Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms
Abstract
1. Introduction
2. Results
2.1. Venom Action on Human Plasma
2.2. Venom Action on Human Fibrinogen
2.3. Fibrinogen Destruction Venom Activity
3. Discussion
4. Materials and Methods
4.1. Venom Sample Preparation
4.2. Plasma and Fibrinogen Preparation
4.3. Thromboelastography Experiments
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kini, R.M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemost. Thromb. 2005, 34, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. The intriguing world of prothrombin activators from snake venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Koh, C.Y. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Pathophysiol. Haemost. Thromb. 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon 1989, 27, 613–635. [Google Scholar] [CrossRef]
- Swenson, S.; Markland, F.S. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef]
- Bourke, L.A.; Youngman, N.J.; Zdenek, C.N.; op den Brouw, B.; Violette, A.; Fourmy, R.; Fry, B.G. Trimeresurus albolabris snakebite treatment implications arising from ontogenetic venom comparisons of anticoagulant function, and antivenom efficacy. Toxicol. Lett. 2020, 327, 2–8. [Google Scholar] [CrossRef]
- Alencar, L.; Martins, M.; Greene, H. Evolutionary history of vipers. eLS 2018, 1–10. [Google Scholar] [CrossRef]
- Wüster, W.; da Graca Salomão, M.; Quijada-Mascareñas, J.A.; Thorpe, R.S.; BBBSP. Origins and evolution of the South American pitviper fauna: Evidence from mitochondrial DNA sequence analysis. In Biology of the Vipers; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 111–128. [Google Scholar]
- Fiorillo, B.F.; Tozetti, A.M.; Martins, M. Habitat use by five species of sympatric pitvipers (Bothrops, Crotalus) in a Brazilian savannah. Herpetol. Notes 2020, 13, 951–960. [Google Scholar]
- Martins, M.; Araujo, M.S.; Sawaya, R.J.; Nunes, R. Diversity and evolution of macrohabitat use, body size and morphology in a monophyletic group of neotropical pitvipers (Bothrops). J. Zool. 2001, 254, 529–538. [Google Scholar] [CrossRef]
- Martins, M.; Marques, O.A.; Sazima, I. Ecological and phylogenetic correlates of feeding habits in neotropical pitvipers of the genus Bothrops. In Biology of the Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., HW, G., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 307–328. [Google Scholar]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Gutiérrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American lancehead pit-vipers: Coagulotoxic venom effects and antivenom neutralisation of Bothrops asper and B. atrox geographical variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Holding, M.L.; Del-Rei, T.H.M.; Rocha, M.M.T.; Mourão, R.H.V.; Chalkidis, H.M.; Prezoto, B.; Gibbs, H.L.; Moura-da-Silva, A.M. Individual variability in Bothrops atrox snakes collected from different habitats in the Brazilian Amazon: New findings on venom composition and functionality. Toxins 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Segura, Á.; Herrera, M.; Villalta, M.; Vargas, M.; Uscanga-Reynell, A.; de León-Rosales, S.P.; Jiménez-Corona, M.E.; Reta-Mares, J.F.; Gutiérrez, J.M.; León, G. Venom of Bothrops asper from Mexico and Costa Rica: Intraspecific variation and cross-neutralization by antivenoms. Toxicon 2012, 59, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: From obvious facts to contingencies. J. Venom Anim. Toxins Incl. Trop. Dis. 2015, 21, 1–17. [Google Scholar] [CrossRef]
- da Costa, M.K.B.; da Fonseca, C.S.; Navoni, J.A.; Freire, E.M.X. Snakebite accidents in Rio Grande do Norte state, Brazil: Epidemiology, health management and influence of the environmental scenario. Trop. Med. Int. Health 2019, 24, 432–441. [Google Scholar] [CrossRef]
