micrurus altirostris (Uruguayan coral snake) venom and its neutralization by commercial coral snake antivenom and specific antiserum raised in rabbits. Clin. Toxicol. 2008, 6, 519. [Google Scholar] [CrossRef]
- Tanaka, G.D.; Furtado, M.F.; Portaro, F.C.; Sant’Anna, O.A.; Tambourgi, D.V. Diversity of Micrurus snake species related to their venom toxic effects and the prospective of antivenom neutralization. PLoS Negl. Trop. Dis. 2010, 4, e622. [Google Scholar] [CrossRef]
- Tanaka, G.D.; Sant’Anna, O.A.; Marcelino, J.R.; Lustoza da Luz, A.C.; Teixeira da Rocha, M.M.; Tambourgi, D.V. Micrurus snake species: Venom immunogenicity, antiserum cross-reactivity and neutralization potential. Toxicon 2016, 117, 59. [Google Scholar] [CrossRef]
- Moreira, K.G.; Prates, M.V.; Andrade, F.A.; Silva, L.P.; Beirão, P.S.; Kushmerick, C.; Naves, L.A.; Bloch, C., Jr. Frontoxins, three-finger toxins from Micrurus frontalis venom, decrease miniature endplate potential amplitude at frog neuromuscular junction. Toxicon 2010, 1, 55. [Google Scholar] [CrossRef]
- da Silva, N.J.; Sites, J.W., Jr. Revision of the Micrurus frontalis Complex (Serpentes: Elapidae). Herpetol. Monogr. 1999, 13, 142. [Google Scholar] [CrossRef]
- Riaño-Umbarila, L.; Olamendi-Portugal, T.; Morelos-Juárez, C.; Gurrola, G.B.; Possani, L.D.; Becerril, B. A novel human recombinant antibody fragment capable of neutralizing Mexican scorpion toxins. Toxicon 2013, 76, 370–376. [Google Scholar] [CrossRef]
- Eskafi, A.H.; Bagheri, K.P.; Behdani, M.; Yamabhai, M.; Shahbazzadeh, D.; Kazemi-Lomedasht, F. Development and characterization of human single chain antibody against Iranian Macrovipera lebetina snake venom. Toxicon 2021, 197, 106. [Google Scholar] [CrossRef] [PubMed]
