Therapeutic Approach to Botulinum Injections for Hemifacial Spasm, Synkinesis and Blepharospasm
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Material and Methods
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aramideh, M.; De Visser, B.W.O.; Brans, J.W.; Koelman, J.H.; Speelman, J.D. Pretarsal application of botulinum toxin for treatment of blepharospasm. J. Neurol. Neurosurg. Psychiatry 1995, 59, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, A.R.; Fasano, A.; Ialongo, T.; Soleti, F.; Fermo, S.L.; Albanese, A. Outcome predictors, efficacy and safety of Botox and Dysport in the long-term treatment of hemifacial spasm. Eur. J. Neurol. 2009, 16, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Neville, C.; Venables, V.; Aslet, M.; Nduka, C.; Kannan, R. An objective assessment of botulinum toxin type A injection in the treatment of post-facial palsy synkinesis and hyperkinesis using the synkinesis assessment questionnaire. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J. Xeomin is free from complexing proteins. Toxicon 2009, 54, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Cakmur, R.; Ozturk, V.; Uzunel, F.; Donmez, B.; Idiman, F. Comparison of preseptal and pretarsal injections of botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J. Neurol. 2002, 249, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sorgun, M.H.; Yilmaz, R.; Akin, Y.A.; Mercan, F.N.; Akbostanci, M.C. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J. Clin. Neurosci. 2015, 22, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.-H.; Lee, J.-H.; Hu, H.-W.; Kim, H.-J. Anatomical Proposal for Botulinum Neurotoxin Injection for Glabellar Frown Lines. Toxins 2022, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Lim, J.K.; Lee, D.K.; Yi, S.D. Botulinum a toxin treatment of hemifacial spasm and blepharospasm. J. Korean Med. Sci. 1993, 8, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Laskawi, R.; Ellies, M.; Drobik, C. Botulinum toxin treatment in patients with hemifacial spasm. Eur. Arch. Otorhinolaryngol. 