Abstract
The aim of this study was to show our therapeutic outcome of botulinum injection to the facial muscles and thereby to find the best therapeutic concept which should be embraced. The decision to treat the lower eyelid with 1-point or 2-points injection was randomly taken as there is no consensus regarding this debate. Injections of the lateral end of the upper eyelid were performed more laterally to the conventional injection point, just lateral to the conjunction of the upper and lower eyelids. Twenty-three patients (12 hemifacial spasm, 6 blepharospasm, 5 post facial palsy synkinesis) were enrolled. Data were retrieved from 112 visits between 2019 and 2022. Overall, 84.9% of the treatments had moderate or marked improvement. The most common side effect was facial weakness (11.8%). Neither ptosis nor diplopia were noted. Two-points regimen in the lower eyelid was associated with a lower risk of facial weakness (p = 0.01), compared to 1-point regimen, with a better therapeutic outcome as reflected by more favorable PGI-C scores (p = 0.04). Injection of the pretarsal segment of the upper eyelid, just onto or even lateral to the conjunction of the upper and lower eyelids, lowers the risk of ptosis.
Key Contribution:
In this study, a new approach is introduced to minimize the risk of side effects following botulinum injections of the facial muscles.
1. Introduction
Treatment with botulinum toxin (BT) for blepharospasm (BS) [1], hemifacial spasm (HFS) [2] and post facial paralysis synkinesis (PFPS) [3] is effective and safe. The efficacy and side effects profile are dependent on the experience and technique of the treating physician. Incobotulinumtoxin A (Xeomin®, INCO) differs from Onabotulinumtoxin A (Botox®, ONA) and abobotulinumtoxin A (Dysport®, ABO) as the formulation of INCO is free from complexing proteins [4]. Two approaches for injections of the orbicularis oculi muscle are being implemented: preseptal and pretarsal injections [1,5]. The pretarsal approach was found to be more effective and safer in terms of the rate of ptosis than the preseptal approach and is now probably being practiced by most centers [1,5]. For the anatomical differences between the two, we refer to the study of Cakmur et al. [5].
Ptosis is perhaps the most common and most debilitating side effect of BT injections into the facial muscles. The rate of occurrence and recurrence of ptosis is variable, ranging from 3.2% to 18% and is significantly dependent on the technique used [1,2,5,6]. Ptosis occurs due to unwanted spread of BT from the injection site, usually in the upper eyelid to the levator palpebrae muscle [1], but sometimes also following injections of the corrugators [7]. Other side effects of facial muscles injection include eye dryness [8], eye weakness [9], ocular itching [10], diplopia [10], blurred vision [1] and hematoma in the injection site [11], which seem to be secondary to either upper or lower eyelid injections. Smile asymmetry or mouth droop occur due to injections of the muscles generating the smile function, i.e., the lower eyelid or lower parts of the face [12]. The decision to treat the lower eyelid with 1-point or 2-points injection is randomly taken and there is no consensus regarding this debate. We aim to show our therapeutic outcome of BT injection into the facial muscles and thereby to find the best therapeutic concept which should be embraced.
2. Results
Thirty-two consecutive patients, diagnosed with either HFS or PFPS or BS, attended our clinic between the years 2019 and 2022. Nine patients were previously treated elsewhere and were excluded. Twenty-three naïve patients (seventeen females) were eventually enrolled and included in the analysis. Data were retrieved from 112 visits (mean of 3.6 ± 2.2 cycles per patient). Demographic and clinical characteristics are presented in Table 1. The majority of patients (n = 12) were diagnosed with HFS, followed by BS (n = 6) and PFPS (n = 5). The mean age at onset (AAO) was 46.6 ± 19.1 years. AAO of the BS group tended to be older than the PFPS group (55.4 ± 16.0 vs. 31.4 ± 12.0 years, p = 0.06). The age at first treatment was 53.8 ± 17.7 years. Treatments were performed using either INCO (53.6%) or ONA (46.4%).

Table 1.
Demographic and clinical characteristics.
The first treatment included a mean dose of 18.2 ± 13.2 U (13.1 ± 6.1 units for the HFS group). The mean dose increased over time. The mean time between injections was 99.3 ± 26.1 days. The overall Hemifacial Spasm 7 (HFS-7) score of the whole group was 4.5 ± 6.5 points and it decreased persistently over time (Figure 1) and so is the Clinician Global Impression of Severity (CGI-S) (Figure 2). The subjective treatment efficacy was 72.2 ± 26.5 percent. The treatment was more effective with increased doses of BT. Effect duration averaged 2.2 ± 0.9 months. The effect was longer for the BS group (p = 0.04).