- Dolab, J.A.; de Roodt, A.R.; de Titto, E.H.; García, S.I.; Funes, R.; Salomón, O.D.; Chippaux, J.-P. Epidemiology of snakebite and use of antivenom in Argentina. Trans R Soc. Trop. Med. Hyg. 2014, 108, 269–276. [Google Scholar] [CrossRef]
- Mota-da-Silva, A.; Colombini, M.; Moura-da-Silva, A.M.; Souza, R.M.; Monteiro, W.M.; Bernarde, P.S. Epidemiological and clinical aspects of snakebites in the upper Juruá River region, western Brazilian Amazonia. Acta Amazonica 2019, 50. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Contreras-Bernal, J.C.; Bisneto, P.F.; Sachett, J.; Mendonça da Silva, I.; Lacerda, M.; Guimarães da Costa, A.; Val, F.; Brasileiro, L.; Sartim, M.A.; et al. Bothrops atrox, the most important snake involved in human envenomings in the amazon: How venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Otero-Patiño, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.L.; Sano-Martins, I.S.; Fan, H.W.; Cardoso, J.L.C.; Theakston, R.D.G.; Warrell, D.A. Haematological evaluation of patients bitten by the jararaca, Bothrops jararaca, in Brazil. Toxicon 2008, 51, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Milani Júnior, R.; Jorge, M.T.; de Campos, F.P.; Martins, F.P.; Bousso, A.; Cardoso, J.L.; Ribeiro, L.A.; Fan, H.W.; França, F.O.; Sano-Martins, I.S.; et al. Snake bites by the jararacuçu (Bothrops jararacussu): Clinicopathological studies of 29 proven cases in São Paulo State, Brazil. QJM 1997, 90, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Maguiña Vargas, C.; Henríquez, C.; Ilquimiche, L.; Mostorino, R.; Gotuzzo Herencia, E.; Legua, P.; Echevarría Zárate, J.; Seas Ramos, C. Ofidismo por Bothrops pictus en el Hospital Nacional Cayetano Heredia: Estudio prospectivo de 23 casos. Folia Dermatol. Peru 1998, 9, 41–48. [Google Scholar]
- Resiere, D.; Mégarbane, B.; Valentino, R.; Mehdaoui, H.; Thomas, L. Bothrops lanceolatus bites: Guidelines for severity assessment and emergent management. Toxins 2010, 2, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Chausson, N.; Uzan, J.; Kaidomar, S.; Vignes, R.; Plumelle, Y.; Bucher, B.; Smadja, D. Thrombotic stroke following snake bites by the “Fer-de-Lance” Bothrops lanceolatus in Martinique despite antivenom treatment: A report of three recent cases. Toxicon 2006, 48, 23–28. [Google Scholar] [CrossRef]
- Thomas, L.; Tyburn, B.; Bucher, B.; Pecout, F.; Ketterle, J.; Rieux, D.; Smadja, D.; Garnier, D.; Plumelle, Y. Prevention of thromboses in human patients with Bothrops Lanceolatus envenoming in Martinique: Failure of anticoagulants and efficacy of a monospecific antivenom. Am. J. Trop. Med. Hyg. 1995, 52, 419–426. [Google Scholar] [CrossRef]
- Numeric, P.; Moravie, V.; Didier, M.; Chatot-Henry, D.; Cirille, S.; Bucher, B.; Thomas, L. Multiple cerebral infarctions following a snakebite by Bothrops caribbaeus. Am. J. Trop. Med. Hyg. 2002, 67, 287–288. [Google Scholar] [CrossRef]
- Smalligan, R.; Cole, J.; Brito, N.; Laing, G.D.; Mertz, B.L.; Manock, S.; Maudlin, J.; Quist, B.; Holland, G.; Nelson, S.; et al. Crotaline snake bite in the Ecuadorian Amazon: Randomised double blind comparative trial of three South American polyspecific antivenoms. BMJ 2004, 329, 1129. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Current challenges for confronting the public health problem of snakebite envenoming in Central America. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Neri-Castro, E.; Bénard-Valle, M.; de León, J.L.; Boyer, L.; Alagón, A. Envenomations by reptiles in Mexico. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2021; pp. 529–542. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.B.; Zdenek, C.N.; Bourke, L.A.; Seneci, L.; Chowdhury, A.; Freitas-de-Sousa, L.A.; de Alcantara Menezes, F.; Moura-da-Silva, A.M.; Tanaka-Azevedo, A.M.; Fry, B.G. Clinical implications of ontogenetic differences in the coagulotoxic activity of Bothrops jararacussu venoms. Toxicol. Lett. 2021, 348, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Frank, N.; Afshar, S. De novo assessment and review of Pan-American pit viper anticoagulant and procoagulant venom activities via kinetomic analyses. Toxins 2019, 11, 94. [Google Scholar] [CrossRef]
- Hofmann, H.; Bon, C. Blood coagulation induced by the venom of Bothrops atrox. 1. Identification, purification, and properties of a prothrombin activator. Biochemistry 1987, 26, 772–780. [Google Scholar] [CrossRef]
- Hofmann, H.; Bon, C. Blood coagulation induced by the venom of Bothrops atrox. 2. Identification, purification, and properties of two factor X activators. Biochemistry 1987, 26, 780–787. [Google Scholar] [CrossRef]
- Hofmann, H.; Dumarey, C.; Bon, C. Blood coagulation induced by Bothrops atrox venom: Idendification and properties of a factor X activator. Biochimie 1983, 65, 201–210. [Google Scholar] [CrossRef]
- Loría, G.D.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.G.; Fox, J.W.; Alape, A.; Gutiérrez, J.M.A. Characterization of ‘basparin A,’ a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 2003, 418, 13–24. [Google Scholar] [CrossRef]
- Sousa, L.F.; Bernardoni, J.L.; Zdenek, C.N.; Dobson, J.; Coimbra, F.; Gillett, A.; Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Fry, B.G. Differential coagulotoxicity of metalloprotease isoforms from Bothrops neuwiedi snake venom and consequent variations in antivenom efficacy. Toxicol. Lett. 2020, 333, 211–221. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.C.P.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.D.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (lancehead pit-vipers) venoms from Brazil: Differential biochemistry and antivenom efficacy resulting from prey-driven venom variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J. On some effects upon the blood produced by the injection of the venom of the Australian black snake (Pseudechis Porphyriacus). J. Physiol. 1893, 15, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Nahas, L.; Kamiguti, A.S.; Barros, M.A.R. Thrombin-like and factor X-activator components of Bothrops snake venoms. Thromb. Haemost. 1979, 41, 314–328. [Google Scholar] [CrossRef]
- Sant’Ana, C.D.; Ticli, F.K.; Oliveira, L.L.; Giglio, J.R.; Rechia, C.G.V.; Fuly, A.L.; Selistre de Araújo, H.S.; Franco, J.J.; Stabeli, R.G.; Soares, A.M.; et al. BjussuSP-I: A new thrombin-like enzyme isolated from Bothrops jararacussu snake venom. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 443–454. [Google Scholar] [CrossRef]
- Magalhães, A.; Magalhães, H.P.B.; Richardson, M.; Gontijo, S.; Ferreira, R.N.; Almeida, A.P.; Sanchez, E.F. Purification and properties of a coagulant thrombin-like enzyme from the venom of Bothrops leucurus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.S.; Rodrigues, R.S.; Izidoro, L.F.M.; Menaldo, D.L.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Fuly, A.L.; Soares, S.G.; Selistre-de-Araújo, H.S.; Barraviera, B.; et al. Biochemical and functional properties of a thrombin-like enzyme isolated from Bothrops pauloensis snake venom. Toxicon 2009, 54, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.V.; Rucavado, A.; Sanz, L.; Calvete, J.J.; Gutiérrez, J.M. Isolation and characterization of a serine proteinase with thrombin-like activity from the venom of the snake Bothrops Asper. Braz. J. Med. Biol. Res. 2008, 41, 12–17. [Google Scholar] [CrossRef][Green Version]
