Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels
Abstract
:1. Introduction
2. Results
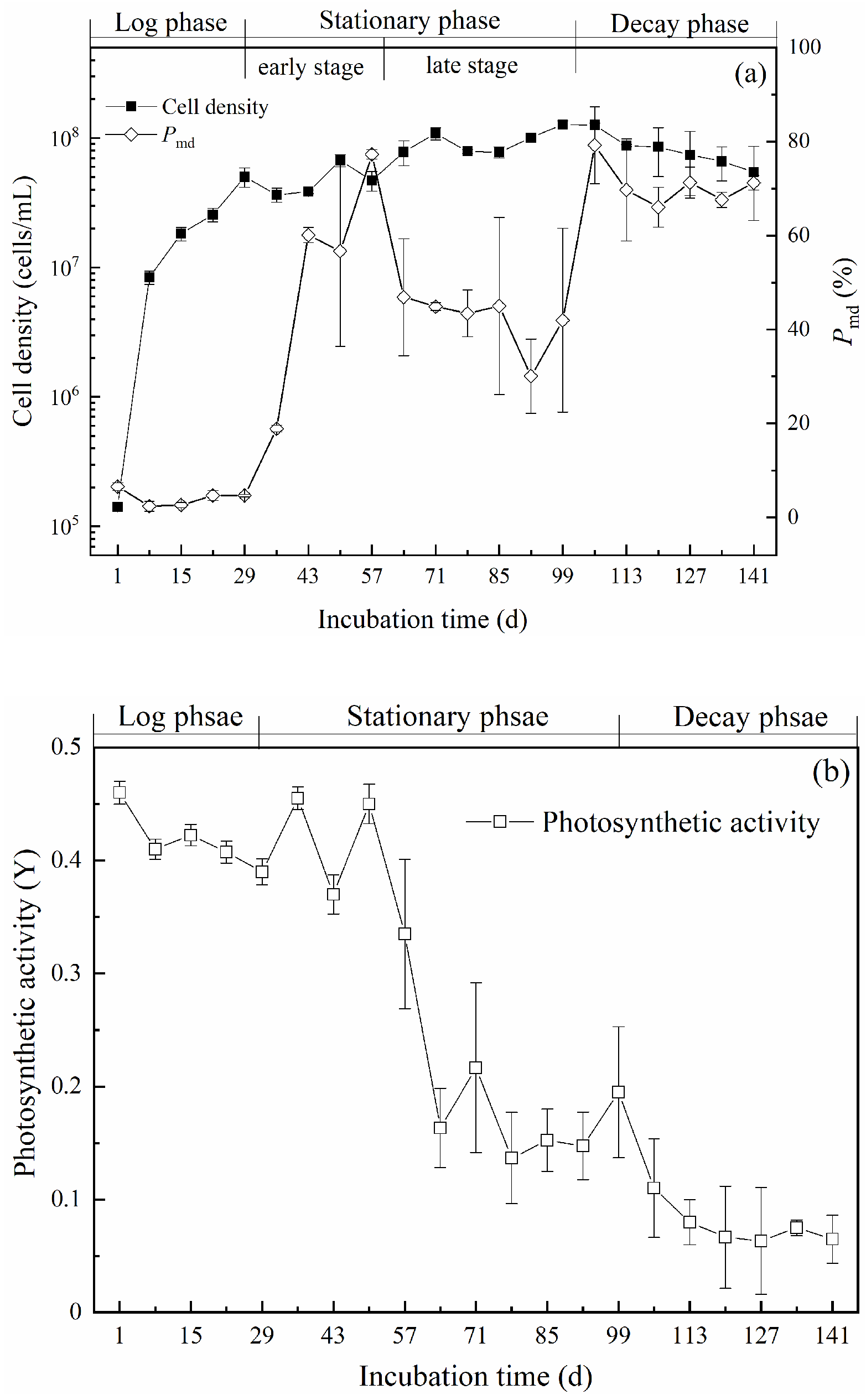
2.1. Growth Characteristics of M. aeruginosa
2.2. Production of β-Cyclocitral
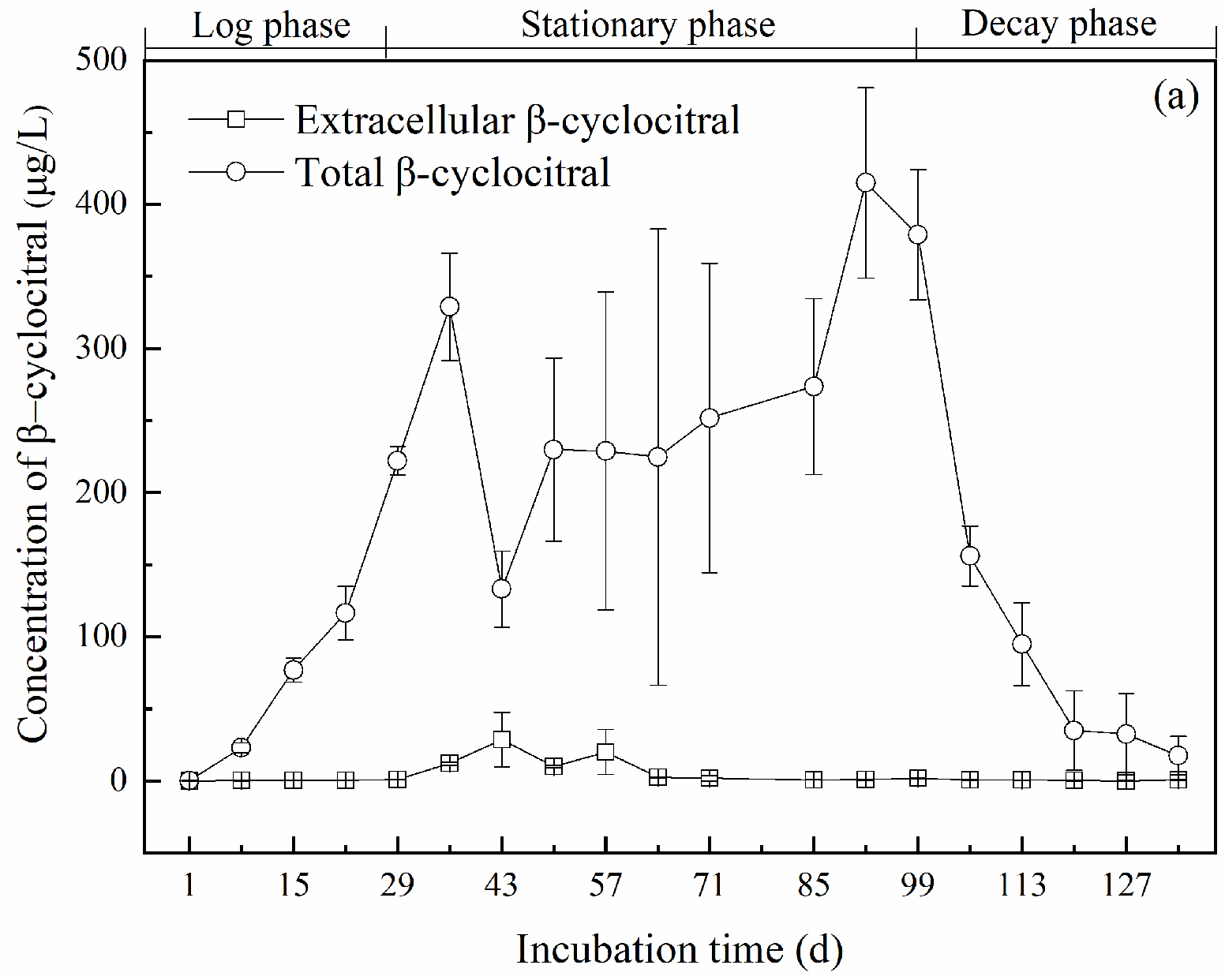
2.2.1. Variations of Intracellular and Extracellular Concentrations of β-Cyclocitral
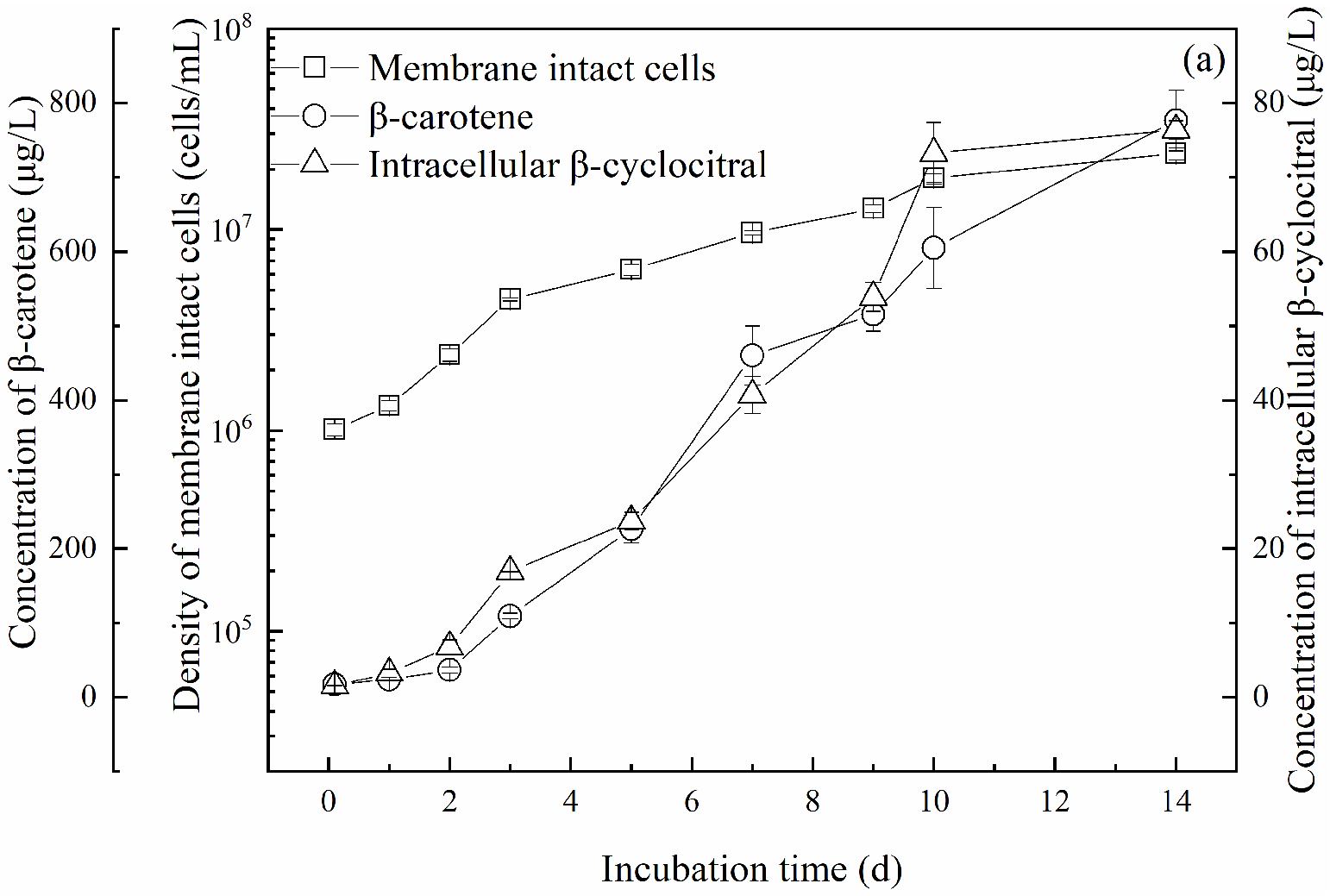
2.2.2. Quota of β-Cyclocitral Produced per Membrane-Intact Cell
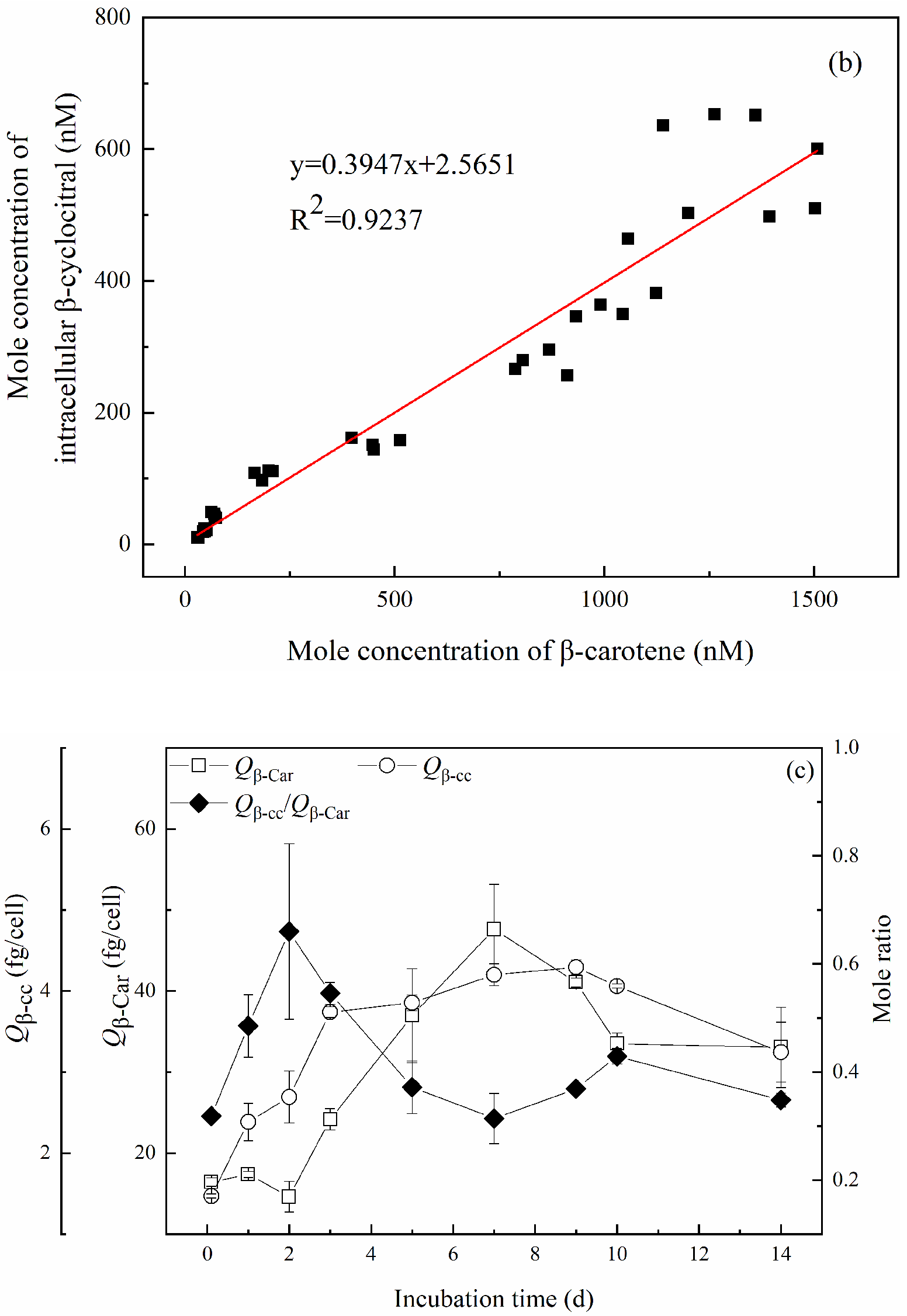
2.3. Variation of β-Cyclocitral along with β-Carotene
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Microorganism
5.2. Flow Cytometric Analysis
5.3. Photosynthetic Activity
5.4. Quantification of Odorous Compounds by HS-SPME–GC–MS
5.5. Quantification of β-Carotene by HPLC
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Broad Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tromas, N.; Fortin, N.; Bedrani, L.; Terrat, Y.; Cardoso, P.; Bird, D.; Greer, C.W.; Shapiro, B.J. Characterising and predicting cyanobacterial blooms in an 8-year amplicon sequencing time course. ISME J. 2017, 11, 1746–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Hamilton, D.P. A systematic literature review of forecasting and predictive models for cyanobacteria blooms in freshwater lakes. Water Res. 2020, 182, 115959. [Google Scholar] [CrossRef] [PubMed]
- du Preez, G.C.; Wepener, V.; Fourie, H.; Daneel, M.S. Irrigation water quality and the threat it poses to crop production: Evaluating the status of the Crocodile (West) and Marico catchments, South Africa. Environ. Monit. Assess. 2018, 190, 127. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.L. Cyanobacterial peptides beyond microcystins–A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Su, M.; Zhu, Y.; Jia, Z.; Liu, T.; Yu, J.; Burch, M.; Yang, M. Identification of MIB producers and odor risk assessment using routine data: A case study of an estuary drinking water reservoir. Water Res. 2021, 192, 116848. [Google Scholar] [CrossRef]
- Shang, L.; Feng, M.; Xu, X.; Liu, F.; Ke, F.; Li, W. Co-occurrence of microcystins and taste-and-odor compounds in drinking water source and their removal in a full-scale drinking water treatment plant. Toxins 2018, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.S.; Lee, C.S.; Srivastava, A.; Oh, H.M.; Ahn, C.Y. Effects of environmental factors on cyanobacterial production of odorous compounds: Geosmin and 2-methylisoborneol. J. Microbiol. Biotechnol. 2017, 27, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.J.; Chen, C.; Ding, J.Q.; Hou, A.; Li, Y.; Niu, Z.B.; Su, X.Y.; Xu, Y.J.; Laws, E.A. The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Geada, P.; Pereira, R.N.; Vasconcelos, V.; Vicente, A.A.; Fernandes, B.D. Assessment of synergistic interactions between environmental factors on Microcystis aeruginosa growth and microcystin production. Algal Res. 2017, 27, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Zimba, P.V.; Grimm, C.C. A synoptic survey of musty/muddy odor metabolites and microcystin toxin occurrence and concentration in southeastem USA channel catfish (Ictalurus punctatus Ralfinesque) production ponds. Aquaculture 2003, 218, 81–87. [Google Scholar] [CrossRef]
