Evaluation of Toxicity of Crude Phlorotannins and Phloroglucinol Using Different Model Organisms
Abstract
1. Introduction
2. Results
2.1. Phloroglucinol
2.2. Larvicidal Effects of Crude Phlorotannin and Phloroglucinol on Survival of Artemia salina Nauplii
2.3. Larvicidal Effect of Crude Phlorotannin and Phloroglucinol on Survival of Daphnia magna Neonates
2.4. Inhibitory Effect of Crude Phlorotannin and Phloroglucinol on Germination of Lactuca Sativa Seed
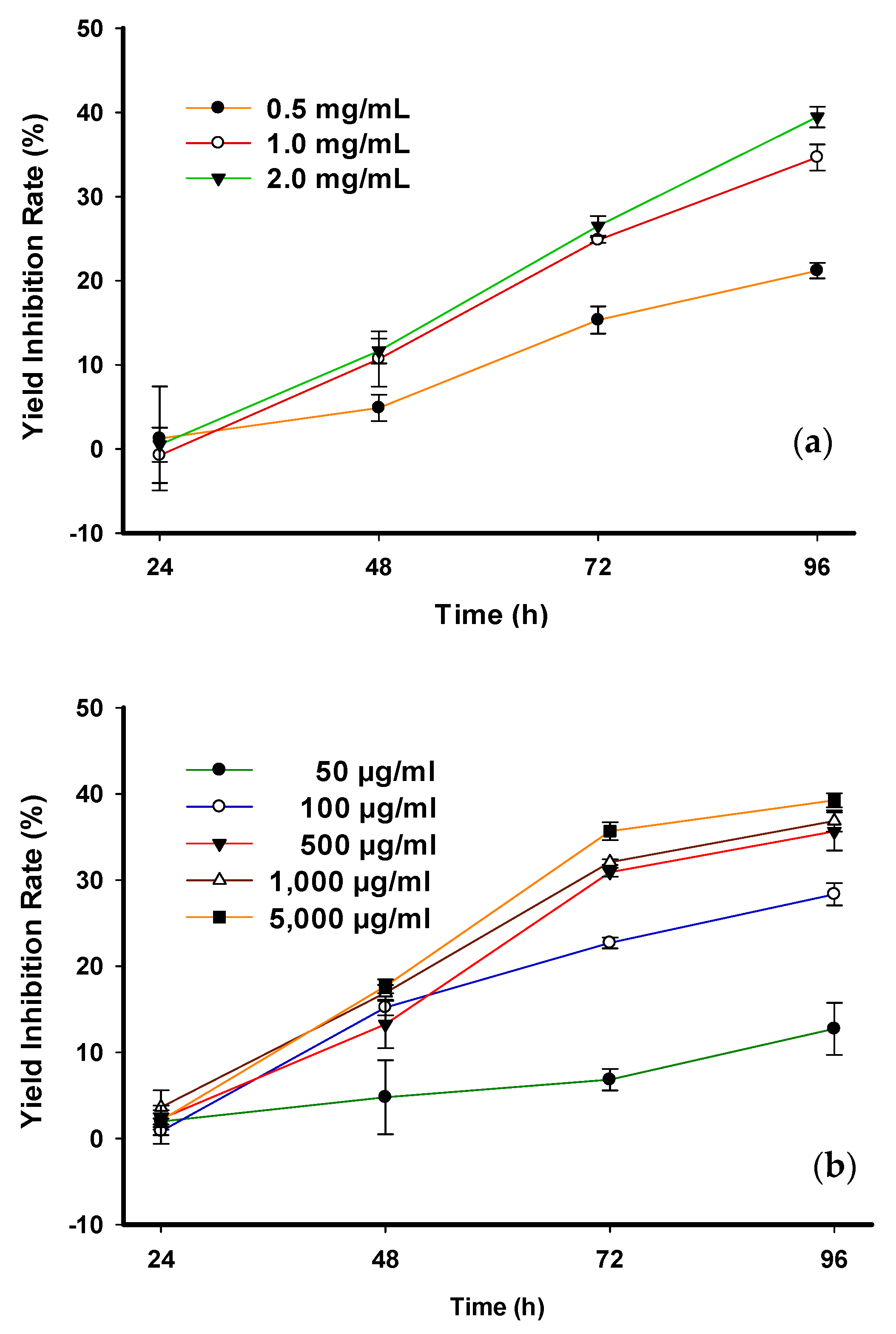
2.5. Inhibitory Effect of Crude Phlorotannin and Phloroglucinol on Freshwater Chlorella vulgaris
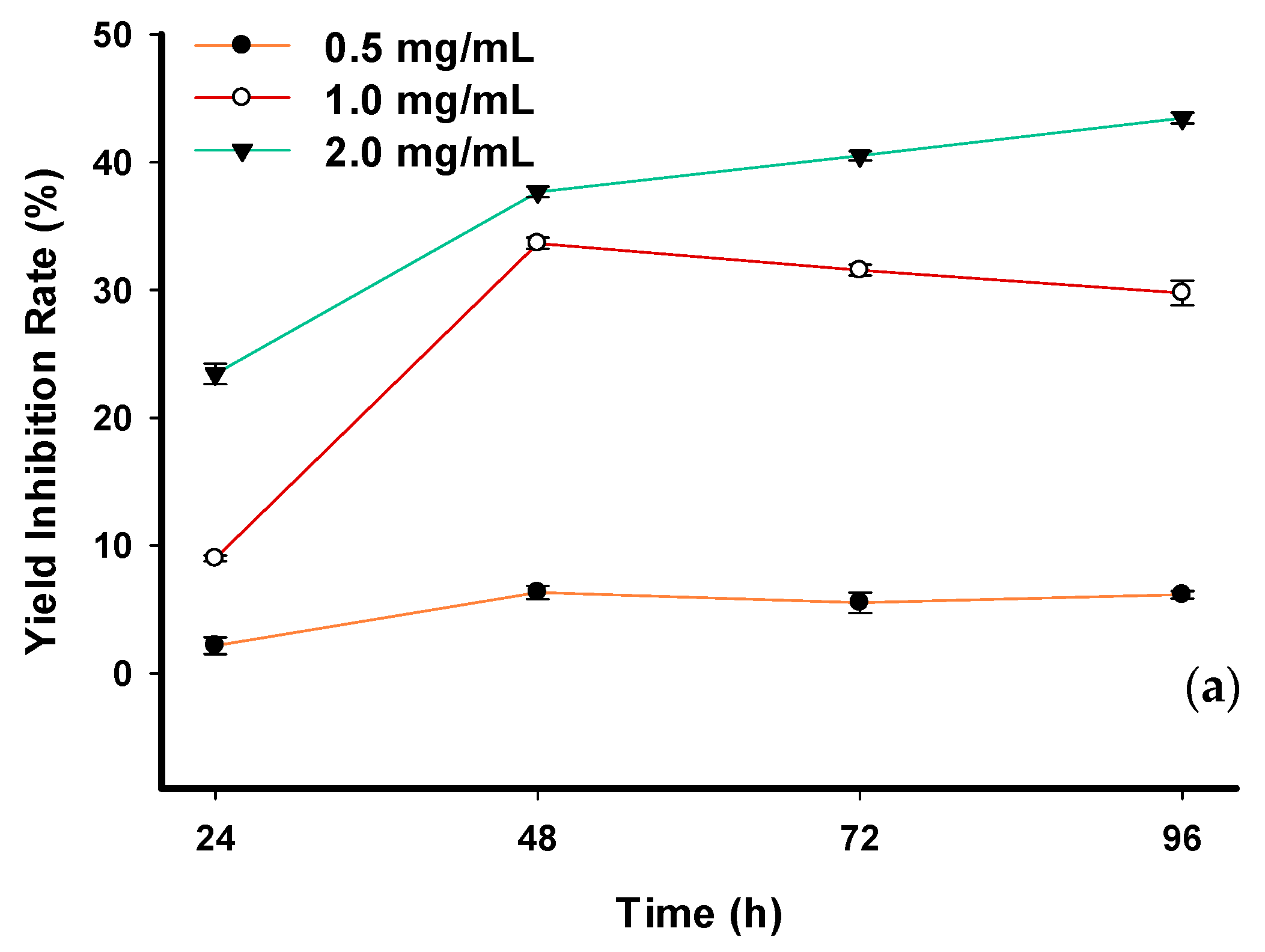
2.6. Inhibitory Effect of Phlorotannin and Phloroglucinol on Seawater Chlorella vulgaris
3. Discussion
3.1. Larvicidal Effect of Crude Phlorotannin and Phloroglucinol on Survival of Artemia salina Nauplii
3.2. Larvicidal Effect of Crude Phlorotannin and Phloroglucinol on Survival of Daphnia magna Neonates
3.3. Inhibitory Effect of Crude Phlorotannin and Phloroglucinol on Germination of Lactuca sativa Seeds
3.4. Inhibitory Effect of Crude Phlorotannin and Phloroglucinol on Freshwater and Seawater Chlorella vulgaris
4. Conclusions
5. Materials and Methods
5.1. Materials
5.1.1. Eclonia cava
5.1.2. Phloroglucinol
5.1.3. Artemia salina Nauplii
5.1.4. Daphnia magna Neonates
5.1.5. Lactuca sativa Seeds
5.1.6. Chlorella vulgaris
5.2. Methods
5.2.1. Crude Phlorotannin Extracts
5.2.2. Phloroglucinol
5.2.3. Artemia salina and Daphnia magna Lethality Test
5.2.4. Lactuca sativa Germination Bioassays
5.2.5. Freshwater and Seawater Chlorella vulgaris Yield Inhibition Test
5.2.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective Effect of Phloroglucinol on Oxidative Stress Induced Cell Damage via Catalase Activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and Identification of Phlorotannins from Ecklonia stolonifera with Antioxidant and Anti-Inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Kageyama, N.; Nakahara, K.; Miki, W. Phlorotannins as Radical Scavengers from the Extract of Sargassum ringgoldianum. Mar. Biotechnol. 2006, 8, 409–414. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant Effects of Phlorotannins Isolated from Ishige okamurae in Free Radical Mediated Oxidative Systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Brock, E.; Nylund, G.M.; Pavia, H. Chemical Inhibition of Barnacle Larval Settlement by the Brown Alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 2007, 337, 165–174. [Google Scholar] [CrossRef][Green Version]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-Inflammatory Activity of Edible Brown Alga Eisenia bicyclis and Its Constituents Fucosterol and Phlorotannins in LPS-Stimulated RAW264.7 Macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Menikea, K.K.; Kyprianou, A.; Samanides, C.G.; Georgiou, S.G.; Koutsokeras, L.; Constantinides, G.; Vyrides, I. Anaerobic Granular Sludge and Zero Valent Scrap Iron (ZVSI) Pre-Treated with Green Tea as a Sustainable System for Conversion of CO2 to CH4. J. Clean. Prod. 2020, 268, 121860. [Google Scholar] [CrossRef]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In Vitro and Cellular Antioxidant Activities of Seaweed Extracts Prepared from Five Brown Seaweeds Harvested in Spring from the West Coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 Reverse Transcriptase and Protease by Phlorotannins from the Brown Alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid.-Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Antifungal and Larvicidal Activities of Phlorotannins from Brown Seaweeds. Mar. Drugs 2021, 19, 223. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef]
- Thangam, T.S.; Kathiresan, K. Mosquito Larvicidal Effect of Seaweed Extracts. Bot. Mar. 1991, 34, 433–436. [Google Scholar] [CrossRef]
- Manilal, A.; Thajuddin, N.; Selvin, J.; Idhayadhulla, A.; Kumar, R.S.; Sujith, S. In Vitro Mosquito Larvicidal Activity of Marine Algae Against the Human Vectors, Culex quinquefasciatus (Say) and Aedes aegypti (Linnaeus) (Diptera: Culicidae). Int. J. Zool. Res. 2011, 7, 272–278. [Google Scholar] [CrossRef]
- Ravikumar, S.; Syed Ali, M.; Margaret Beula, J. Mosquito Larvicidal Efficacy of Seaweed Extracts against Dengue Vector of Aedes aegypti. Asian Pac. J. Trop. Biomed. 2011, 1, S143–S146. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hirota, H.; Kato, H.; Fusetani, N. Phlorotannins and Snlfoquinovosyl Diacylglycerols: Promoters of Larval Metamorphosis in Ascidians, Isolated from the Brown Alga Sargassum thunbergii. Fish. Sci. 1994, 60, 319–321. [Google Scholar] [CrossRef][Green Version]
- Lau, S.C.K.; Qian, P.Y. Phlorotannins and Related Compounds as Larval Settlement Inhibitors of the Tube-Building Polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 1997, 159, 219–227. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kwon, E.-H.; Choi, J.-S.; Hong, S.-Y.; Shin, H.-W.; Hong, Y.-K. Antifouling Activity of Seaweed Extracts on the Green Alga Enteromorpha prolifera and the Mussel Mytilus edulis. J. Appl. Phycol. 2001, 13, 117–125. [Google Scholar] [CrossRef]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal Effect of Phlorotannins from the Brown Alga Ecklonia kurome on Red Tide Microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Birrell, C.L.; McCook, L.J.; Willis, B.L.; Harrington, L. Chemical Effects of Macroalgae on Larval Settlement of the Broadcast Spawning Coral Acropora millepora. Mar. Ecol. Prog. Ser. 2008, 362, 129–137. [Google Scholar] [CrossRef]
- Kim, Y.D.; Choi, J.S. Larvicidal Effects of Korean Seaweed Extracts on Brine Shrimp Artemia salina. J. Anim. Plant Sci. 2017, 27, 1039–1046. [Google Scholar]
- Nakajima, N.; Sugimoto, N.; Ohki, K.; Kamiya, M. Diversity of Phlorotannin Profiles among Sargassasacean Species Affecting Variation and Abundance of Epiphytes. Eur. J. Phycol. 2016, 51, 307–316. [Google Scholar] [CrossRef]