- Petretski, J.; Kanashiro, M.; Silva, C.; Alves, E.; Kipnis, T. Two related thrombin-like enzymes present in Bothrops atrox venom. Braz. J. Med. Biol. Res. 2000, 33, 1293–1300. [Google Scholar] [CrossRef]
- Wellmann, I.A.M.; Ibiapina, H.N.S.; Sachett, J.A.G.; Sartim, M.A.; Silva, I.M.; Oliveira, S.S.; Tarragô, A.M.; Moura-da-Silva, A.M.; Lacerda, M.V.G.; Ferreira, L.C.d.L.; et al. Correlating fibrinogen consumption and profiles of inflammatory molecules in human envenomation’s by Bothrops atrox in the Brazilian Amazon. Front. Immunol. 2020, 11, 1874. [Google Scholar] [CrossRef]
- Larréché, S.; Chippaux, J.-P.; Chevillard, L.; Mathé, S.; Résière, D.; Siguret, V.; Mégarbane, B. Bleeding and thrombosis: Insights into pathophysiology of Bothrops venom-related hemostasis disorders. Int. J. Mol. Sci. 2021, 22, 9643. [Google Scholar] [CrossRef]
- Seneci, L.; Zdenek, C.N.; Chowdhury, A.; Rodrigues, C.F.B.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Fry, B.G. A clot twist: Extreme variation in coagulotoxicity mechanisms in Mexican neotropical rattlesnake venoms. Front. Immunol. 2021, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Seneci, L.; Zdenek, C.N.; Bourke, L.A.; Cochran, C.; Sánchez, E.E.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Frank, N.; Fry, B.G. A symphony of destruction: Dynamic differential fibrinogenolytic toxicity by rattlesnake (Crotalus and Sistrurus) venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 245, 109034. [Google Scholar] [CrossRef]
- Youngman, N.J.; Peng, Y.-H.; Harris, R.J.; Jones, L.; Llinas, J.; Haworth, M.; Gillett, A.; Fry, B.G. Differential coagulotoxic and neurotoxic venom activity from species of the arboreal viperid snake genus Bothriechis (palm-pitvipers). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 256, 109326. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Sousa E Silva, M.C.C.; Morena, P.; Nahas, L. The anticoagulant effect of Bothrops castelnaudi snake venom (Castelnaud’s pit viper). Toxicon 1985, 23, 383–391. [Google Scholar] [CrossRef]
- Lotto, N.P.; de Albuquerque Modesto, J.C.; Sant’Anna, S.S.; Grego, K.F.; Guarnieri, M.C.; Lira-da-Silva, R.M.; Santoro, M.L.; Oguiura, N. The absence of thrombin-like activity in Bothrops erythromelas venom is due to the deletion of the snake venom thrombin-like enzyme gene. PLoS ONE 2021, 16, e0248901. [Google Scholar] [CrossRef]
- Kaaber, A.B.; Jans, Ø.; Dziegiel, M.H.; Stensballe, J.; Johansson, P.I. Managing patients on direct factor Xa inhibitors with rapid thrombelastography. Scand. J. Clin. Lab. Investig. 2021, 81, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, L.T.; Nascimento, B.; Shankarakutty, A.K.; Rizoli, S.; Adhikari, N.K.J. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit. Care 2014, 18, 518. [Google Scholar] [CrossRef]
- Leeper, C.M.; Gaines, B.A. Viscoelastic hemostatic assays in the management of the pediatric trauma patient. Semin. Pediatric Surg. 2017, 26, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, A.; Karczmarska-Wódzka, A.; Sikora, J.; Sobczak, P.; Lemanowicz, A.; Filipska, K.; Ślusarz, R. Hypercoagulability as measured by thrombelastography may be associated with the size of acute ischemic infarct—A pilot study. Diagnostics 2021, 11, 712. [Google Scholar] [CrossRef]