- Jüttner, F.; Watson, S.B.; von Elert, E.; Köster, O. β-Cyclocitral, a Grazer Defence Signal Unique to the Cyanobacterium Microcystis. J. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef]
- Jüttner, F.; Höflacher, B. Evidence of β-carotene 7,8(7′,8′) oxygenase (β-cyclocitral, crocetindial generating) in Microcystis. Arch. Microbiol. 1985, 141, 337–343. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, M.; Yang, L.; Wang, Y.; Wang, Y.; Meng, Y.; Liu, J.; Zuo, Z. Effects of high light and temperature on Microcystis aeruginosa cell growth and β-cyclocitral emission. Ecotoxicol. Environ. Saf. 2020, 192, 110313. [Google Scholar] [CrossRef]
- Arii, S.; Yamashita, R.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.-I. Differences in susceptibility of cyanobacteria species to lytic volatile organic compounds and influence on seasonal succession. Chemosphere 2021, 284, 131378. [Google Scholar] [CrossRef]
- Harada, K.-I.; Ozaki, K.; Tsuzuki, S.; Kato, H.; Hasegawa, M.; Kuroda, E.K.; Arii, S.; Tsuji, K. Blue color formation of cyanobacteria with β-cyclocitral. J. Chem. Ecol. 2009, 35, 1295–1301. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.I. Cyanobacterial blue color formation during lysis under natural conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675. [Google Scholar] [CrossRef] [Green Version]
- Zhong, F.; Gao, Y.; Yu, T.; Zhang, Y.; Xu, D.; Xiao, E.; He, F.; Zhou, Q.; Wu, Z. The management of undesirable cyanobacteria blooms in channel catfish ponds using a constructed wetland: Contribution to the control of off-flavor occurrences. Water Res. 2011, 45, 6479–6488. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Min, Q.; Ren, R.; Liu, J.; Sun, X.; Ping, X.; Chen, J. The relationships between odors and environmental factors at bloom and non-bloom area in Lake Taihu, China. Chemosphere 2019, 218, 569–576. [Google Scholar]