1994, 251, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Farish, S.; Taylor, H.; O’Day, J. Blepharospasm and hemifacial spasm. Randomized trial to determine the most appropriate location for botulinum toxin injections. Ophthalmology 1997, 104, 865–868. [Google Scholar] [CrossRef][Green Version]
- Cillino, S.; Raimondi, G.; Guépratte, N.; Damiani, S.; Cillino, M.; Di Pace, F.; Casuccio, A. Long-term efficacy of botulinum toxin A for treatment of blepharospasm, hemifacial spasm, and spastic entropion: A multicentre study using two drug-dose escalation indexes. Eye 2010, 24, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Auada Souto, M.P.; Souto, L.R.M. An unusual adverse event of botulinum toxin injection in the lower face. J. Cosmet. Dermatol. 2021, 20, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Lolekha, P.; Choolam, A.; Kulkantrakorn, K. A comparative crossover study on the treatment of hemifacial spasm and blepharospasm: Preseptal and pretarsal botulinum toxin injection techniques. Neurol. Sci. 2017, 38, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.; Sauveur, G.D.S. Tear osmolarity, dry eye syndrome, blepharospasm and botulinum neurotoxin. J. Fr. Ophtalmol. 2021, 44, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Poungvarin, N.; Viriyavejakul, A.; Komoltri, C. Placebo-controlled double-blind cross-over study of botulinum A toxin in hemifacial spasm. Park. Relat. Disord. 1995, 1, 85–88. [Google Scholar] [CrossRef]
- Kent, R.; Robertson, A.; Quinones Aguilar, S.; Tzoulis, C.; Maltman, J. Real-World Dosing of OnabotulinumtoxinA and IncobotulinumtoxinA for Cervical Dystonia and Blepharospasm: Results from TRUDOSE and TRUDOSE II. Toxins 2021, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Contarino, M.F.; Van Den Dool, J.; Balash, Y.; Bhatia, K.; Giladi, N.; Koelman, J.H.; Lokkegaard, A.; Marti, M.J.; Postma, M.; Relja, M.; et al. Clinical Practice: Evidence-Based Recommendations for the Treatment of Cervical Dystonia with Botulinum Toxin. Front. Neurol. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [PubMed]