- Drevets, P.D.; Tien, L.; LaCoursiere, R.J.; Burgbacher, T.E.; Fox, E.D. Rattlesnake Envenomation in a Venom-Naive Man with Significant Coagulopathy and Severe Oropharyngeal Edema Requiring Emergent Surgical Airway. J. Curr. Surg. 2021, 11, 15–20. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Chowdhury, A.; Haw, G.Y.H.; Violette, A.; Fourmy, R.; Christ, T.; Vonk, F.J.; Fry, B.G. Taxon-selective venom variation in adult and neonate Daboia russelii (Russell’s Viper), and antivenom efficacy. Toxicon 2022, 205, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Wagner, M.T.; Frank, N. Mechanisms responsible for the anticoagulant properties of neurotoxic Dendroaspis venoms: A viscoelastic analysis. Int. J. Mol. Sci. 2020, 21, 2082. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake venomics of the Lesser Antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus: Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-Bernat, S.; Pérez, A.; De Morais-Zani, K.; Sant’Anna, S.S.; Hatakeyama, D.M.; Tasima, L.J.; De Souza, M.B.; Kayano, A.M.; Zavaleta, A.; et al. Danger in the canopy. Comparative proteomics and bioactivities of the venoms of the South American palm pit viper Bothrops bilineatus subspecies bilineatus and smaragdinus and Antivenomics of B. b. bilineatus (Rondônia) venom against the Brazilian pentabothropic antivenom. J. Proteome Res. 2020, 19, 3518–3532. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.R.; Teixeira-Ferreira, A.; Vargas, F.F.R.; Guerra-Duarte, C.; Costal-Oliveira, F.; Stransky, S.; Lopes-de-Souza, L.; Dutra, A.A.A.; Yarlequé, A.; Bonilla, C.; et al. Proteomic profile, biological activities and antigenic analysis of the venom from Bothriopsis bilineata smaragdina (“loro machaco”), a pitviper snake from Peru. J. Proteom. 2018, 187, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kuch, U.; Mebs, D.; Gutiérrez, J.M.; Freire, A. Biochemical and biological characterization of Ecuadorian pitviper venoms (genera Bothriechis, Bothriopsis, Bothrops and Lachesis). Toxicon 1996, 34, 714–717. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Freitas, T.V.; Ferreira-Alves, D.L.; Velarde, D.T.; Diniz, M.R.; Cordeiro, M.N.; Agostini-Cotta, G.; Diniz, C.R. Biological activities of venoms from South American snakes. Toxicon 1992, 30, 95–103. [Google Scholar] [CrossRef]
- Alencar, L.R.V.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 2016, 105, 50–62. [Google Scholar] [CrossRef]
- Carrasco, P.A.; Mattoni, C.I.; Leynaud, G.C.; Scrocchi, G.J. Morphology, phylogeny and taxonomy of South American bothropoid pitvipers (Serpentes, Viperidae). Zool. Scr. 2012, 41, 109–124. [Google Scholar] [CrossRef]
- Fenwick, A.M.; Gutberlet, R.L.; Evans, J.A.; Parkinson, C.L. Morphological and molecular evidence for phylogeny and classification of South American pitvipers, genera Bothrops, Bothriopsis, and Bothrocophias (Serpentes: Viperidae). Zool. J. Linn. Soc. 2009, 156, 617–640. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal viperid snake genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Coimbra, F.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Basal but divergent: Clinical implications of differential coagulotoxicity in a clade of Asian vipers. Toxicol. In Vitro 2019, 58, 195–206. [Google Scholar] [CrossRef]
- Clauss, A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957, 17, 237–246. [Google Scholar] [CrossRef]
- Bogarín, G.; Romero, M.; Rojas, G.; Lutsch, C.; Casadamont, M.; Lang, J.; Otero, R.; Gutiérrez, J.M.A. Neutralization, by a monospecific Bothrops lanceolatus antivenom, of toxic activities induced by homologous and heterologous Bothírops snake venoms. Toxicon 1999, 37, 551–557. [Google Scholar] [CrossRef]
- Theakston, R.D.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull。 World Health Organ. 1983, 61, 949–956. [Google Scholar]