- Choi, J.S.; Choi, I.S. Inhibitory Effect of Marine Green Algal Extracts on Germination of Lactuca sativa Seeds. J. Environ. Biol. 2016, 37, 207–213. [Google Scholar]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A Review of Extraction Methods, Structural Characteristics, Bioactivities, Bioavailability, and Future Trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins Are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A Critical Review of Analytical Methods Used for the Chemical Characterisation and Quantification of Phlorotannin Compounds in Brown Seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the Genus Artemia in Ecotoxicity Testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, V.; Katramados, I.; Newman, J.D. Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii. Biosensors 2011, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Manfra, L.; Savorelli, F.; Pisapia, M.; Magaletti, E.; Cicero, A.M. Long-Term Lethal Toxicity Test with the Crustacean Artemia franciscana. J. Vis. Exp. 2012, 35, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A Review of Toxicity Testing Protocols and Endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna Model in the Toxicity Assessment of Pharmaceuticals: A Review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef]
- Barata, C.; Baird, D.J. Determining the Ecotoxicological Mode of Action of Toxicants from Measurements on Individuals: Results from Short Duration Chronic Tests with Daphnia magna Straus. Aquat. Toxicol. 2000, 48, 195–209. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant Enzyme Activities and Lipid Peroxidation in the Freshwater Cladoceran Daphnia magna Exposed to Redox Cycling Compounds. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2005, 140, 175–186. [Google Scholar] [CrossRef]
- Dietrich, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Single and Combined Toxicity of Pharmaceuticals at Environmentally Relevant Concentrations in Daphnia magna—A Multigenerational Study. Chemosphere 2010, 79, 60–66. [Google Scholar] [CrossRef]
- De Liguoro, M.; Maraj, S.; Merlanti, R. Transgenerational Toxicity of Flumequine over Four Generations of Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 169, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, R.; Daniel, D.; de Alkimin, G.D.; Nunes, B. Multi-Parametric Analysis of Ciprofloxacin Toxicity at Ecologically Relevant Levels: Short- and Long-Term Effects on Daphnia magna. Environ. Toxicol. Pharmacol. 2020, 74, 103295. [Google Scholar] [CrossRef] [PubMed]
- De Coen, W.M.; Janssen, C.R.; Segner, H. The Use of Biomarkers in Daphnia magna Toxicity Testing V. In Vivo Alterations in the Carbohydrate Metabolism of Daphnia magna Exposed to Sublethal Concentrations of Mercury and Lindane. Ecotoxicol. Environ. Saf. 2001, 48, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, M. Germination Inhibitors from the Brown Alga Sargassum crassifolium (Phaeophyta, Sargassaceae). Bot. Mar. 1985, 28, 501–504. [Google Scholar] [CrossRef]
- Moller, M.; Smith, M.L. The Significance of the Mineral Component of Seaweed Suspensions on Lettuce (Lactuca sativa L.) Seedling Growth. J. Plant Physiol. 1998, 153, 658–663. [Google Scholar] [CrossRef]