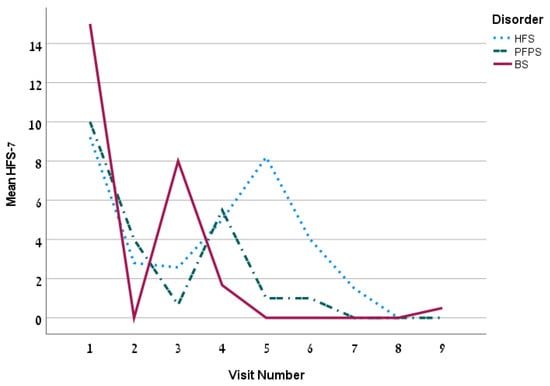
Figure 1.
The mean HFS-7 by visit for the 3 disorders.
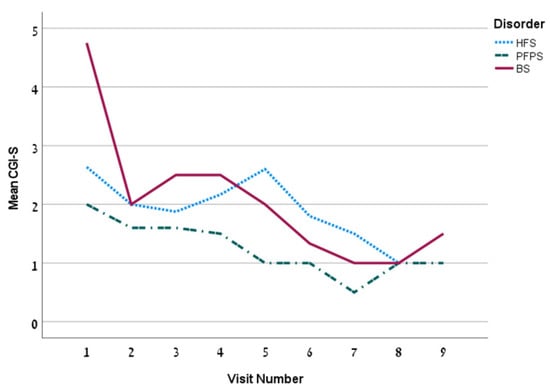
Figure 2.
The mean CGI-S by visit for the 3 disorders.
As for the Patient Global impression of Change (PGI-C) score, marked improvement was most frequent and was scored 55 times (59.1%), followed by moderate and mild improvement (in 25.8% and 9.7%, respectively). On five occasions (5.4%), the patient reported no change. Overall, 84.9% of the treatments had moderate or marked improvement. There was no difference in PGI-C among the different disorders. However, following a head-to-head comparison between 1-point and 2-points injection regimen, PGI-C scores were more favorable in the 2-points regimen as 97.3% had marked or moderate improvement versus 75.4% in the 1-point regimen (p = 0.04).
Injection of the lateral-upper portion of the major zygomatic muscle was rarely performed and seemed to have no added value compared to that of its lower portion.
Analysis on HFS alone showed non-significant differences in the doses of both types of BT (19.8 ± 14.3 units for ONA vs. 18.0 ± 11.3 units for INCO, p = 0.90), non-significant difference in subjective treatment efficacy (71.0 ± 24.7 vs. 72.8 ± 31.7%, respectively; p = 0.44), and non-significant difference in the intervals between injections (105.3 ± 36.0 vs. 90.1 ± 10.2 days, respectively; p = 0.64).
Side effects from BT injections occurred in 25.3% of 93 visits. No significant difference in the rate of “any side effect” was noted between 1-point and 2-points approach. The most common side effect was facial weakness with a rate of 11.8%, followed by dry eye (9.7%) and hematoma at the injection site (5.4%) and lacrimation (5.4%) (Table 2). Interestingly, neither ptosis nor diplopia were noted. As for the side effect of facial weakness, most occurred following the 1-point approach, while much lower rates were noted after the 2-points approach (20.4% vs. 2.7%, respectively, p = 0.01) (Table 2).

Table 2.
Side effects by disorder for all and by the number of injection points in lower eyelid.
3. Discussion
In this study, we aimed to evaluate the therapeutic effect of patients treated with BT for dystonia and hemispasm/synkinesis of the face. There was a markedly favorable therapeutic effect of BT treatment, with a fair side effects profile. Ptosis, probably the most common and debilitating side effect, being present in 3.2–18% [1,2,5,6], was not reported even in one single case. We suggest that injection of the pretarsal segment of the upper eyelid, just onto or even lateral to the conjunction of the upper and lower eyelids, can avoid infiltration of BT to the levator palpebrae muscle, without compromising the therapeutic effect. Lolekha et al. also had no ptosis, following 40 pretarsal injections [13]. In the study of Lolekha et al. the dose injected to each point on the upper eyelid was limited to 2.5 units only. We used maximal doses of 3 units per injection point. Nonetheless, our off-the-records analysis on non-naïve patients, who were treated with doses as high as 8 units ONA and 30 units of ABO per injection point (lateral and medial upper eyelid), was not related to ptosis or diplopia. Diplopia, an occasionally encountered and debilitating side effect, occurring in up to 5% [9], was not noted in our study. This is probably due to strict avoidance of injection of the far medial part of the lower eyelid, leading to diffusion of the toxin into the inferior oblique or the inferior rectus. Table 3 presents clinical outcomes found in our study and those of others. Blurred vision, a side effect which was found to occur in up to 10% in BS patients [1], was not reported in our cohort.