- Lister, C.; Arbuckle, K.; Jackson, T.N.W.; Debono, J.; Zdenek, C.N.; Dashevsky, D.; Dunstan, N.; Allen, L.; Hay, C.; Bush, B.; et al. Catch a tiger snake by its tail: Differential toxicity, co-factor dependence and antivenom efficacy in a procoagulant clade of Australian venomous snakes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 202, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, B.; Guedes, T.B.; Carrasco, P.A.; Melville, J. A complex biogeographic history of diversification in Neotropical lancehead pitvipers (Serpentes, Viperidae). Zool. Scr. 2020, 49, 145–158. [Google Scholar] [CrossRef]
- Youngman, N.J.; Carlsson, D.J.; Jones, L.; Neri-Castro, E.; Alagón, A.; Fry, B.G. Cloud serpent coagulotoxicity: The biochemical mechanisms underpinning the anticoagulant actions of Mixcoatlus and Ophryacus venoms. Toxicon 2022, 211, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Valenzuela, D.; Mora-Obando, D.; Fernández, M.L.; Loaiza-Lange, A.; Gibbs, H.L.; Lomonte, B. Proteomic and toxicological profiling of the venom of Bothrocophias campbelli, a pitviper species from Ecuador and Colombia. Toxicon 2014, 90, 15–25. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Preciado, L.M.; Fernández, J.; Camacho, E.; Lomonte, B.; Castro, F.; Cañas, C.A.; Galvis, C.; Castaño, S. Snake venomics, experimental toxic activities and clinical characteristics of human envenomation by Bothrocophias myersi (Serpentes: Viperidae) from Colombia. J. Proteom. 2020, 220, 103758. [Google Scholar] [CrossRef]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Gonzalez-Kozlova, E.; Zarria-Romero, J.; Lazo, F.; Rodríguez, E.; Magalhães, H.P.B.; Chávez-Olortegui, C.; Oliveira, L.S.; Alvarenga, V.G.; et al. Fibrinogen-clotting enzyme, pictobin, from Bothrops pictus snake venom. Structural and functional characterization. Int. J. Biol. Macromol. 2020, 153, 779–795. [Google Scholar] [CrossRef]
- Romero-Vargas, F.; Rocha, T.; Cruz-Höfling, M.; Rodrigues-Simioni, L.; Ponce-Soto, L. Biochemical characterization of a PLA2 Btae TX-I isolated from Bothriopsis taeniata snake venom: A pharmacological and morphological study. J. Clin. Toxicol. 2014, 4. [Google Scholar] [CrossRef]
- Floriano, R.S.; Carregari, V.C.; de Abreu, V.A.; Kenzo-Kagawa, B.; Ponce-Soto, L.A.; da Cruz-Höfling, M.A.; Hyslop, S.; Marangoni, S.; Rodrigues-Simioni, L. Pharmacological study of a new Asp49 phospholipase A2 (Bbil-TX) isolated from Bothriopsis bilineata smargadina (forest viper) venom in vertebrate neuromuscular preparations. Toxicon 2013, 69, 191–199. [Google Scholar] [CrossRef]
- Rodrigues-Simioni, L.; Floriano, R.S.; Rostelato-Ferreira, S.; Sousa, N.C.; Marangoni, S.; Ponce-Soto, L.A.; Carregari, V.C.; Hyslop, S. Presynaptic action of Bothriopsis bilineata smargadina (forest viper) venom in vitro. Toxicon 2011, 58, 140–145. [Google Scholar] [CrossRef]
- Jackson, T.N.W.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P.; et al. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef]
- da Fonseca, W.L.; de Souza Oliveira, A.; Correa, R.R.; Bernarde, P.S. Caudal luring in the Neotropical two-striped forest pitviper Bothrops bilineatus smaragdinus Hoge, 1966 in the western Amazon. Herpetol. Notes 2019, 12, 365–374. [Google Scholar]
- Venegas, P.J.; Chávez Arribasplata, J.C.; Almora, E.; Grilli, P.G.; Duran, V. New observations on diet of the South American two-striped forest-pitviper Bothrops bilineatus smaragdinus (Hoge, 1966). Cuad Herpetol. 2019. [Google Scholar] [CrossRef]
