Intramuscular Innervation of the Supraspinatus Muscle Assessed Using Sihler’s Staining: Potential Application in Myofascial Pain Syndrome
Abstract
:1. Introduction
2. Materials and Methods
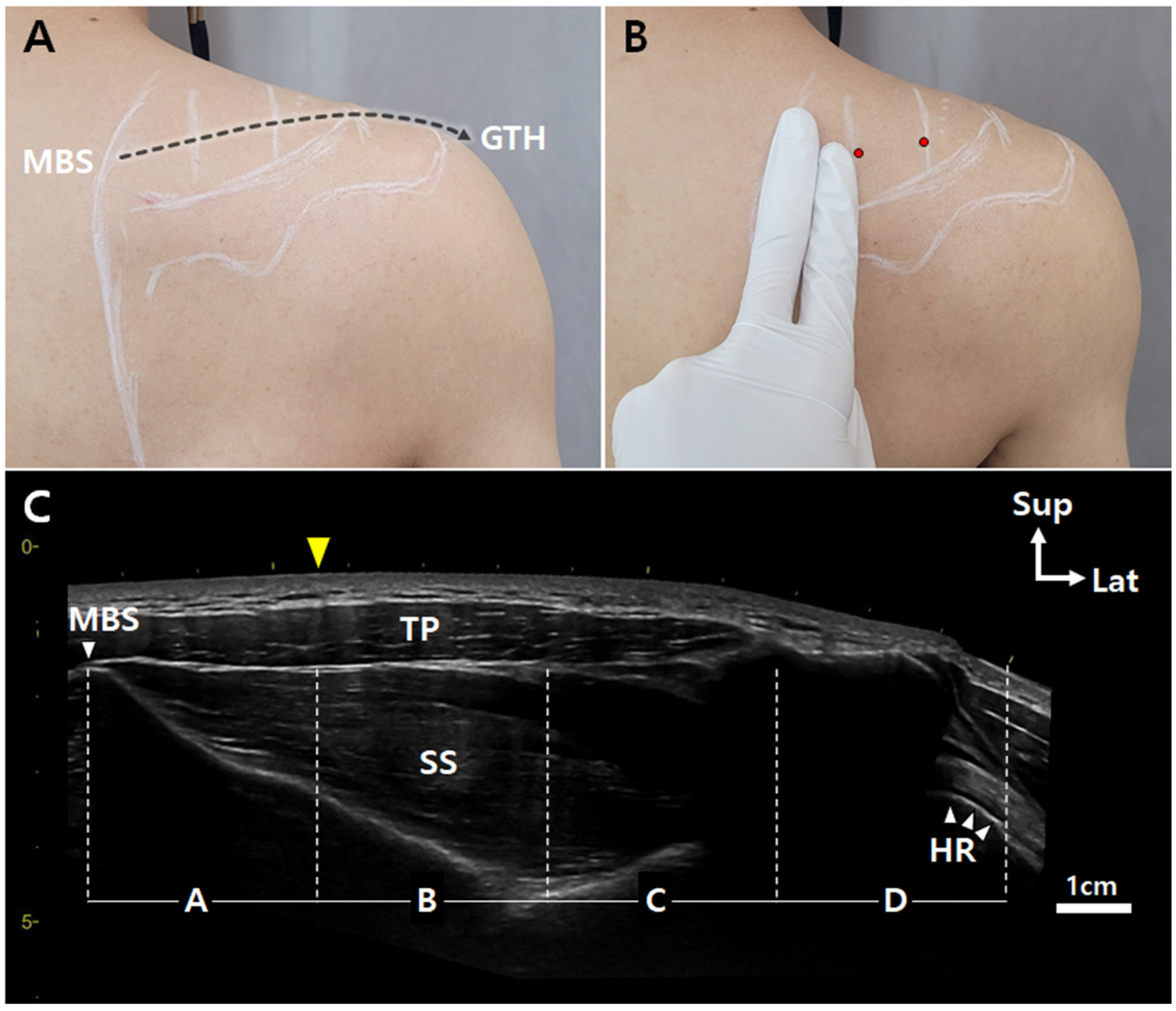
2.1. Harvesting Specimens
2.2. Modified Sihler’s Staining
- −
- Fixation solution: 10% unneutralized formalin;
- −
- Maceration solution: 3% aqueous potassium hydroxide (KOH) (add 1 mL of 3% hydrogen peroxide to every 1 L of 3% KOH solution for depigmentation);
- −
- Sihler I solution: one volume of glycerin, one volume of glacial acetic acid, and six volumes of 1% aqueous chloral hydrate;
- −
- Sihler II. solution: one volume of Ehrlich’s hematoxylin, one volume of glycerin, and six volumes of 1% aqueous chloral hydrate;
- −
- Neutralization solution: 0.05% lithium carbonate solution;
- −
- Clearing solution: 70–99% formamide.