Table 3.
Clinical data from our study and from other previous studies on the therapeutic outcomes of botulinum toxin for HFS and BS.
The rates of facial weakness (11.8%) and dry eye (9.7%) were relatively frequent, compared to the rates in the study of Sorgun et al. (3.6%, 0.3%, respectively) [6], which showed excellent side effects profile. Dry eye is a particularly frequent side effect in BS [14] and was absent in all our HFS patients. Sorgun et al. performed only one injection of 5 units in the lateral part of the lower eyelid, avoiding injection in the medial part. This methodology contradicts our statement that the 2-points injection is safer. The study of Lolekha et al. which adhered to the 2-points injection of the lower eyelid, supported our finding as relatively few side effects occurred following pretarsal injections, including hematoma (5%) and lacrimation (3.8%) [13]. Another option to minimize side effects, while we extrapolate our results and those of Sorgun et al. [6], is to inject the lateral part of the lower eyelid with higher doses and that of the medial part with lower doses. It is of note that in our analysis on naïve patients, only one patient had facial weakness after 2-point injection regimen, but this patient was also injected with 3 units in the upper part of major zygomaticus.
From all the above-mentioned, it seems that the 2-points injection technique of the lower eyelid is safe and preferable over 1-point injection, to avoid facial weakness of the injected side. The 2-points technique seems also to be more effective. It may make sense to inject the lower eyelid with no more than 3 units per injection site, and particular caution should be implemented when the medial part is injected.
The mean subjective treatment efficacy of 71.8% in HFS seen in our study is rather similar to that of Sorgun et al. [6]. Additionally, 84.9% and 97.3% of the treatments had moderate to marked response to treatment according to the PGI-C score for all and for the 2-points regimen, respectively and this outcome is comparable with others [2,5,15] (Table 3).
The main limitations of this study were its small sample and the short-lasting follow-up time. Hence, no firm conclusion should be drawn and a further larger study should be ensued in order to confirm its findings. The size of the study could be larger, but we decided to perform the analysis on our naïve patients only, since some of our patients were satisfied with their old pattern of injection from the previous clinic and preferred to have mild facial weakness as a consequence of the BT treatment.
We assume that more cycles would lead to a better therapeutic outcome and to less side effects. On the other hand, the short follow-up may omit cases in which injections of more sites are necessary, with possible secondary side effects that are not reflected in a short-term follow-up.
The assessment was incomplete due to language barriers in some patients. The effect duration time was inaccurate as in some patients it did not subside until next treatment.
The study cohort was heterogenous; hence, any conclusion should be taken with caution. The use of more than one toxin in a study is sub-optimal, based on the statement of Kent et al. [16], which found that there was no fixed-dose ratio conversion between INCO and ONA, and consistent with the product label and recommendations from regulatory agencies, the potency units of ONA are not interchangeable with other BT type A products. On the other hand, many studies used more than one type of toxin, and the conversion ratio of 1:1 between ONA and INCO is widely accepted [17,18,19]. To clarify this question, we performed another sub-analysis on HFS alone, in which no significant differences between ONA and INCO were found in the mean dose, in the mean interval between the injections and in the subjective treatment efficacy. Indeed, we did not make different decisions between both types of BT in our dosage calculation prior to each treatment.
4. Conclusions
This study is a proof of concept, in which we introduce a technical approach to reduce the risk of side effects to the minimum, without compromising the therapeutic effect. The extra-lateral injection of the upper eyelid prevents ptosis, and perhaps diplopia, without a deleterious impact on efficacy. The same holds true for the 2-points approach of lower eyelid injection, which reduces the risk of iatrogenic facial weakness. Furthermore, a larger study may shed light on this proof of concept.