- Silva de Oliveira, S.; Campos Alves, E.; dos Santos Santos, A.; Freitas Nascimento, E.; Tavares Pereira, J.P.; Mendonça da Silva, I.; Sachett, J.; dos Santos Ibiapina, H.N.; Santos Sarraf, L.K.; Contreras Bernal, J.C.; et al. Bothrops snakebites in the Amazon: Recovery from hemostatic disorders after Brazilian antivenom therapy. Clin. Toxicol. 2020, 58, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Silva de Oliveira, S.; C Alves, E.; S Santos, A.; F Nascimento, E.; T Pereira, J.P.; M Silva, I.; A G Sachett, J.; S Sarraff, L.K.; Freitas-de-Sousa, L.A.; Colombini, M.; et al. Bleeding disorders in Bothrops atrox envenomations in the Brazilian Amazon: Participation of hemostatic factors and the impact of tissue factor. Toxins 2020, 12, 554. [Google Scholar] [CrossRef]
- Silva, A.; Hodgson, W.C.; Tasoulis, T.; Isbister, G.K. Rodent lethality models are problematic for evaluating antivenoms for human envenoming. Front. Pharmacol. 2022, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Bos, M.H.A.; Nouwens, A.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Habu coagulotoxicity: Clinical implications of the functional diversification of Protobothrops snake venoms upon blood clotting factors. Toxicol. In Vitro 2019, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
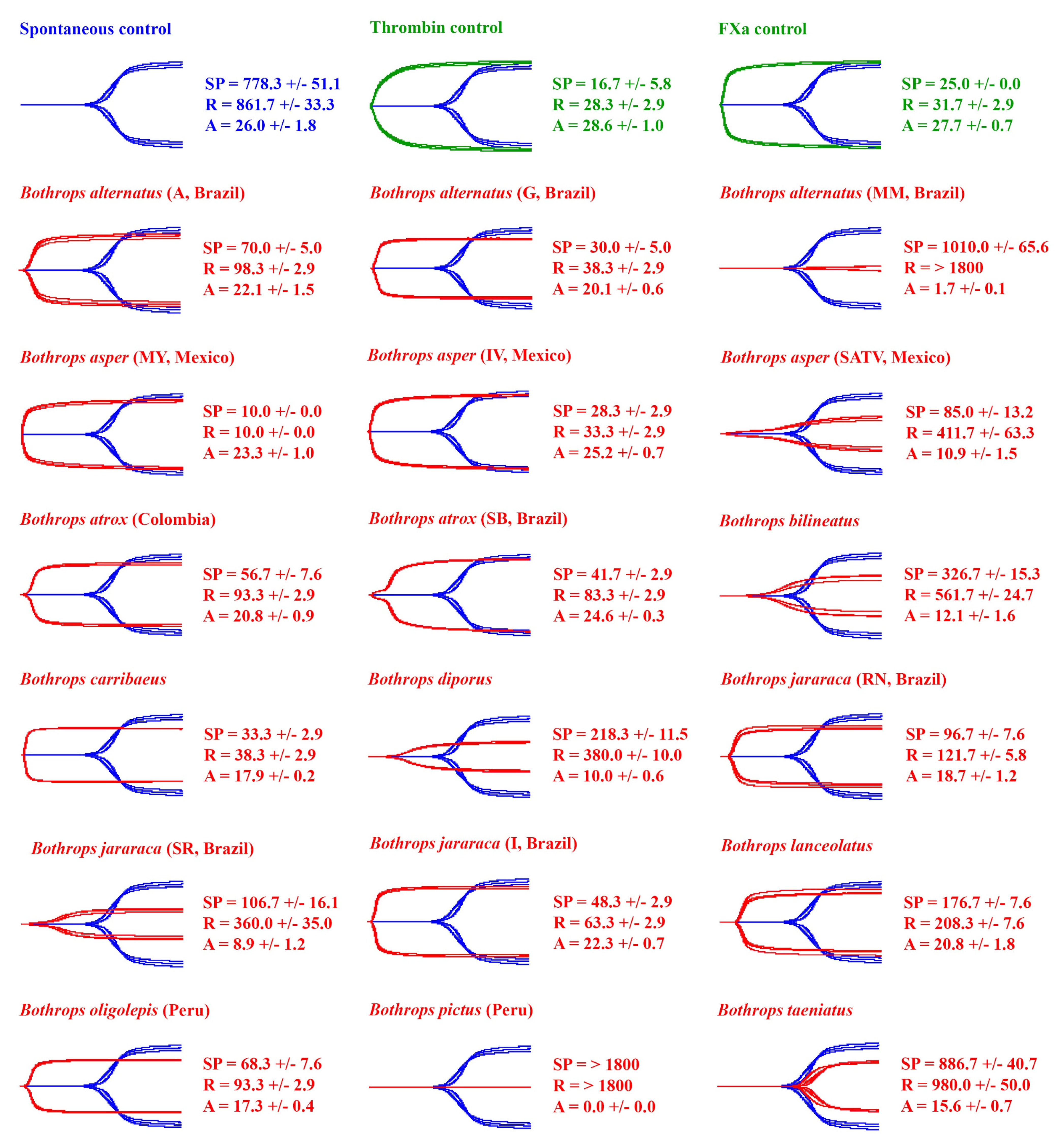

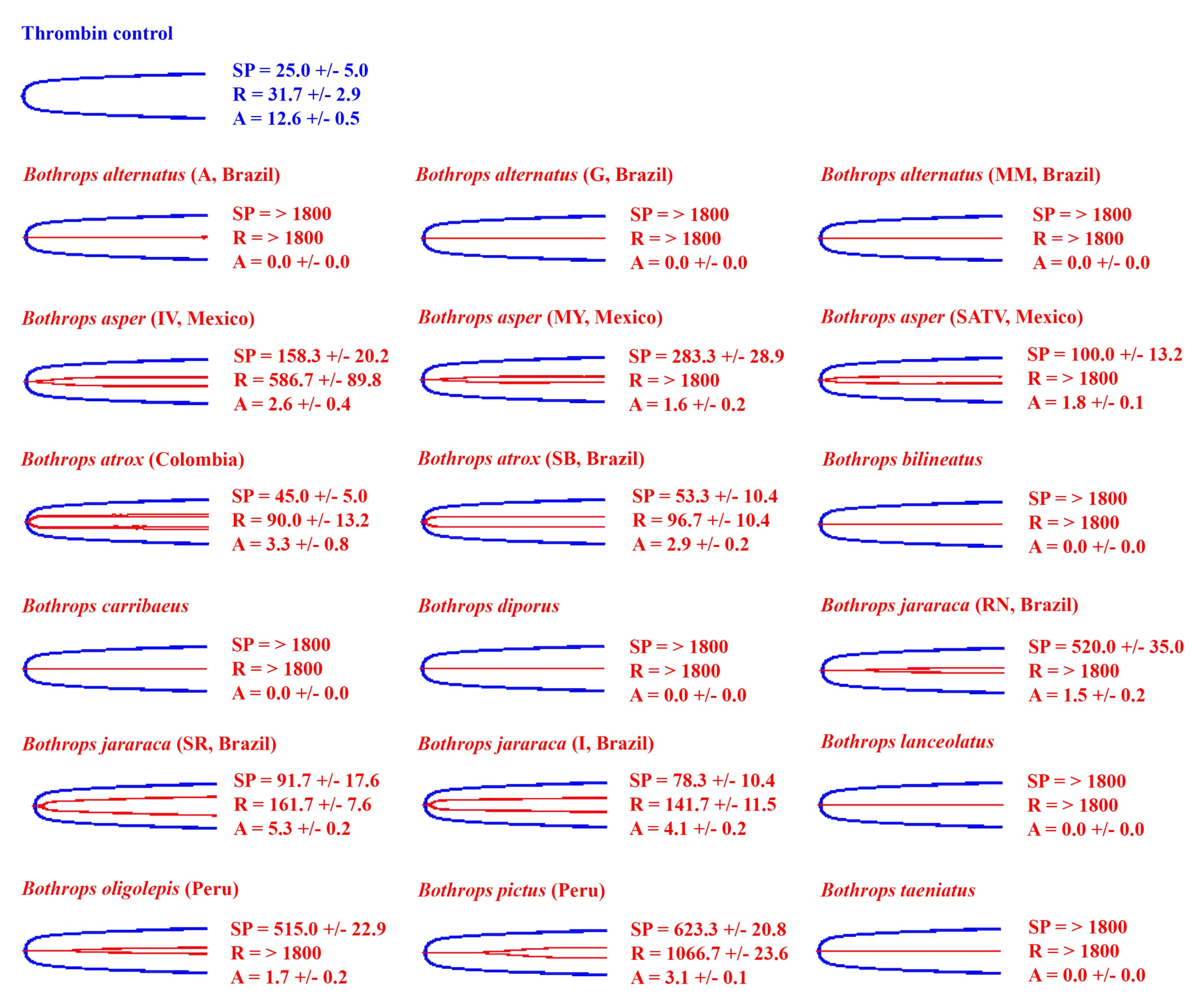

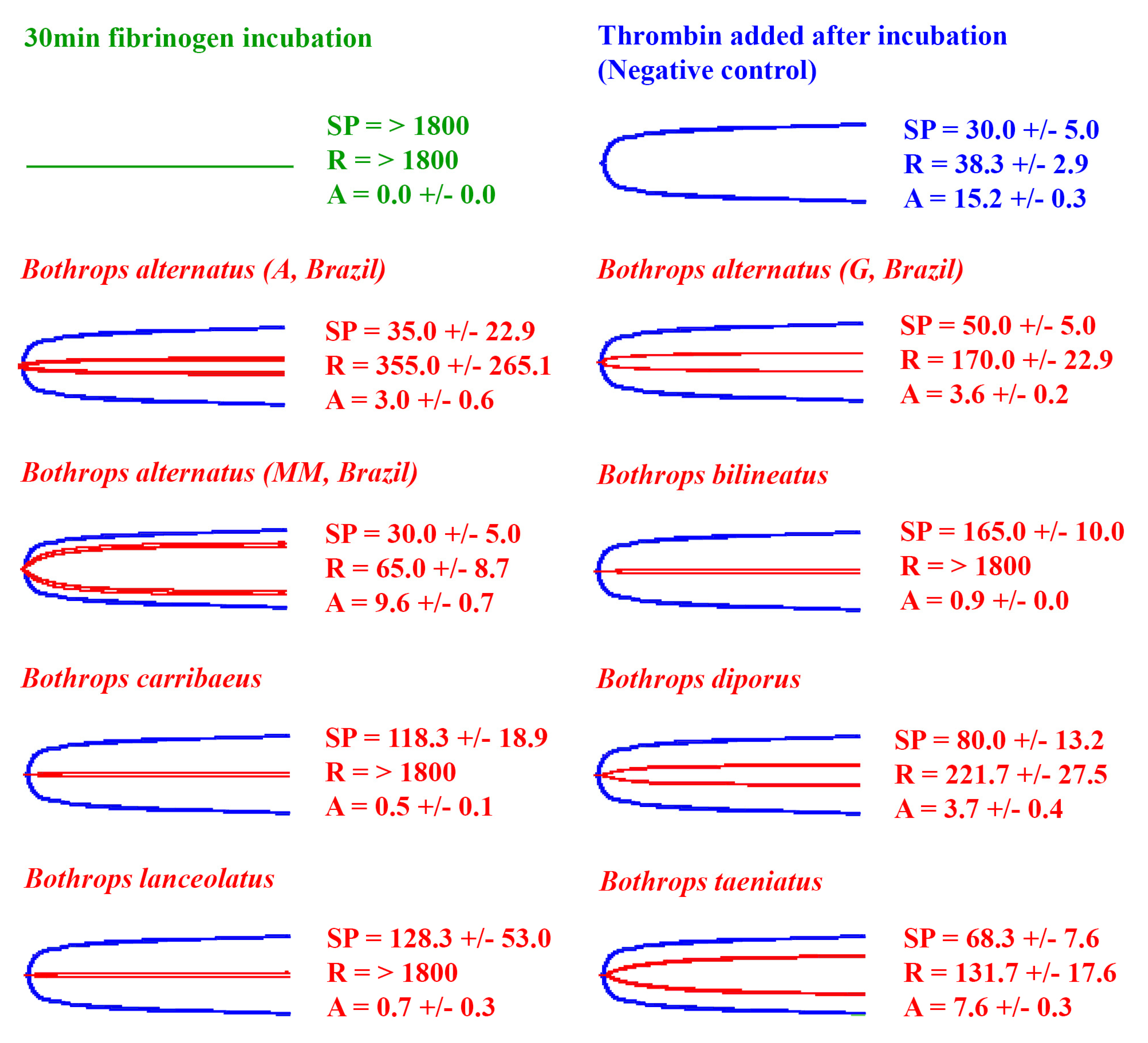
| Species | Pooled (n) or Individual | Locality | Locality Abbreviation in Figures (If Used) | Sex | Approx Age (yrs) | Source, and Wild-Caught or Captive Bred Stock |
|---|---|---|---|---|---|---|
| B. alternatus | Individual | Mogi Mirim–SP, Brazil | MM, Brazil | F | 9 | Instituto Butantan, wild-caught in 2009 |
| B. alternatus | Individual | Guararena–SP, Brazil | G, Brazil | F | 7 | Instituto Butantan, captive-born in 2011, 17a specimen from litter |
| B. alternatus | Individual | Araraquara–SP, Brazil | A, Brazil | F | 5 | Instituto Butantan, wild-caught in 2013 |
| B. atrox | Individual | São Bento–MA, Brazil | SB, Brazil | F | 12 | Instituto Butantan, wild-caught in 2009 |
| B. atrox | Pooled (2) | Balbira–AM, Brazil x São Bento–MA, Brazil | BxSB, Brazil | F | 8 | Institute Butantan, captive-born in 2010, l1a specimen from litter |
| Balbira–AM, Brazil x São Bento–MA, Brazil | F | 8 | Institute Butantan, captive-born in 2010, 7a specimen from litter | |||
| B. atrox | Pooled (66) | French Guiana | - | M + F | Adults | Latoxan, captive-born and wild-caught snakes |
| B. atrox | Pooled (n values not supplied) | Alto Marañon, Peru (Amazon rainforest) | Peru | UNKN | Adults | EFS, wild-caught |
| B. atrox | UNKN | Suriname | - | UNKN | Adults | Kentucky reptile zoo, unknown |
| B. atrox | UNKN | Colombia | - | UNKN | Adults | Kentucky reptile zoo, unknown |
| B. asper | Pooled (40) | Costa Rica (Pacific region) | Costa Rica | UNKN | Adults | JMG, wild-caught |
| B. asper | Pooled (2) | Ecuador | - | M | Adults | Latoxan, captive-born |
| B. asper | Individual | Mérida, Yucatán, Mexico | MY, Mexico | UNKN | Adult | UNAM, wild-caught |
| B. asper | Individual | San Andres, Tuxtla, Veracruz, Mexico | SATV, Mexico | UNKN | Adult | UNAM, wild-caught |
| B. asper | Individual | Ixtaczoquitlan, Veracruz, Mexico | IV, Mexico | UNKN | Young adult | UNAM, wild-caught |
| B. barnetti | Pooled (n values not supplied) | Talara, Department of Tubmes, Peru | Peru | UNKN | Adults | UNMSM, wild-caught |
| B. bilineatus | Pooled (2) | UNKN | - | F + F | Adults | M-toxins, imported |
| B. caribbaeus | UNKN | St. Lucia, West Indies | - | UNKN | Adults | Kentucky reptile zoo, wild-caught |
| B. diporus | UNKN | UNKN | - | UNKN | Adults | VEL, imported |
| B. jararacussu | Pooled (2) | Juquitiba–SP | J, Brazil | F | 3 | Instituto Butantan, wild-caught in 2015 |
| Juquitiba–SP | F | 3 | Instituto Butantan, wild-caught in 2015 | |||
| B. jararacussu | Individual | Cubatão–SP | C, Brazil | F | 3 | Instituto Butantan, wild-caught in 2015 |