- Serna, M.; Hernández, F.; Coll, F.; Amorós, A. Brassinosteroid Analogues Effect on Yield and Quality Parameters of Field-Grown Lettuce (Lactuca sativa L.). Sci. Hortic. 2012, 143, 29–37. [Google Scholar] [CrossRef]
- Aragão, F.B.; Duarte, I.D.; Fantinato, D.E.; Galter, I.N.; Silveira, G.L.; dos Reis, G.B.; Andrade-Vieira, L.F.; Matsumoto, S.T. Toxicogenetic of Tebuconazole Based Fungicide through Lactuca sativa Bioassays. Ecotoxicol. Environ. Saf. 2021, 213, 111985. [Google Scholar] [CrossRef]
- Ladhari, A.; Zarrelli, A.; Di Meo, M.C.; Ghannem, M.; Ben Mimoun, M. Physiological Mechanisms and Adaptation Strategies of Lactuca sativa L. in Response to Olea europaea L. and Ficus carica L. Allelochemicals. S. Afr. J. Bot. 2022, 147, 106–118. [Google Scholar] [CrossRef]
- Ouyang, H.L.; Kong, X.Z.; He, W.; Qin, N.; He, Q.S.; Wang, Y.; Wang, R.; Xu, F.L. Effects of Five Heavy Metals at Sub-Lethal Concentrations on the Growth and Photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 2012, 57, 3363–3370. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The Effect of Cadmium on the Growth and Antioxidant Response for Freshwater Algae Chlorella vulgaris. Springerplus 2016, 5, 1290. [Google Scholar] [CrossRef]
- Dauda, S.; Chia, M.A.; Bako, S.P. Toxicity of Titanium Dioxide Nanoparticles to Chlorella vulgaris Beyerinck (Beijerinck) 1890 (Trebouxiophyceae, Chlorophyta) under Changing Nitrogen Conditions. Aquat. Toxicol. 2017, 187, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Biodegradation of Levofloxacin by an Acclimated Freshwater Microalga, Chlorella vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic Effects of Boscalid on the Growth, Photosynthesis, Antioxidant System and Metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of Carbamazepine Using Freshwater Microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the Determination of Its Metabolic Fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Kim, J.R.; Govindwar, S.P.; Jeon, B.H. Insights into Microalgae Mediated Biodegradation of Diazinon by Chlorella vulgaris: Microalgal Tolerance to Xenobiotic Pollutants and Metabolism. Algal Res. 2016, 20, 126–134. [Google Scholar] [CrossRef]
- Zhao, F.; Xiang, Q.; Zhou, Y.; Xu, X.; Qiu, X.; Yu, Y.; Ahmad, F. Evaluation of the Toxicity of Herbicide Topramezone to Chlorella vulgaris: Oxidative Stress, Cell Morphology and Photosynthetic Activity. Ecotoxicol. Environ. Saf. 2017, 143, 129–135. [Google Scholar] [CrossRef]
- Ayesha; Hira; Sultana, V.; Jehan, A.; Ehteshamul-Haque, S. In Vitro Cytotoxicity of Seaweeds from Karachi Coast on Brine Shrimp. Pak. J. Bot. 2010, 42, 3555–3560. [Google Scholar]