- Manson, E.Z.; Kyama, M.C.; Gikunju, J.K.; Kimani, J.; Kimotho, J.H. Evaluation of lethality and cytotoxic effects induced by Naja ashei (large brown spitting cobra) venom and the envenomation-neutralizing efficacy of selected commercial antivenoms in Kenya. Toxicon 2022, 14, 100125. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Calvete, J.J. Strategies in ‘snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 28, 23. [Google Scholar] [CrossRef]
- Espino-Solis, G.P.; Riaño-Umbarila, L.; Becerril, B.; Possani, L.D. Antidotes against venomous animals: State of the art and prospectives. J. Proteom. 2009, 2, 183. [Google Scholar] [CrossRef]
- Hedegaard, L.; Laustsen, A.H.; Pus, U.; Wade, J.; Villar, P.; Boddum, K.; Slavny, P.; Masters, E.W.; Arias, A.S.; Oscoz, S.; et al. In vitro discovery of a human monoclonal antibody that neutralizes lethality of cobra snake venom. MAbs 2022, 14, 2085536. [Google Scholar] [CrossRef]
- Juste, M.; Martin-Eauclaire, M.F.; Devaux, C.; Billiald, P.; Aubrey, N. Using a recombinant bispecific antibody to block Na+ -channel toxins protects against experimental scorpion envenoming. Cell Mol. Life 2007, 2, 206. [Google Scholar] [CrossRef]
- di Tommaso, A.; Juste, M.O.; Martin-Eauclaire, M.F.; Dimier-Poisson, I.; Billiald, P.; Aubrey, N. Diabody mixture providing full protection against experimental scorpion envenoming with crude Androctonus australis venom. J. Biol. Chem. 2012, 287, 14149. [Google Scholar] [CrossRef]
- Figueiredo, L.F.; Dias-Lopes, C.; Alvarenga, L.M.; Mendes, T.M.; Machado-de-Ávila, R.A.; McCormack, J.; Minozzo, J.C.; Kalapothakis, E.; Chavez-Olórtegui, C. Innovative immunization protocols using chimeric recombinant protein for the production of polyspecific loxoscelic antivenom in horses. Toxicon 2014, 86, 59. [Google Scholar] [CrossRef]
- Alvarenga, L.M.; Zahid, M.; di Tommaso, A.; Juste, M.O.; Aubrey, N.; Billiald, P.; Muzard, J. Engineering venom’s toxin-neutralizing antibody fragments and its therapeutic potential. Toxins 2014, 6, 2541. [Google Scholar] [CrossRef]