| B. jararaca | Individual | Rio Negrino–SC | RN, Brazil | F | 1 | Instituto Butantan, wild-caught in 2017 |
| B. jararaca | Individual | São Roque–SP | SR, Brazil | F | 3 | Instituto Butantan, wild-caught in 2015 |
| B. jararaca | Individual | Ibiúna–SP | I, Brazil | F | 3 | Instituto Butantan, wild-caught in 2015 |
| B. lanceolatus | UNKN | Martinique | - | UNKN | Adults | Latoxan, imported |
| B. leucurus | Pooled (3) | Porto Seguro–BA | PS, Brazil | F | 3 | Instituto Butantan, captive-born in 2016, 5a specimen from litter |
| Porto Seguro–BA | F | 3 | Instituto Butantan, captive-born in 2016, 13a specimen from litter | |||
| Porto Seguro–BA | M | 3 | Instituto Butantan, captive-born in 2016, 14a specimen from litter | |||
| B. mattogrossensis | Pooled (3) | Porto Murtinho–MS | PM, Brazil | F | 6 | Instituto Butantan, captive-born in 2012, 4a specimen from litter |
| Porto Murtinho–MS | F | 9 | Instituto Butantan, wild-caught in 2009 | |||
| Porto Murtinho–MS | F | 9 | Instituto Butantan, wild-caught in 2009 | |||
| B. mattogrossensis | UNKN | Bolivia | - | UNKN | Adults | VEL, imported |
| B. moojeni | Individual | Palmas–TO | P, Brazil | F | 13 | Instituto Butantan, wild-caught in 2005 |
| B. moojeni | Pooled (2) | Gaúcha do Norte–MT | GdN, Brazil | F | 8 | Instituto Butantan, captive-born in 2010, 13a specimen from litter |
| Gaúcha do Norte–MT | F | 8 | Instituto Butantan, captive-born in 2010, 5a specimen from litter | |||
| B. neuwiedi | Individual | Salto Pirapora–SP | SP, Brazil | M | 6 | Instituto Butantan, captive-born in 2013, 3a specimen from litter |
| B. neuwiedi | Individual | Curitiba–PR | C, Brazil | F | 2 | Instituto Butantan, wild-caught in 2017 |
| B. neuwiedi | Individual | Munhoz–MG | M, Brazil | F | 4 | Instituto Butantan, wild-caught in 2015 |
| B. oligolepis | Pooled (3) | La Merced, Chanchamayo, Peru (central rainforest región) | Peru | UNKN | Adults | UNMSM, wild-caught |
| B. pauloensis | Pooled (3) | São Simão–SP | SS, Brazil | F | 6 | Instituto Butantan, captive-born in 2012, 6a specimen from litter |
| São Simão–SP | F | 7 | Instituto Butantan, captive-born in 2011, 2a specimen from litter | |||
| São Simão–SP | F | 7 | Instituto Butantan, captive-born in 2011, 6a specimen from litter | |||
| B. pictus | Pooled (7) | Districts of Carabayllo and Comas, Peru | Peru | UNKN | Adults | UNMSM, wild-caught |
| B. pubescens | UNKN | Uruguay | - | UNKN | Adults | VEL, imported |
| B. taeniatus | UNKN | UNKN | - | UNKN | Adults | VEL, imported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourke, L.A.; Zdenek, C.N.; Tanaka-Azevedo, A.M.; Silveira, G.P.M.; Sant’Anna, S.S.; Grego, K.F.; Rodrigues, C.F.B.; Fry, B.G. Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms. Toxins 2022, 14, 297. https://doi.org/10.3390/toxins14050297
Bourke LA, Zdenek CN, Tanaka-Azevedo AM, Silveira GPM, Sant’Anna SS, Grego KF, Rodrigues CFB, Fry BG. Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms. Toxins. 2022; 14(5):297. https://doi.org/10.3390/toxins14050297
Chicago/Turabian StyleBourke, Lachlan Allan, Christina N. Zdenek, Anita Mitico Tanaka-Azevedo, Giovanni Perez Machado Silveira, Sávio Stefanini Sant’Anna, Kathleen Fernandes Grego, Caroline Fabri Bittencourt Rodrigues, and Bryan Grieg Fry. 2022. "Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms" Toxins 14, no. 5: 297. https://doi.org/10.3390/toxins14050297
APA StyleBourke, L. A., Zdenek, C. N., Tanaka-Azevedo, A. M., Silveira, G. P. M., Sant’Anna, S. S., Grego, K. F., Rodrigues, C. F. B., & Fry, B. G. (2022). Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms. Toxins, 14(5), 297. https://doi.org/10.3390/toxins14050297