- Yoo, J.; Ahn, B.; Oh, J.J.; Han, T.; Kim, W.K.; Kim, S.; Jung, J. Identification of Toxicity Variations in a Stream Affected by Industrial Effluents Using Daphnia magna and Ulva pertusa. J. Hazard. Mater. 2013, 260, 1042–1049. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, Y.; von Gersdorff Jørgensen, L.; Strobel, B.W.; Hansen, H.C.B.; Cedergreen, N. Where Does the Toxicity Come from in Saponin Extract? Chemosphere 2018, 204, 243–250. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Amaral, C.; Fernandes, J.M.C.; Fraga, I.; Semitela, S.; Braga, F.; Coimbra, A.M.; Dias, A.A.; Bezerra, R.M.; Sampaio, A. Hazardous Impact of Vinasse from Distilled Winemaking By-Products in Terrestrial Plants and Aquatic Organisms. Ecotoxicol. Environ. Saf. 2019, 183, 109493. [Google Scholar] [CrossRef]
- Yu, K.X.; Wong, C.L.; Ahmad, R.; Jantan, I. Larvicidal Activity, Inhibition Effect on Development, Histopathological Alteration and Morphological Aberration Induced by Seaweed Extracts in Aedes aegypti (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2015, 8, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, A.C.S.; Cruz-Alcalde, A.; Mazón, C.S.; Martí, C.B.; Diaz-Cruz, M.S. Nano-TiO2 Phototoxicity in Fresh and Seawater: Daphnia magna and Artemia sp. as Proxies. Water 2021, 13, 55. [Google Scholar] [CrossRef]
- Fonseca, R.R.; Souza Filho, A.P.S.; Villaca̧, R.C.; Teixeira, V.L. Inhibitory Effects against Pasture Weeds in Brazilian Amazonia of Natural Products from the Marine Brown Alga Dictyota menstrualis. Nat. Prod. Commun. 2013, 8, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Baroud, S.; Tahrouch, S.; El Mehrach, K.; Sadki, I.; Fahmi, F.; Hatimi, A. Effect of Brown Algae on Germination, Growth and Biochemical Composition of Tomato Leaves (Solanum lycopersicum). J. Saudi Soc. Agric. Sci. 2021, 20, 337–343. [Google Scholar] [CrossRef]
- Thorsen, M.K.; Woodward, S.; McKenzie, B.M. Kelp (Laminaria digitata) Increases Germination and Affects Rooting and Plant Vigour in Crops and Native Plants from an Arable Grassland in the Outer Hebrides, Scotland. J. Coast. Conserv. 2010, 14, 239–247. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Ismail, M.; Hamouda, M. Influence of Some Brown Seaweed Extracts on Germination and Cytological Responses of Trigonella foenum-graecum L. BioTechnol. Indian J. 2016, 12, 104. [Google Scholar]
- Xiong, J.Q.; Kurade, M.B.; Kim, J.R.; Roh, H.S.; Jeon, B.H. Ciprofloxacin Toxicity and Its Co-Metabolic Removal by a Freshwater Microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Beratto-Ramos, A.; Castillo-Felices, R.d.P.; Troncoso-Leon, N.A.; Agurto-Muñoz, A.; Agurto-Muñoz, C. Selection Criteria for High-Value Biomass: Seasonal and Morphological Variation of Polyphenolic Content and Antioxidant Capacity in Two Brown Macroalgae. J. Appl. Phycol. 2019, 31, 653–664. [Google Scholar] [CrossRef]
- Milhem, M.M.; Al-Hiyasat, A.S.; Darmani, H. Toxicity Testing of Restorative Dental Materials Using Brine Shrimp Larvae (Artemia salina). J. Appl. Oral Sci. 2008, 16, 297–301. [Google Scholar] [CrossRef][Green Version]
- Zhang, F.; Lv, X.; Jia, H.; Huang, C.; Wei, J.; Ding, Z.; Wang, F.; Wang, J. Toxicity of the Novel Fungicide Oxathiapiprolin to Chlorella vulgaris: Assessments at Different Levels of Biological Organization. Chemosphere 2021, 291, 132752. [Google Scholar] [CrossRef]