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501. [Google Scholar] [CrossRef]
- Liu, X.; Shi, C.; Xu, X.; Li, X.; Xu, Y.; Huang, H.; Zhao, Y.; Zhou, Y.; Shen, H.; Chen, C.; et al. Spatial distributions of β-cyclocitral and β-ionone in the sediment and overlying water of the west shore of Taihu Lake. Sci. Total Environ. 2017, 579, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, X.; Chen, H.; Mao, Z.; Shen, R.; Zeng, Q.; Ge, Y. Co-occurrence of multiple cyanotoxins and taste-and-odor compounds in the large eutrophic Lake Taihu, China: Dynamics, driving factors, and challenges for risk assessment. Environ. Pollut. 2022, 294, 118594. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, T.F.; Zhang, T.; Li, C.; Gao, N. Characterization of typical taste and odor compounds formed by Microcystis aeruginosa. J. Environ. Sci. 2013, 25, 1539–1548. [Google Scholar] [CrossRef]
- Ye, C.; Yang, Y.; Xu, Q.; Ying, B.; Zhang, M.; Gao, B.; Ni, B.; Yakefu, Z.; Bai, Y.; Zuo, Z. Volatile organic compound emissions from Microcystis aeruginosa under different phosphorus sources and concentrations. Phycol. Res. 2018, 66, 15–22. [Google Scholar] [CrossRef]
- Zuo, Z.; Yang, L.; Chen, S.; Ye, C.; Han, Y.; Wang, S.; Ma, Y. Effects of nitrogen nutrients on the volatile organic compound emissions from Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 161, 214–220. [Google Scholar] [CrossRef]