5. Material and Methods
Consecutive patients who attended routinely the Movement Disorders Clinic and were diagnosed with HFS, PFPS or BS were enrolled. The diagnosis was made by a movement disorders specialist (GY). Only naïve patients were enrolled and those treated earlier at another clinic were excluded. Patients were treated in intervals of 3 months (except for the second cycle in which a booster was given, if needed, 3 weeks after first treatment). The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Shaare Zedek Medical Center (protocol code 0406, date of approval 21 May 2019). Informed consent was signed by each patient, prior to enrollment. Demographic data were noted, including age at injection date, AAO, gender, the side involved. Prior to each treatment, patients completed a questionnaire which included the subjective efficacy in percentage of the previous treatment at maximal effect (where 0% is no benefit and 100% is benefit to an asymptomatic state). In addition, effect duration and side effects of the previous treatment, if occurred, were also noted. Prior to each treatment, the following forms were also completed: PGI-C, CGI-S and the HFS-7. Clinical data, including the BT type, the dose and points of the injections were noted. Patients were treated with either ONA or INCO, diluted with 50 units per milliliter (mL) in a 31G-syringe. Patients received the same type of BT each treatment. The doses for each injection were chosen for each individual, based on clinical impression of the severity, ranging from 1 unit to 5 units per injection point. We followed the accepted line of 1:1 ratio between ONA and INCO in the treatment plan [17]. Possible injection points are depicted in Figure 3 and illustrated in Figure 4. The orbicularis oculi muscle included the following injection points: the pretarsal nasal (medial) and temporal (lateral) ends of the upper eyelid, the lateral point (temporal area), just beneath and lateral to the eyebrow and 2 cm lateral from the eye, the pretarsal nasal (medial) and temporal (lateral) ends of the lower eyelid, just on the edge of the orbital bone. In the 1-point regimen the lower eyelid injection was under the eye, midway between both inner and outer edges. The 2-points regimen was in both midway and lateral position, just under the outer edge. It is of note that injections in the lateral end of the upper eyelid were performed more laterally, compared to the conventional injection point, just lateral to the conjunction of the upper and lower eyelids. To minimize the risk of local hematoma of the lower eyelid, one should use the thumb and index fingers to stretch the skin in opposing horizontal directions, while injecting between the fingers the subcutaneous layer under the skin as superficially as possible. Other points of injection were the major zygomatic muscle (either the inferior or the upper-lateral portion), minor zygomatic muscle (also called the zygomatic head of the levator labii superior) and the risorius muscle. To best localize the minor and major zygomatic, as well as the risorius muscles, one should palpate the muscles and wait for muscular twitches. In cases of infrequent twitches, anatomical location is roughly estimated.
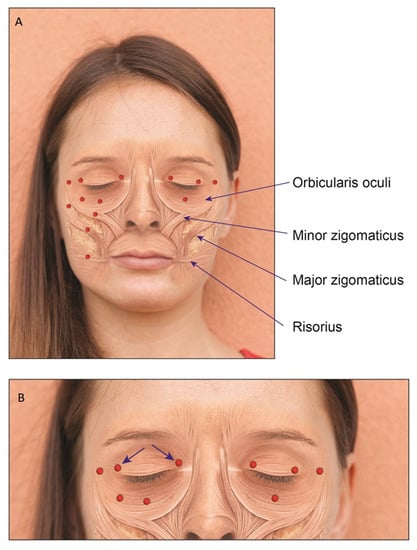
Figure 3.
(A) Injection sites and anatomical locations of the relevant muscles of the upper and the lower face. The red dots indicate the injection points. Right side is our method of injection in the upper eyelid and 2-points injection of the lower eyelid. Left is the conventional method to inject the upper eyelid, with 1-point injection of the lower eyelid. (B) Zoom in focused on injections of the orbicularis oculi: the arrows reflect the direction of the tip of the needle. The direction of the needle for other parts is not important.
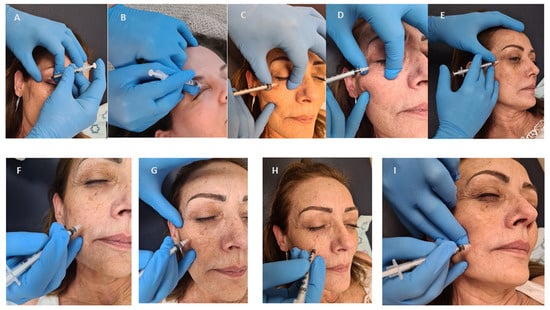
Figure 4.
Location of injections and technique: (A) lateral upper eyelid (B) medial upper eyelid (C) medial lower eyelid (D) lateral lower eyelid (E) lateral part of orbicularis oculi (F) lower part of the major zygomaticus (G) upper part of the major zygomaticus (H) minor zygomaticus (I) risorius.
Statistical Analysis
Demographic data were calculated using descriptive and frequency tables. Differences of continuous measures were calculated using Kruskal–Wallis and Mann–Whitney non-parametric tests and for categorical measures using chi-square tests. The p-value of the differences in complication rates among the disorders was defined as ≤0.01, using a correction for multiple comparisons. For other comparisons, p-value ≤ 0.05 was defined as statistically significant. The analysis was performed by SPSS v.28.