- Yuan, N.; Pei, Y.; Bao, A.; Wang, C. The Physiological and Biochemical Responses of Daphnia magna to Dewatered Drinking Water Treatment Residue. Int. J. Environ. Res. Public Health 2020, 17, 5863. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Kim, Y.; Song, J.; Shim, T.; Cho, K.; Jung, J. Evaluation of the Combined Effect of Elevated Temperature and Cadmium Toxicity on Daphnia magna Using a Simplified DEBtox Model. Environ. Pollut. 2021, 291, 118250. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Chopra, A.K.; Durgapal, N.C.; Kumar, A. Evaluation of Daphnia magna as an Indicator of Toxicity and Treatment Efficacy of Municipal Sewage Treatment Plant. J. Appl. Sci. Environ. Manag. 2007, 11, 61–67. [Google Scholar] [CrossRef]
- Silva, A.; Figueiredo, S.A.; Sales, M.G.; Delerue-Matos, C. Ecotoxicity Tests Using the Green Algae Chlorella vulgaris-A Useful Tool in Hazardous Effluents Management. J. Hazard. Mater. 2009, 167, 179–185. [Google Scholar] [CrossRef]
- Choi, J.S.; Bae, H.J.; Kim, S.J.; Choi, I.S. In Vitro Antibacterial and Anti-Inflammatory Properties of Seaweed Extracts against Acne Inducing Bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar]
- Vinayak, R.C.; Sudha, S.A.; Chatterji, A. Bio-Screening of a Few Green Seaweeds from India for Their Cytotoxic and Antioxidant Potential. J. Sci. Food Agric. 2011, 91, 2471–2476. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A Microwell Cytotoxicity Assay Using Artemia salina (Brine Shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Fushimi, Y.; Shigemori, H. An Allelopathic Substance in Red Pine Needles (Pinus densiflora). J. Plant Physiol. 2009, 166, 442–446. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Hussain, J.; Ali, L.; Kamran, M.; Waqas, M.; Lee, I.J. Rhizonin A from Burkholderia sp. KCTC11096 and Its Growth Promoting Role in Lettuce Seed Germination. Molecules 2012, 17, 7980–7988. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis Seed Germination. A Comparative Study of Wild-Type and Gibberellin-Deficient Seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef]
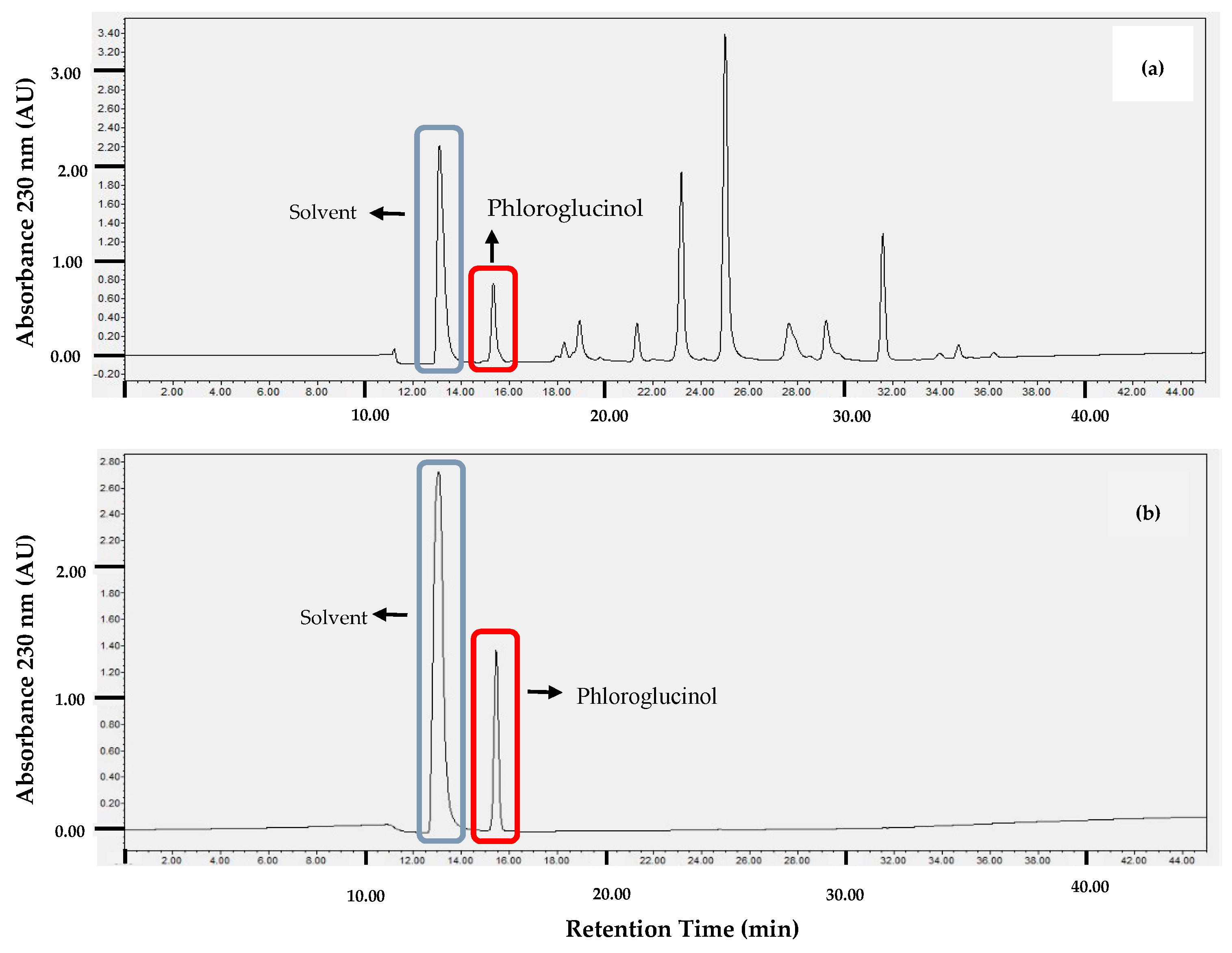

| Incubation Time (h) | Survival of Artemia salina Nauplii | ||||||
| Control | Vehicle (2.5% Methanol) | Crude Phlorotannin (mg/mL) | |||||
| 0.1 | 1.0 | 5.0 | 10.0 | 50.0 | |||
| 24 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.00 ± 0.0 | 81.3 ± 7.5 * | 0.0 ± 0.0 * |
| 48 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 98.8 ± 2.5 | 80.0 ± 10.0 * | 0.0 ± 0.0 * |
| 72 | 90.0 ± 4.1 | 88.8 ± 2.5 | 83.8 ± 6.29 | 82.5 ± 2.9 * | 85.0 ± 4.1 | 37.5 ± 6.5 * | 0.0 ± 0.0 * |
| Incubation Time (h) | Survival of Artemia salina Nauplii (%) | ||||||
| Control | Phloroglucinol (mg/mL) | ||||||
| 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | |||
| 24 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 68.3 ± 2.9 * | 1.7 ± 2.9 * | 0.0 ± 0.0 * | |
| 48 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 48.3 ± 7.6 * | 0.0 ± 0.0 * | 0.0 ± 0.0 * | |