- Fixation: The SS specimens underwent fixation for approximately a week in a 10% unneutralized formalin solution.
- Maceration: The fixed specimens were washed under running water overnight. Then, the SS specimens were macerated and depigmented for 4 weeks in a maceration solution.
- Decalcification: The macerated SS specimens were placed in Sihler I solution for 48 h for decalcification.
- Staining: Once the SS specimens were decalcified, they were stained in Sihler II solution for a week. This step stained all tissues, including nerve and muscle fibers.
- Destaining: The stained SS specimens were again placed in Sihler I solution for approximately 1–3 h. This step destained the muscle fibers, excluding the stained nerves.
- Neutralization: The destained SS specimens were neutralized under running water for 30 min. They were then placed in 0.05% lithium carbonate for 30 min.
- Clearing: As a preparatory step for clearing, the SS specimens were cleared with increasing concentrations (70%, 80%, 90%, and 99%) of formamide.
3. Results
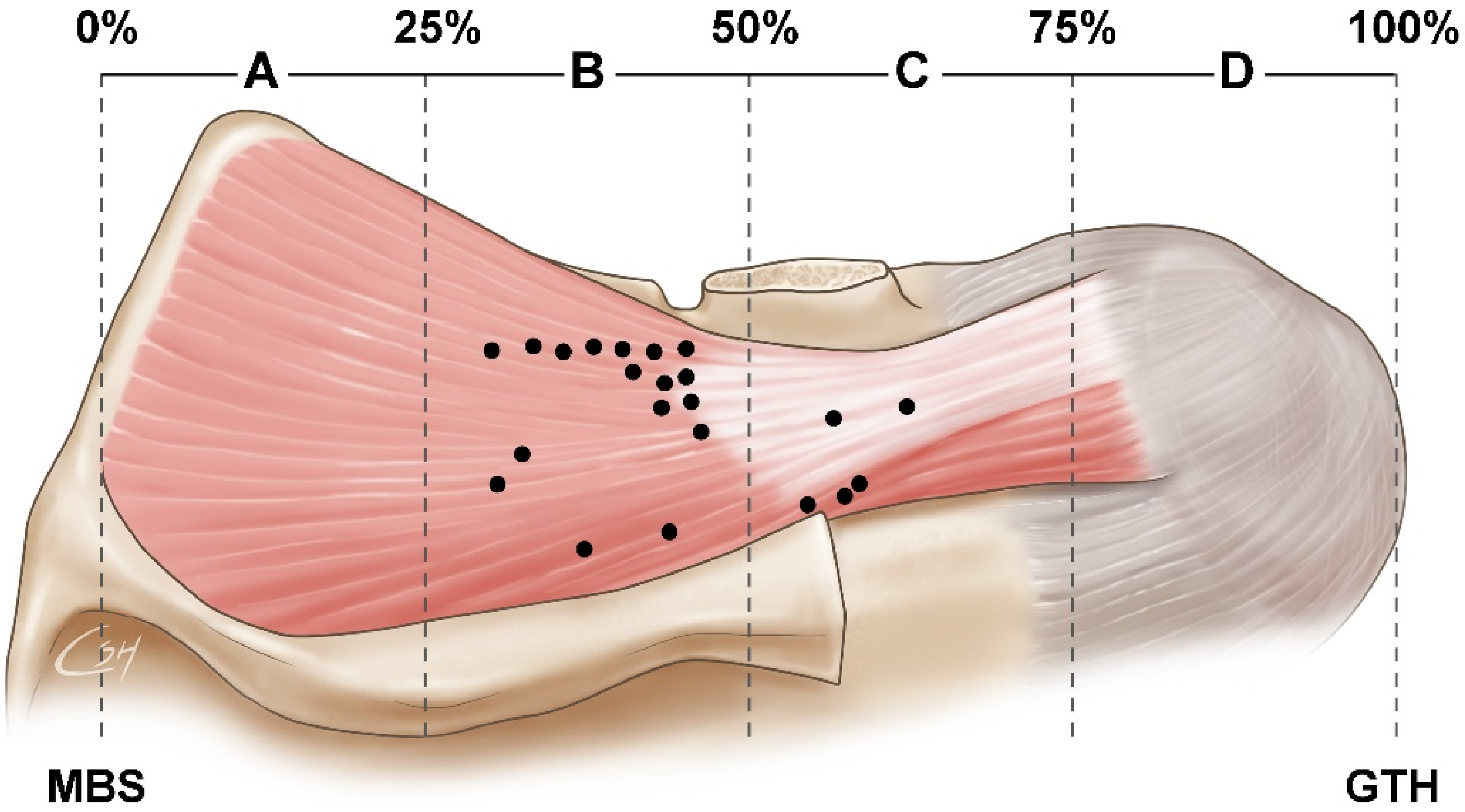
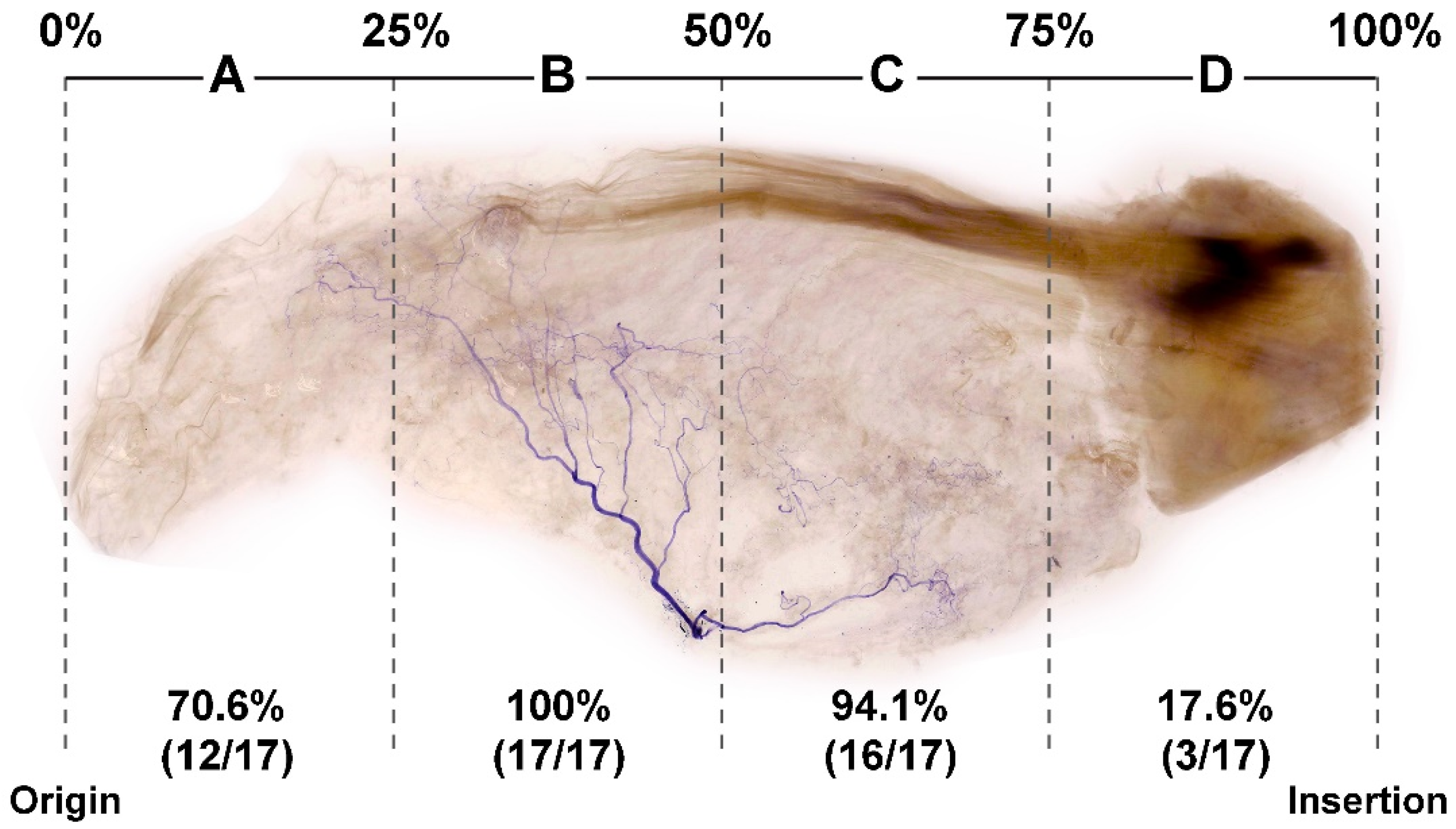
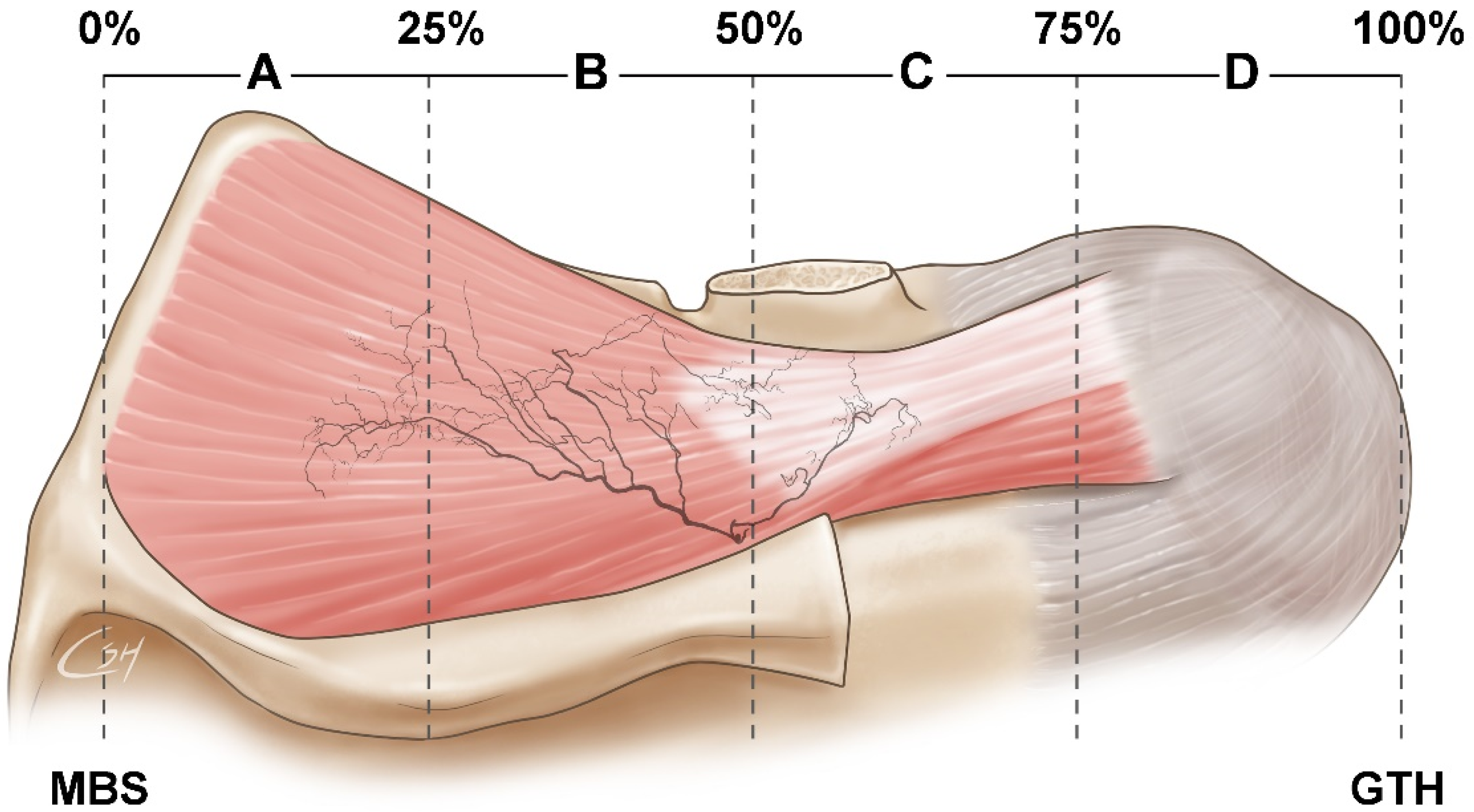
3.1. Locations of Nerve Entry Points of the SS
3.2. Concentration of Nerve Endings in the SS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTP | myofascial tender point |
| BoNT | botulinum neurotoxin |
| SS | supraspinatus muscle |
| MBS | medial border of the scapula |
| GTH | greater tubercle of the humerus |
| NMJ | neuromuscular junction |
| IMNE | intramuscular nerve ending |
| TP | trapezius |
| HR | humerus |
References