- Carmo, A.O.; Chatzaki, M.; Horta, C.C.; Magalhães, B.F.; Oliveira-Mendes, B.B.; Chávez-Olórtegui, C.; Kalapothakis, E. Evolution of alternative methodologies of scorpion antivenoms production. Toxicon 2015, 97, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Manson, E.Z.; Kyama, M.C.; Kimani, J.; Bocian, A.; Hus, K.K.; Petrilla, V.; Legáth, J.; Kimotho, J.H. Development and Characterization of Anti-Naja ashei Three-Finger Toxins (3FTxs)-Specific Monoclonal Antibodies and Evaluation of Their In Vitro Inhibition Activity. Toxins 2022, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- El Ayeb, M.; Rochat, H. Production of monoclonal antibodies. Scorpion antitoxins: Characterization and molecular mechanisms of neutralization. Arch. Inst. Pasteur Tunis. 1988, 65, 29. [Google Scholar] [PubMed]
- Licea, A.F.; Becerril, B.; Possani, L.D. Fab fragments of the monoclonal antibody BCF2 are capable of neutralizing the whole soluble venom from the scorpion Centruroides noxius Hoffmann. Toxicon 1996, 34, 843. [Google Scholar] [CrossRef]
- Chavanayarn, C.; Thanongsaksrikul, J.; Thueng-In, K.; Bangphoomi, K.; Sookrung, N.; Chaicumpa, W. Humanized-single domain antibodies (VH/VHH) that bound specifically to Naja kaouthia phospholipase A2 and neutralized the enzymatic activity. Toxins 2012, 4, 554–567. [Google Scholar] [CrossRef]
- Kazemi-Lomedasht, F.; Yamabhai, M.; Sabatier, J.M.; Behdani, M.; Zareinejad, M.R.; Shahbazzadeh, D. Development of a human scFv antibody targeting the lethal Iranian cobra (Naja oxiana) snake venom. Toxicon 2019, 171, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Vital Brazil, O.; Vieira, R.J. Neostigmine in the treatment of snake accidents caused by Micrurus frontalis: Report of two cases. Rev. Inst. Med. Trop. S. Paulo 1996, 38, 61–67. [Google Scholar] [CrossRef]