| 72 | 100.0 ± 0.0 | 96.7 ± 2.9 | 96.7 ± 5.8 | 31.7 ± 2.9 * | 0.0 ± 0.0 * | 0.0 ± 0.0 * | |
| Incubation Time (h) | Survival of D. magna Neonates (%) | |||||
| Control | Vehicle (2.5% Methanol) | Phlorotannin Extract (mg/mL) | ||||
| 0.5 | 1.0 | 2.0 | ||||
| 24 | 95.0 ± 5.8 | 92.5 ± 5.0 | 91.3 ± 2.5 | 63.8 ± 6.3 * | 16.3 ± 4.9 * | |
| 48 | 87.5 ± 8.7 | 85.0 ± 7.1 | 38.8 ± 11.1 * | 20.0 ± 12.2 * | 0.0 ± 0.0 * | |
| 72 | 87.5 ± 8.7 | 85.0 ± 7.1 | 31.3 ± 7.5 * | 7.5 ± 6.5 * | 0.0 ± 0.0 * | |
| Incubation Time (h) | Survival of Daphnia magna Neonate (%) | |||||
| Control | Phloroglucinol (mg/mL) | |||||
| 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | ||
| 24 | 96.7 ± 2.9 | 95.0 ± 0.0 | 98.3 ± 2.9 | 68.3 ± 2.9 * | 28.3 ± 12.6 * | 0.0 ± 0.0 * |
| 48 | 93.3 ± 2.9 | 86.7 ± 2.9 * | 83.3 ± 7.6 * | 61.7 ± 5.8 * | 25.0 ± 8.7 * | 0.0 ± 0.0 * |
| 72 | 93.3 ± 2.9 | 80.0 ± 5.0 * | 73.3 ± 7.6 * | 61.7 ± 2.9 * | 16.7 ± 5.8 * | 0.0 ± 0.0 * |
| Incubation Time (h) | Germination of L. sativa Seed (%) (Radicle Length of L. sativa Seed (mm)) | |||||
| Vehicle | Crude Phlorotannin (mg/mL) | |||||
| 1.0 | 10.0 | 50.0 | ||||
| 24 | 67.5 ± 9.8 | 40.0 ± 14.1 * | 2.5 ± 5.0 * | 2.5 ± 5.0 * | ||
| (1.91 ± 0.75) | (0.93 ± 0.49) | (0.08 ± 0.15) * | (0.15 ± 0.29) * | |||
| 48 | 85.0 ± 5.8 | 57.5 ± 9.6 * | 37.5 ± 20.6 * | 17.5 ± 17.1 * | ||
| (9.04 ± 1.13) | (6.82 ± 1.32) * | (1.16 ± 0.63) * | (1.56 ± 1.86) * | |||
| 72 | 90.0 ± 8.2 | 70.0 ± 8.2 * | 57.5 ± 22.2% * | 35.0 ± 19.2 * | ||
| (17.78 ± 1.68) | (15.16 ± 2.75) | (2.06 ± 1.04) * | (4.24 ± 3.43) * | |||
| 96 | 95.0 ± 5.8 | 70.0 ± 8.2 * | 70.0 ± 11.6 * | 52.5 ± 9.6 * | ||
| (25.29 ± 2.39) | (22.33 ± 1.87) | (3.40 ± 1.98) * | (7.89 ± 4.86) * | |||
| Incubation Time (h) | Germination of L. sativa Seed (%) (Radicle Length of L. sativa Seed (mm)) | |||||
| Vehicle | Phloroglucinol(µg/mL) | |||||
| 50 | 100 | 500 | 1000 | 5000 | ||
| 24 | 46.7 ± 5.8 | 50.0 ± 24.5 | 50.0 ± 0.0 | 20.0 ± 17.3 | 0.0 ± 0.0 * | 0.0 ± 0.0 * |
| (2.05 ± 0.38) | (2.35 ± 1.07) | (2.21 ± 0.50) | (0.72 ± 0.57) * | (0.00 ± 0.00) * | (0.00 ± 0.00) * | |
| 48 | 83.3 ± 15.3 | 70.0 ± 20.0 | 73.3 ± 15.3 | 60.0 ± 0.0 | 13.3 ± 5.8 * | 0.0 ± 0.0 * |
| (10.82 ± 1.35) | (7.76 ± 2.97) | (7.24 ± 0.96) * | (4.21 ± 1.02) * | (0.39 ± 0.67) * | (0.00 ± 0.00) * | |
| 72 | 90.0 ± 10.0 | 76.7 ± 15.3 | 86.7 ± 5.8 | 63.3 ± 5.8 * | 30.0 ± 10.0 * | 0.0 ± 0.0 * |
| (19.78 ± 1.10) | (13.83 ± 5.54) | (14.92 ± 2.04) * | (8.03 ± 0.37) * | (1.92 ± 0.97) * | (0.00 ± 0.00) * | |
| 96 | 96.7 ± 5.8 | 76.7 ± 15.3 | 86.7 ± 5.8 | 80.0 ± 0.0 * | 53.3 ± 5.8 * | 0.0 ± 0.0 * |
| (29.78 ± 1.03) | (20.05 ± 7.83) | (21.89 ± 2.50) * | (11.73 ± 0.53) * | (3.53 ± 0.67) * | (0.00 ± 0.00) * | |
| Exposure Time (h) | Cell Density (CD) of Freshwater C. vulgaris (106 cells/mL) | |||||
| Control | Crude Phlorotannin (mg/mL) | |||||
| 0.5 | 1.0 | 2.0 | ||||
| 0 | 1.22 ± 0.04 | 1.36 ± 0.08 | 1.33 ± 0.05 | 1.28 ± 0.07 | ||
| 24 | 1.46 ± 0.34 | 1.37 ± 0.08 | 1.49 ± 0.23 | 1.44 ± 0.28 | ||
| 48 | 2.31 ± 0.19 | 2.11 ± 0.10 | 1.82 ± 0.28 | 1.77 ± 0.18 * | ||