- Kalb, R.L. Evaluation and treatment of shoulder pain. Hosp. Pract. 1998, 33, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hains, G.; Descarreaux, M.; Hains, F. Chronic shoulder pain of myofascial origin: A randomized clinical trial using ischemic compression therapy. J. Manipulative Physiol. Ther. 2010, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, D.A.; Koes, B.W.; de Jong, B.A.; Bouter, L.M. Shoulder disorders in general practice: Incidence, patient characteristics, and management. Ann. Rheum. Dis. 1995, 54, 959–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, A.; Pokorna, A. Prevalence of musculoskeletal low back pain among registered nurses: Results of an online survey. J. Clin. Nurs. 2021, 30, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.; Sparer, E.H.; Tran, B.N.; Ruan, Q.Z.; Dennerlein, J.T.; Singhal, D.; Lee, B.T. Prevalence of Work-Related Musculoskeletal Disorders Among Surgeons and Interventionalists: A Systematic Review and Meta-analysis. JAMA Surg. 2018, 153, e174947. [Google Scholar] [CrossRef]
- Ben Ayed, H.; Yaich, S.; Trigui, M.; Hmida, M.B.; Jemaa, M.B.; Ammar, A.; Jedidi, J.; Karray, R.; Feki, H.; Mejdoub, Y.; et al. Prevalence, Risk Factors and Outcomes of Neck, Shoulders and Low-Back Pain in Secondary-School Children. J. Res. Health Sci. 2019, 19, e00440. [Google Scholar]
- Bron, C.; de Gast, A.; Dommerholt, J.; Stegenga, B.; Wensing, M.; Oostendorp, R.A. Treatment of myofascial trigger points in patients with chronic shoulder pain: A randomized, controlled trial. BMC Med. 2011, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Villafane, J.H.; Lopez-Royo, M.P.; Herrero, P.; Valdes, K.; Cantero-Téllez, R.; Pedersini, P.; Negrini, S. Prevalence of Myofascial Trigger Points in Poststroke Patients With Painful Shoulders: A Cross-Sectional Study. Phys. Med. Rehabil. 2019, 11, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.C.; Wu, W.T.; Han, D.S.; Chang, K.V. Comparative Effectiveness of Botulinum Toxin Injection for Chronic Shoulder Pain: A Meta-Analysis of Randomized Controlled Trials. Toxins 2020, 12, 251. [Google Scholar] [CrossRef]