- Kaeley, N.; Prasad, H., Jr.; Singhal, A.; Subhra Datta, S.; Galagali, S.S. Snakebite Causing Facial and Lingual Tremors: A Case Report. Cureus 2022, 14, e27798. [Google Scholar] [CrossRef]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery. Adv. Healthc. Mater. 2021, 10, e2001812. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Annex 5—Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO Technical Report Series; No. 1004; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Gutiérrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688. [Google Scholar] [CrossRef] [PubMed]
- Marinetti, G.V. The action of phospholipase A on lipoproteins. Biochim. Biophys. Acta 1965, 98, 554–565. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.M.; Arruda, E.Z.; Murakami, Y.L.; Moraes, R.A.; El-Kik, C.Z.; Tomaz, M.A.; Fernandes, F.F.; Oliveira, C.Z.; Soares, A.M.; Giglio, J.R.; et al. Evaluation of three Brazilian antivenom ability to antagonize myonecrosis and hemorrhage induced by Bothrops snake venoms in a mouse model. Toxicon 2007, 50, 196. [Google Scholar] [CrossRef]
- Melo, P.A.; Homsi-Brandeburgo, M.I.; Giglio, J.R.; Suarez-Kurtz, G. Antagonism of the myotoxic effects of Bothrops jararacussu venom and bothropstoxin by polyanions. Toxicon 1993, 31, 285. [Google Scholar] [CrossRef]
- Melo, P.A.; Nascimento, M.C.; Mors, W.B.; Suarez-Kurtz, G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon 1994, 32, 595. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef]

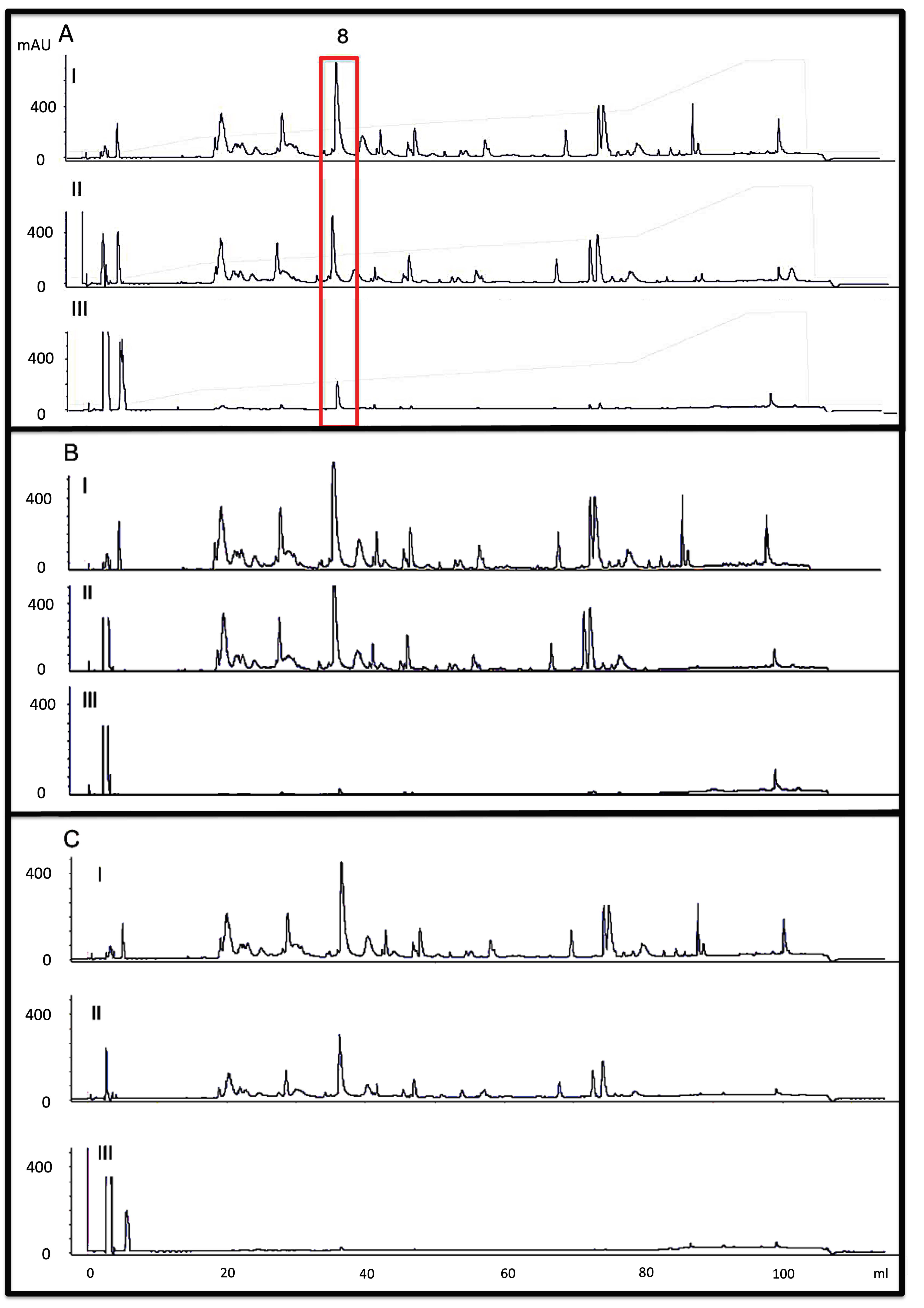
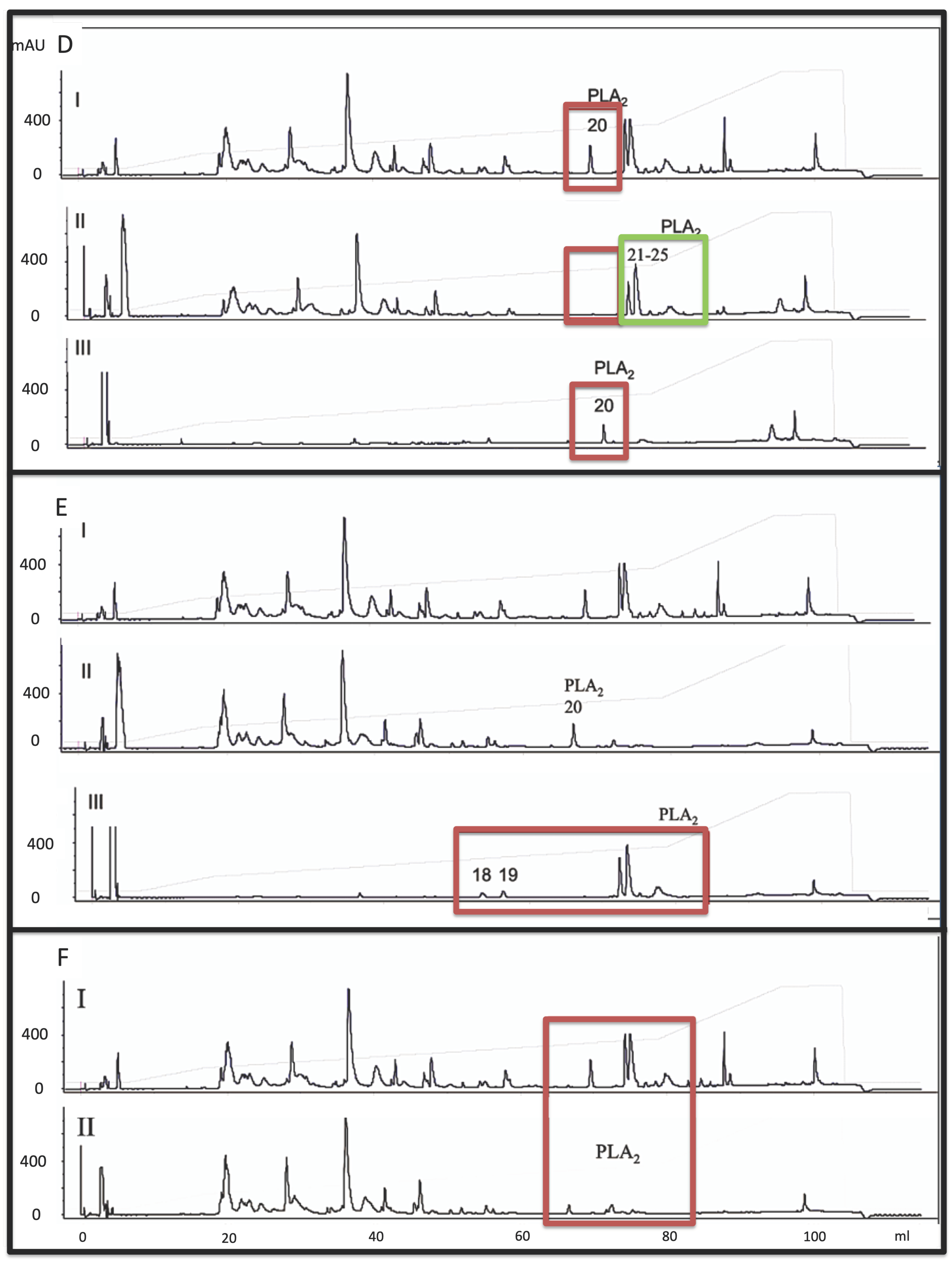


| Gel Filtration Sample (LD50 μg/Animal) | RP-HPLC Analyses | Protein Identification * |
|---|---|---|
| P1 | 31 | LAO |
| P2 | 31 | LAO |
| P3 | 18, 19, 20, 21, 22 | PLA2 |
| P4 (3.3) | 17 20, 21, 23–25 | 3FTx (10.1%) PLA2 |