- Drugă, B.; Buda, D.M.; Szekeres, E.; Chiş, C.; Chiş, I.; Sicora, C. The impact of cation concentration on Microcystis (cyanobacteria) scum formation. Sci. Rep. 2019, 9, 3017. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, P.; Nie, Y. Concentration and bioaccumulation of cyanobacterial bioactive and odorous metabolites occurred in a large, shallow chinese lake. Bull. Environ. Contam. Toxicol. 2014, 93, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Feng, J.; Song, Y.; Ni, M.; Dietrich, A.M.; Chen, C.; Li, Q.; Gao, N. Release behavior of odor contaminants derived from Microcystis aeruginosa in rivers and a non-strict anaerobic aqueous system. J. Water Supply Res. Technol. AQUA 2015, 64, 812–823. [Google Scholar] [CrossRef]
- Gomes, T.; Xie, L.; Brede, D.; Lind, O.C.; Solhaug, K.A.; Salbu, B.; Tollefsen, K.E. Sensitivity of the green algae Chlamydomonas reinhardtii to gamma radiation: Photosynthetic performance and ROS formation. Aquat. Toxicol. 2017, 183, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. USA. 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, X.; Au, D.W.T.; Mao, X.; Yuan, K. The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae. Chemosphere 2010, 78, 541–547. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. Chlorophyll Fluoresc. 2004, 1367, 137–147. [Google Scholar]
- Tao, Y.; Mao, X.; Hu, J.; Mok, H.O.L.; Wang, L.; Au, D.W.T.; Zhu, J.; Zhang, X. Mechanisms of photosynthetic inactivation on growth suppression of Microcystis aeruginosa under UV-C stress. Chemosphere 2013, 93, 637–644. [Google Scholar] [CrossRef]