- Kang, J.J.; Kim, J.; Park, S.; Kim, T.H.; Kim, D.K. Feasibility of Ultrasound-Guided Trigger Point Injection in Patients with Myofascial Pain Syndrome. Healthcare 2019, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Shin, J.C.; Kim, W.S.; Chang, W.H.; Lee, S.C. Application of ultrasound-guided trigger point injection for myofascial trigger points in the subscapularis and pectoralis muscles to post-mastectomy patients: A pilot study. Yonsei Med. J. 2014, 55, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Qin, B.; Yang, F.; Yu, T.; Yu, J.; Wang, J.; Zheng, H. Lidocaine Injection in the Intramuscular Innervation Zone Can Effectively Treat Chronic Neck Pain Caused by MTrPs in the Trapezius Muscle. Pain Physician 2015, 18, E815–E826. [Google Scholar] [PubMed]
- Cheshire, W.P.; Abashian, S.W.; Mann, D.J. Botulinum toxin in the treatment of myofascial pain syndrome. Pain 1994, 59, 65–69. [Google Scholar] [CrossRef]
- De Andres, J.; Cerda-Olmedo, G.; Valia, J.C.; Monsalve, V.; Lopez-Alarcón; Minguez, A. Use of botulinum toxin in the treatment of chronic myofascial pain. Clin. J. Pain 2003, 19, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wang, D. An update on botulinum toxin A injections of trigger points for myofascial pain. Curr. Pain Headache Rep. 2014, 18, 386. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, J.M. Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study. Toxins 2018, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Miguel, C.; Cirera, A. Retrospective study of the clinical effect of incobotulinumtoxinA for the management of myofascial pain syndrome in refractory patients. Toxicon 2021, 203, 117–120. [Google Scholar] [CrossRef]