| Total | HFS | PFPS | BS | p-Value Total | |||
|---|---|---|---|---|---|---|---|
| N (% females) | 23 (73.9) | 12 (58.3) | 5 (100.0) | 6 (83.3) | 0.17 | ||
| Affected side | Left | 6 | 4 | 2 | 0 | <0.001 | |
| Right | 11 | 8 | 3 | 0 | |||
| Bilateral | 6 | 0 | 0 | 6 | |||
| Visit number | 112 | 49 | 28 | 35 | 0.99 | ||
| Time between injections, (days), mean ± SD * | 99.3 ± 26.1 | 99.4 ± 29.1 | 92.1 ± 12.4 | 104.3 ± 29.6 | 0.82 | ||
| Number of cycles, mean ± SD | 3.6 ± 2.2 | 3.2 ± 2.0 | 3.8 ± 2.4 | 3.9 ± 2.4 | 0.37 | ||
| AAO (y), mean ± SD | 46.6 ± 19.1 | 49.3 ± 20.0 | 31.4 ± 12.0 | 55.4 ± 16.0 | 0.06 | ||
| Age at first treatment (y), mean ± SD | 53.8 ± 17.7 | 56.6 ± 16.3 | 36.6 ± 12.4 | 62.5 ± 16.5 | 0.02 | ||
| Time from onset to treatment (y), mean ± SD | 5.6 ± 8.7 | 6.8 ± 11.6 | 5.2 ± 3.3 | 3.2 ± 2.3 | 0.64 | ||
| Total dose (U), mean ± SD | 27.0 ± 23.4 | 18.9 ± 12.8 | 9.0 ± 4.4 | 52.9 ± 22.1 | <0.001 | ||
| Effect duration (months), mean + SD | 2.2 ± 0.9 | 2.1 ± 0.9 | 2.0 ± 0.8 | 2.5 ± 1.0 | 0.04 | ||
| Subjective treatment efficacy (%), mean ± SD | 72.2 ± 26.5 | 71.8 ± 27.6 | 73.5 ± 30.9 | 71.5 ± 21.4 | 0.59 | ||
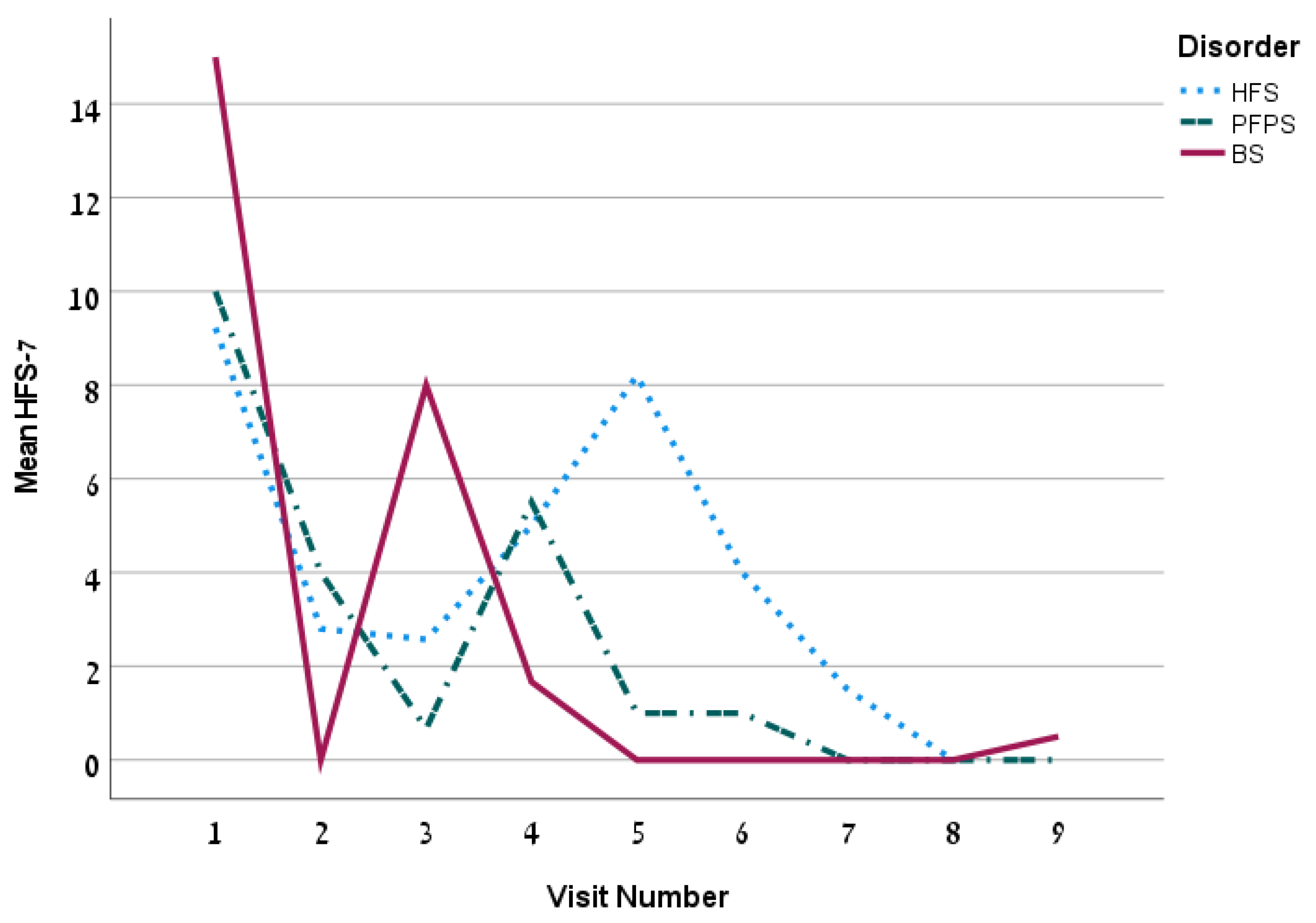
| HFS-7, mean ± SD | 4.5 ± 6.5 | 5.3 ± 6.4 | 3.4 ± 5.7 | 4.0 ± 7.5 | 0.38 | ||
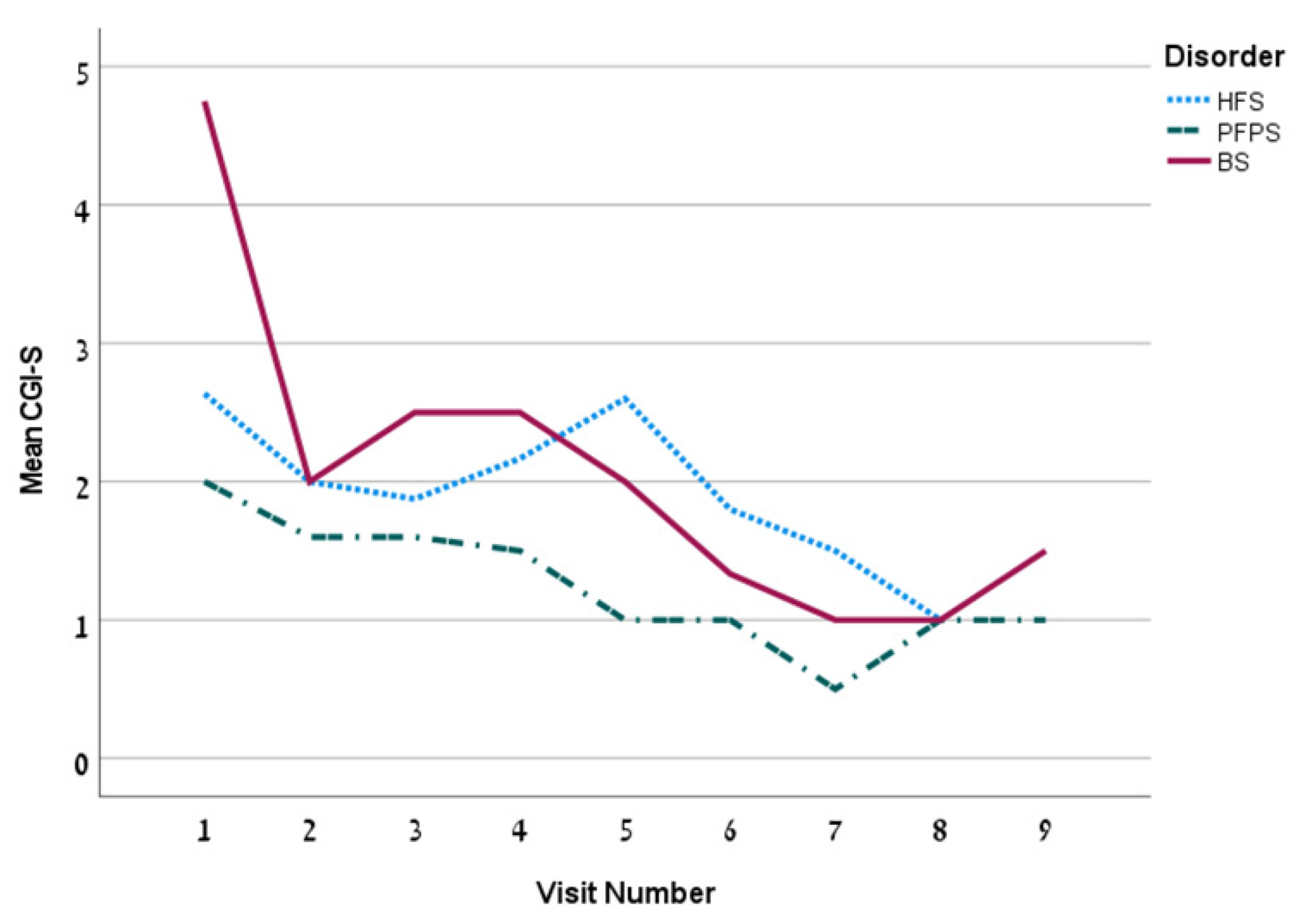
| CGI-S, mean ± SD | 2.0 ± 1.4 | 2.2 ± 1.2 | 1.4 ± 0.8 | 2.3 ± 1.8 | 0.03 | ||
| PGI-C, n (%) | Marked improvement | 55 (59.1) | 20 (50.0) | 16 (69.6) | 19 (63.3) | 0.43 | |
| Moderate improvement | 24 (25.8) | 13 (32.5) | 4 (17.4) | 7 (23.3) | |||
| Mild improvement | 9 (9.7) | 4 (10.0) | 1 (4.3) | 4 (13.3) | |||
| No Change | 5 (5.4) | 3 (7.5) | 2 (8.7) | 0 (0.0) | |||
| Worse | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Complication’s Rate (in Percentage) by Disorder (n = 93) | Complication’s Rate (in Percentage) According to the Number of Injection Points in the Lower Eyelid | |||||||