| P5 | 30 1–5, 7, 8, 10, 13, 16 20, 21, 22, 23 | LIPA 3FTx PLA2 |
| P6 (6.92) | 1–9, 13 21 | 3FTx PLA2 (6.3%) |
| P7 (7.07) | 1–9, 13 21 | 3FTx PLA2 (6.3%) |
| P8 (2.97) | 2, 3, 5, 8, 1. | 3FTx |
| Monoclonal | Target | |
|---|---|---|
| 3FTx | PLA2 | |
| 1E8 | ++ 1 | |
| 2G2 | ++ | |
| 2B1 | ++ | |
| 3B2 | +++ | |
| 4B3 | +++ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Netto, C.; Strauch, M.A.; Monteiro-Machado, M.; Teixeira-Araújo, R.; Fonseca, J.G.; Leitão-Araújo, M.; Machado-Alves, M.L.; Sanz, L.; Calvete, J.J.; Melo, P.A.; et al. Monoclonal-Based Antivenomics Reveals Conserved Neutralizing Epitopes in Type I PLA2 Molecules from Coral Snakes. Toxins 2023, 15, 15. https://doi.org/10.3390/toxins15010015
Corrêa-Netto C, Strauch MA, Monteiro-Machado M, Teixeira-Araújo R, Fonseca JG, Leitão-Araújo M, Machado-Alves ML, Sanz L, Calvete JJ, Melo PA, et al. Monoclonal-Based Antivenomics Reveals Conserved Neutralizing Epitopes in Type I PLA2 Molecules from Coral Snakes. Toxins. 2023; 15(1):15. https://doi.org/10.3390/toxins15010015
Chicago/Turabian StyleCorrêa-Netto, Carlos, Marcelo A. Strauch, Marcos Monteiro-Machado, Ricardo Teixeira-Araújo, Juliana Guzzo Fonseca, Moema Leitão-Araújo, Maria Lúcia Machado-Alves, Libia Sanz, Juan J. Calvete, Paulo A. Melo, and et al. 2023. "Monoclonal-Based Antivenomics Reveals Conserved Neutralizing Epitopes in Type I PLA2 Molecules from Coral Snakes" Toxins 15, no. 1: 15. https://doi.org/10.3390/toxins15010015
APA StyleCorrêa-Netto, C., Strauch, M. A., Monteiro-Machado, M., Teixeira-Araújo, R., Fonseca, J. G., Leitão-Araújo, M., Machado-Alves, M. L., Sanz, L., Calvete, J. J., Melo, P. A., & Zingali, R. B. (2023). Monoclonal-Based Antivenomics Reveals Conserved Neutralizing Epitopes in Type I PLA2 Molecules from Coral Snakes. Toxins, 15(1), 15. https://doi.org/10.3390/toxins15010015