- Cogne, M.; Creuze, A.; Petit, H.; Delleci, C.; Dehail, P.; de Seze, M. Number of botulinum toxin injections needed to stop requests for treatment for chronic lateral epicondylar tendinopathy. A 1-year follow-up study. Ann. Phys. Rehabil. Med. 2019, 62, 336–341. [Google Scholar] [CrossRef]
- Childers, M.K. Targeting the neuromuscular junction in skeletal muscles. Am. J. Phys. Med. Rehabil. 2004, 83, S38–S44. [Google Scholar] [CrossRef]
- Childers, M.K.; Brashear, A.; Jozefczyk, P.; Reding, M.; Alexander, D.; Good, D.; Walcott, J.M.; Jenkins, S.W.; Turkel, C.; Molloy, P.T. Dose-dependent response to intramuscular botulinum toxin type A for upper-limb spasticity in patients after a stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1063–1069. [Google Scholar] [CrossRef]
- Ramirez-Castaneda, J.; Jankovic, J.; Comella, C.; Dashtipour, K.; Fernandez, H.H.; Mari, Z. Diffusion, spread, and migration of botulinum toxin. Mov. Disord. 2013, 28, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.; Simons, D. Myofascial Trigger Points: Translating Molecular Theory into Manual Therapy. J. Man. Manip. Ther. 2006, 14, 232–239. [Google Scholar] [CrossRef]
- Gracies, J.M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum toxin dilution and endplate targeting in spasticity: A double-blind controlled study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16.e2. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Verhaegen, A.; Pans, S.; Molenaers, G. Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: Muscle atrophy after motor end plate-targeted injections. Res. Dev. Disabil. 2013, 34, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.H.; Lee, H.J.; Lee, J.H.; Lee, K.L.; Kim, H.J. Effective botulinum neurotoxin injection in treating iliopsoas spasticity. Clin. Anat. 2021, 34, 431–436. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Choi, Y.J.; Lee, J.H.; Hu, K.S.; Kim, H.J. Intramuscular Neural Distribution of Rhomboid Muscles: Evaluation for Botulinum Toxin Injection Using Modified Sihler’s Method. Toxins 2020, 12, 289. [Google Scholar] [CrossRef]
- Simons, D.G.; Hong, C.Z.; Simons, L.S. Endplate potentials are common to midfiber myofacial trigger points. Am. J. Phys. Med. Rehabil. 2002, 81, 212–222. [Google Scholar] [CrossRef]
- Hong, C.-Z. Myofascial Trigger Points: Pathophysiology and Correlation with Acupuncture Points. Acupunct. Med. 2000, 18, 41–47. [Google Scholar] [CrossRef]
- Donnelly, J.M. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Wolters Kluwer Health: Philadelphia, PA, USA, 2019. [Google Scholar]
- Ge, H.Y.; Nie, H.; Madeleine, P.; Danneskiold-Samsøe, B.; Graven-Nielsen, T.; Arendt-Nielsen, L. Contribution of the local and referred pain from active myofascial trigger points in fibromyalgia syndrome. Pain 2009, 147, 233–240. [Google Scholar] [CrossRef]
- Saxena, A.; Chansoria, M.; Tomar, G.; Kumar, A. Myofascial pain syndrome: An overview. J. Pain Palliat. Care Pharmacother. 2015, 29, 16–21. [Google Scholar] [CrossRef]
- Lluch, E.; Arguisuelas, M.D.; Coloma, P.S.; Palma, F.; Rey, A.; Falla, D. Effects of deep cervical flexor training on pressure pain thresholds over myofascial trigger points in patients with chronic neck pain. J. Manip. Physiol. Ther. 2013, 36, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Javanshir, K.; Ortega-Santiago, R.; Mohseni-Bandpei, M.A.; Miangolarra-Page, J.C.; Fernández-de-Las-Peñas, C. Exploration of somatosensory impairments in subjects with mechanical idiopathic neck pain: A preliminary study. J. Manip. Physiol. Ther. 2010, 33, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Parratte, B.; Tatu, L.; Vuillier, F.; Diop, M.; Monnier, G. Intramuscular distribution of nerves in the human triceps surae muscle: Anatomical bases for treatment of spastic drop foot with botulinum toxin. Surg. Radiol. Anat. 2002, 24, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Haladaj, R.; Wysiadecki, G.; Tubbs, R.S. Intramuscular innervation of the lateral rectus muscle evaluated using sihler’s staining technique: Potential application to strabismus surgery. Clin. Anat. 2020, 33, 585–591. [Google Scholar] [CrossRef]
- Mu, L.; Sanders, I. The human cricothyroid muscle: Three muscle bellies and their innervation patterns. J. Voice 2009, 23, 21–28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Lee, J.-H.; Yi, K.-H.; Kim, H.-J. Intramuscular Innervation of the Supraspinatus Muscle Assessed Using Sihler’s Staining: Potential Application in Myofascial Pain Syndrome. Toxins 2022, 14, 310. https://doi.org/10.3390/toxins14050310
Lee H-J, Lee J-H, Yi K-H, Kim H-J. Intramuscular Innervation of the Supraspinatus Muscle Assessed Using Sihler’s Staining: Potential Application in Myofascial Pain Syndrome. Toxins. 2022; 14(5):310. https://doi.org/10.3390/toxins14050310
Chicago/Turabian StyleLee, Hyung-Jin, Ji-Hyun Lee, Kyu-Ho Yi, and Hee-Jin Kim. 2022. "Intramuscular Innervation of the Supraspinatus Muscle Assessed Using Sihler’s Staining: Potential Application in Myofascial Pain Syndrome" Toxins 14, no. 5: 310. https://doi.org/10.3390/toxins14050310
APA StyleLee, H.-J., Lee, J.-H., Yi, K.-H., & Kim, H.-J. (2022). Intramuscular Innervation of the Supraspinatus Muscle Assessed Using Sihler’s Staining: Potential Application in Myofascial Pain Syndrome. Toxins, 14(5), 310. https://doi.org/10.3390/toxins14050310