| Complication | Total | HFS | PFPS | BS | p-Value | 1-Point Regimen (n = 49) | 2-Points Regimen (n = 37) | p-Value |
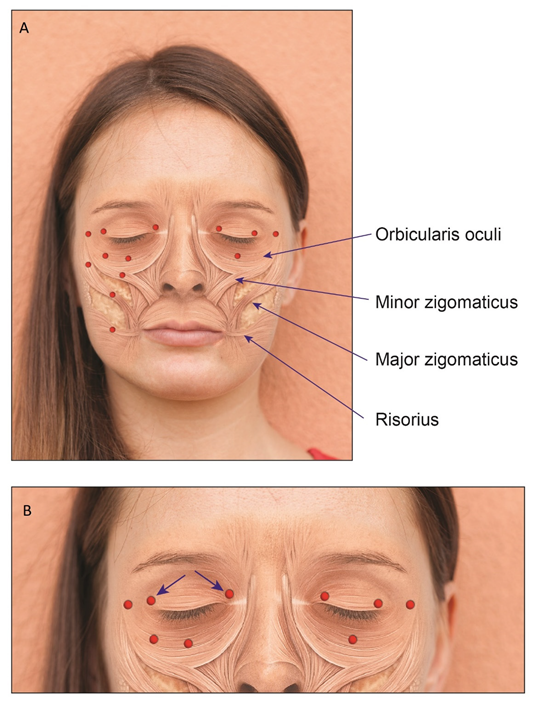
| Facial weakness | 11.8 | 15.0 | 17.4 | 3.3 | 0.21 | 20.4 | 2.7 | 0.01 |
| Hematoma at injection site | 5.4 | 12.5 | 0 | 0 | 0.03 | 4.1 | 8.1 | 0.37 |
| Dry eye | 9.7 | 0 | 13.0 | 20.0 | 0.02 | 8.2 | 10.8 | 0.48 |
| Lacrimation | 5.4 | 5.0 | 8.7 | 3.3 | 0.69 | 2.0 | 8.1 | 0.21 |
| Eye size asymmetry | 1.1 | 2.5 | 0 | 0 | 0.51 | 2.0 | 0 | 0.57 |
| Ptosis | 0 | 0 | 0 | 0 | NA | 0 | 0 | NA |
| Diplopia | 0 | 0 | 0 | 0 | NA | 0 | 0 | NA |
| Any side effect per visit | 25.3 | 12.6 | 4.6 | 8.0 | 0.51 | 26.5 | 23.7 | 0.48 |
| Comparison of PGI-C scores (in percentage) between 1-point and 2-points injections of the lower eyelid | ||||||||
| 1-point regimen | 2-points regimen | |||||||
| Marked improvement | 54.6 | 68.4 | 0.04 | |||||
| Moderate improvement | 20.8 | 28.9 | ||||||
| Mild improvement | 9.3 | 2.6 | ||||||
| No change | 8.3 | 0 | ||||||
| Author | N | Diagnosis | Efficacy | Side Effects |
|---|---|---|---|---|
| Our study 2022 | 23 | HFS, PFPS, BS | Subjective treatment efficacy: 72.2% Moderate to marked improvement: 84.9% | Facial weakness; 11.8% (for 2-points regimen 2.7%), hematoma: 5.4%, dry eye: 9.7%, lacrimation: 5.4% eye size asymmetry: 1.1% |
| Cakmur et al., 2002 [5] | 53 | HFS, BS | 86–96% success (HFS), 90–97% (BS) | Ptosis: 7–18% (HFS), 13–16% (BS), blurred vision: 1–2% (BS), hematoma (not detailed) |
| Sorgun et al., 2015 [6] | 68 | HFS | 73.7% improvement | Hematoma: 4.9%, facial asymmetry: 3.6% Ptosis: 3.6%, diplopia: 3.2%, eye weakness: 2.3% |
| Bentivoglio et al., 2009 [2] | 108 | HFS | 94% success (Yes/no benefit) | Ptosis: 3.2% (ONA), 8.7% (ABO), lacrimation: 4.3% (ONA), 1.7% (ABO), irritation of conjuctivae: 2.8 (ONA), 0.6% (ABO), hematoma: 2.4% (ONA), 1.7% (ABO), blurred vision: 1.8% (ONA), 1.2% (ABO), diplopia: 1.2% (ONA), 2.3% (ABO) |