- Tao, Y.; Hou, D.; Zhou, T.; Cao, H.; Zhang, W.; Wang, X. UV-C suppression on hazardous metabolites in Microcystis aeruginosa: Unsynchronized production of microcystins and odorous compounds at population and single-cell level. J. Hazard. Mater. 2018, 359, 281–289. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Q.P.; Dong, H.R. Determination of lycopene and β-carotene by high-performance liquid chromatography using sudan I as internal standard. J. Chromatogr. B 2006, 838, 44–49. [Google Scholar] [CrossRef]


| Samples | β-cyclocitral Concentrations (μg/L) | Qβ-cc (fg/Cell) | Ref |
|---|---|---|---|
| Laboratory samples | – | 7.15–11.72 | [14] |
| Laboratory samples | 2000 | 41–865 | [24] |
| Surface scum | 1400 | 3.3 | [20] |
| Fish pond | 1.94 ± 1.26 | - | [21] |
| Lake Taihu | 0.35 (0.01) 1 | - | [22] |
| Lake Taihu | 1.37 (0.01–0.28) 1 | - | [23] |
| Lake Taihu | 0.15–12 (14.4–342) 2 | - | [24] |
| Lake Chaohu | ~0.75 3 | - | [30] |
| Source water | 0.06–2.12 | <0.21 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, Y.; Hou, D.; Teng, F.; Cai, Z.; Tao, Y. Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels. Toxins 2022, 14, 201. https://doi.org/10.3390/toxins14030201
Wang X, Zhu Y, Hou D, Teng F, Cai Z, Tao Y. Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels. Toxins. 2022; 14(3):201. https://doi.org/10.3390/toxins14030201
Chicago/Turabian StyleWang, Xuejian, Yinjie Zhu, Delin Hou, Fei Teng, Zhonghua Cai, and Yi Tao. 2022. "Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels" Toxins 14, no. 3: 201. https://doi.org/10.3390/toxins14030201
APA StyleWang, X., Zhu, Y., Hou, D., Teng, F., Cai, Z., & Tao, Y. (2022). Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels. Toxins, 14(3), 201. https://doi.org/10.3390/toxins14030201