Author Contributions
Conceptualization, G.Y.; methodology, G.Y.; software, G.Y.; validation, G.Y., G.R. and R.E.; formal analysis, G.Y.; investigation, G.Y.; data curation, G.Y.; writing—original draft preparation, G.Y.; writing—review and editing, G.Y., A.J., G.R. and R.E.; supervision, R.E.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Shaare Zedek Medical Center (protocol code 0406, date of approval 21 May 2019).
Informed Consent Statement
Each participant signed an informed consent prior to enrollment.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
Gilad Yahalom received consultancy fees from Abbvie Biopharmaceuticals Inc. and Medison Pharma. No other disclosures were reported.
References
- Aramideh, M.; De Visser, B.W.O.; Brans, J.W.; Koelman, J.H.; Speelman, J.D. Pretarsal application of botulinum toxin for treatment of blepharospasm. J. Neurol. Neurosurg. Psychiatry 1995, 59, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, A.R.; Fasano, A.; Ialongo, T.; Soleti, F.; Fermo, S.L.; Albanese, A. Outcome predictors, efficacy and safety of Botox and Dysport in the long-term treatment of hemifacial spasm. Eur. J. Neurol. 2009, 16, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Neville, C.; Venables, V.; Aslet, M.; Nduka, C.; Kannan, R. An objective assessment of botulinum toxin type A injection in the treatment of post-facial palsy synkinesis and hyperkinesis using the synkinesis assessment questionnaire. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J. Xeomin is free from complexing proteins. Toxicon 2009, 54, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Cakmur, R.; Ozturk, V.; Uzunel, F.; Donmez, B.; Idiman, F. Comparison of preseptal and pretarsal injections of botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J. Neurol. 2002, 249, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sorgun, M.H.; Yilmaz, R.; Akin, Y.A.; Mercan, F.N.; Akbostanci, M.C. Botulinum toxin injections for the treatment of hemifacial spasm over 16 years. J. Clin. Neurosci. 2015, 22, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.-H.; Lee, J.-H.; Hu, H.-W.; Kim, H.-J. Anatomical Proposal for Botulinum Neurotoxin Injection for Glabellar Frown Lines. Toxins 2022, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Lim, J.K.; Lee, D.K.; Yi, S.D. Botulinum a toxin treatment of hemifacial spasm and blepharospasm. J. Korean Med. Sci. 1993, 8, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Laskawi, R.; Ellies, M.; Drobik, C. Botulinum toxin treatment in patients with hemifacial spasm. Eur. Arch. Otorhinolaryngol. 1994, 251, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Farish, S.; Taylor, H.; O’Day, J. Blepharospasm and hemifacial spasm. Randomized trial to determine the most appropriate location for botulinum toxin injections. Ophthalmology 1997, 104, 865–868. [Google Scholar] [CrossRef][Green Version]
- Cillino, S.; Raimondi, G.; Guépratte, N.; Damiani, S.; Cillino, M.; Di Pace, F.; Casuccio, A. Long-term efficacy of botulinum toxin A for treatment of blepharospasm, hemifacial spasm, and spastic entropion: A multicentre study using two drug-dose escalation indexes. Eye 2010, 24, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Auada Souto, M.P.; Souto, L.R.M. An unusual adverse event of botulinum toxin injection in the lower face. J. Cosmet. Dermatol. 2021, 20, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Lolekha, P.; Choolam, A.; Kulkantrakorn, K. A comparative crossover study on the treatment of hemifacial spasm and blepharospasm: Preseptal and pretarsal botulinum toxin injection techniques. Neurol. Sci. 2017, 38, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.; Sauveur, G.D.S. Tear osmolarity, dry eye syndrome, blepharospasm and botulinum neurotoxin. J. Fr. Ophtalmol. 2021, 44, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Poungvarin, N.; Viriyavejakul, A.; Komoltri, C. Placebo-controlled double-blind cross-over study of botulinum A toxin in hemifacial spasm. Park. Relat. Disord. 1995, 1, 85–88. [Google Scholar] [CrossRef]
- Kent, R.; Robertson, A.; Quinones Aguilar, S.; Tzoulis, C.; Maltman, J. Real-World Dosing of OnabotulinumtoxinA and IncobotulinumtoxinA for Cervical Dystonia and Blepharospasm: Results from TRUDOSE and TRUDOSE II. Toxins 2021, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Contarino, M.F.; Van Den Dool, J.; Balash, Y.; Bhatia, K.; Giladi, N.; Koelman, J.H.; Lokkegaard, A.; Marti, M.J.; Postma, M.; Relja, M.; et al. Clinical Practice: Evidence-Based Recommendations for the Treatment of Cervical Dystonia with Botulinum Toxin. Front. Neurol. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).