| 72 | 3.41 ± 0.06 | 2.47 ± 0.15 * | 1.89 ± 0.06 * | 1.79 ± 0.11 * | ||
| 96 | 4.80 ± 0.19 | 3.26 ± 0.18 * | 2.24 ± 0.17 * | 2.02 ± 0.12 * | ||
| Exposure Time (h) | Cell Density (CD) of Freshwater C. vulgaris (106 cells/mL) | |||||
| Control | Phloroglucinol (µg/L) | |||||
| 50 | 100 | 500 | 1000 | 5000 | ||
| 0 | 1.27 ± 0.02 | 1.24 ± 0.04 | 1.26 ± 0.10 | 1.22 ± 0.08 | 1.28 ± 0.07 | 1.30 ± 0.07 |
| 24 | 1.20 ± 0.08 | 1.12 ± 0.08 | 1.17 ± 0.08 | 1.11 ± 0.11 | 1.06 ± 0.09 | 1.12 ± 0.02 |
| 48 | 1.83 ± 0.08 | 1.61 ± 0.26 | 1.14 ± 0.05 | 1.23 ± 0.09 | 1.06 ± 0.03 * | 1.03 ± 0.05 * |
| 72 | 3.80 ± 0.09 | 3.35 ± 0.12 * | 2.32 ± 0.09 * | 1.78 ± 0.08 * | 1.71 ± 0.06 * | 1.47 ± 0.12 * |
| 96 | 4.34 ± 0.17 | 3.44 ± 0.30 * | 2.34 ± 0.18 * | 1.82 ± 0.23 * | 1.74 ± 0.19 * | 1.57 ± 0.08 * |
| Exposure Time (h) | Cell Density (CD) of Seawater C. vulgaris (106 cells/mL) | |||||
| Control | Crude Phlorotannin (mg/mL) | |||||
| 0.5 | 1.0 | 2.0 | ||||
| 0 | 1.41 ± 0.07 | 1.35 ± 0.13 | 1.40 ± 0.17 | 1.25 ± 0.22 | ||
| 24 | 2.76 ± 0.05 | 2.64 ± 0.09 | 2.30 ± 0.02 * | 1.51 ± 0.06 * | ||
| 48 | 3.72 ± 0.09 | 3.31 ± 0.09 * | 1.55 ± 0.06 * | 1.31 ± 0.05 * | ||
| 72 | 3.83 ± 0.10 | 3.47 ± 0.05 * | 1.77 ± 0.04 * | 1.18 ± 0.08 * | ||
| 96 | 4.47 ± 0.06 | 4.01 ± 0.08 * | 2.32 ± 0.09 * | 1.33 ± 0.07 * | ||
| Exposure Time (h) | Cell Density (CD) of Seawater C. vulgaris (106 cells/mL) | |||||
| Control | Phloroglucinol (µg/L) | |||||
| 50 | 100 | 500 | 1000 | 5000 | ||
| 0 | 1.26 ± 0.07 | 1.29 ± 0.08 | 1.26 ± 0.02 | 1.24 ± 0.04 | 1.21 ± 0.07 | 1.19 ± 0.06 |
| 24 | 1.62 ± 0.07 | 1.67 ± 0.01 | 1.50 ± 0.03 * | 1.47 ± 0.07 * | 1.37 ± 0.03 * | 1.33 ± 0.03 * |
| 48 | 1.86 ± 0.07 | 1.69 ± 0.04 * | 1.68 ± 0.05 * | 1.35 ± 0.06 * | 1.21 ± 0.07 * | 1.14 ± 0.02 * |
| 72 | 3.44 ± 0.11 | 3.03 ± 0.07 * | 2.79 ± 0.06 * | 1.94 ± 0.07 * | 1.78 ± 0.08 * | 1.32 ± 0.04 * |
| 96 | 4.14 ± 0.16 | 3.32 ± 0.12 * | 2.97 ± 0.17 * | 2.52 ± 0.11 * | 2.42 ± 0.05 * | 1.80 ± 0.16 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harwanto, D.; Negara, B.F.S.P.; Tirtawijaya, G.; Meinita, M.D.N.; Choi, J.-S. Evaluation of Toxicity of Crude Phlorotannins and Phloroglucinol Using Different Model Organisms. Toxins 2022, 14, 312. https://doi.org/10.3390/toxins14050312
Harwanto D, Negara BFSP, Tirtawijaya G, Meinita MDN, Choi J-S. Evaluation of Toxicity of Crude Phlorotannins and Phloroglucinol Using Different Model Organisms. Toxins. 2022; 14(5):312. https://doi.org/10.3390/toxins14050312
Chicago/Turabian StyleHarwanto, Dicky, Bertoka Fajar Surya Perwira Negara, Gabriel Tirtawijaya, Maria Dyah Nur Meinita, and Jae-Suk Choi. 2022. "Evaluation of Toxicity of Crude Phlorotannins and Phloroglucinol Using Different Model Organisms" Toxins 14, no. 5: 312. https://doi.org/10.3390/toxins14050312
APA StyleHarwanto, D., Negara, B. F. S. P., Tirtawijaya, G., Meinita, M. D. N., & Choi, J.-S. (2022). Evaluation of Toxicity of Crude Phlorotannins and Phloroglucinol Using Different Model Organisms. Toxins, 14(5), 312. https://doi.org/10.3390/toxins14050312