| Aramideh et al., 1995 [1] | 45 | BS | Pretarsal: 95% success (Yes/no benefit) | Diplopia: 10%, Blurred vision: 10% |
| Price et al., 1997 [10] | 92 | HFS, BS | Effect not measured | Lacrimation: 4–18%, ocular irritation: 4–18%, ptosis: 1–13%, diplopia: 1–5% |
| Lolekha et al., 2017 [14] | 40 | HFS, BS | Satisfaction rating scale: 73.8–82.8% | Ptosis: 3.8%, lacrimation: 3.8%, Hematoma: 5%, irritation: 6.3% |
| Poungvarin et al., 1995 [16] | 55 | HFS | Excellent response (80.95%) Moderate response (7.14%) | Facial weakness: 7.14%, local pain: 4.76%, lacrimation: 2.38% |
| Park et al., 1993 [8] | 112 | HFS, BS | Excellent response (98.6%) | Dry eyes: 19.8% (HFS), 27.3% (BS), mouth droop: 19.8% (HFS), ptosis: 10.9% (HFS), 27.3% (BS), lid edema: 5% (HFS), 0.9% (BS), fatigue: 4% (HFS), diplopia: 2% (HFS), 0.9% (BS), hematoma: 2% (HFS) |
| Cillino et al., 2010 [11] | 131 | HFS, BS | Effect not measured | Hematoma: 31.5% (BS), 31% (HFS), ptosis: (19.2% (BS), 17.2% (HFS), diplopia: 5.4% (BS), 8.6% (HFS), photophobia: 1.4% (BS), 3.4% (HFS), dry eyes: 2.7% (BS), 1.7% (HFS), mouth droop: 1.7% (HFS), blurred vision: 1.4% (BS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahalom, G.; Janah, A.; Rajz, G.; Eichel, R. Therapeutic Approach to Botulinum Injections for Hemifacial Spasm, Synkinesis and Blepharospasm. Toxins 2022, 14, 362. https://doi.org/10.3390/toxins14050362
Yahalom G, Janah A, Rajz G, Eichel R. Therapeutic Approach to Botulinum Injections for Hemifacial Spasm, Synkinesis and Blepharospasm. Toxins. 2022; 14(5):362. https://doi.org/10.3390/toxins14050362
Chicago/Turabian StyleYahalom, Gilad, Amir Janah, Gustavo Rajz, and Roni Eichel. 2022. "Therapeutic Approach to Botulinum Injections for Hemifacial Spasm, Synkinesis and Blepharospasm" Toxins 14, no. 5: 362. https://doi.org/10.3390/toxins14050362
APA StyleYahalom, G., Janah, A., Rajz, G., & Eichel, R. (2022). Therapeutic Approach to Botulinum Injections for Hemifacial Spasm, Synkinesis and Blepharospasm. Toxins, 14(5), 362. https://doi.org/10.3390/toxins14050362




