Influence of Endosperm Starch Composition on Maize Response to Fusarium temperatum Scaufl. & Munaut
Abstract
:1. Introduction
2. Results
2.1. Fusarium Ear Rot Infection
2.2. Mycotoxin Occurrence
2.3. Maize Type
3. Discussions
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Materials
4.3. Plant Infection Assay
4.4. Inoculum Preparation
4.5. Mycotoxin Analysis
4.6. Amylose Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesterházy, Á.M.; Lemmens, L.M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Jabłońska, E.; Piątek, K.; Wit, M.; Mirzwa-Mróz, E.; Wakulinski, W. Molecular diversity of the Fusarium fujikuroi species complex from maize. Eur. J. Plant Pathol. 2020, 158, 859–877. [Google Scholar] [CrossRef]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol. Ecol. 2010, 73, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Zhang, J.B.; Li, H.P.; Gong, A.D.; Xue, S.; Agboola, R.S.; Liao, Y.C. Molecular identification, mycotoxin production and comparative pathogenicity of Fusarium temperatum isolated from maize in China. J. Phytopathol. 2014, 162, 147–157. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, W.; Pan, Y.; Xu, J.; Xu, J.S.; Chen, W.Q.; Feng, J. First report of Fusarium temperatum causing Fusarium ear rot on maize in Northern China. Plant Dis. 2014, 98, 1273. [Google Scholar] [CrossRef]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wang, X.; Chen, G.; Sun, S.; Yang, Y.; Zhu, Z.; Duan, C. The major Fusarium species causing maize ear and kernel rot and their toxigenicity in Chongqing, China. Toxins 2018, 10, 90. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.X.; Du, Q.; Tang, Z.L.; Li, S.C.; Wang, B.B. First report of maize ear rot caused by Fusarium sacchari in China. Plant Dis. 2019, 103, 2674. [Google Scholar] [CrossRef]
- Scauflaire, J.; Gourgue, M.; Munaut, F. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia 2011, 103, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Scauflaire, J.; Mahieu, O.; Louvieaux, J.; Foucart, G.; Renard, F.; Munaut, F. Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. Eur. J. Plant Pathol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Scauflaire, J.; Gourgue, M.; Callebaut, A.; Munaut, F. Fusarium temperatum, a mycotoxin-producing pathogen of maize. Eur. J. Plant Pathol. 2012, 133, 911–922. [Google Scholar] [CrossRef]
- Pintos Varela, C.; Aguín Casal, O.; Chaves Padin, M.; Ferreiroa Martinez, V.; Sainz Oses, M.J.; Scauflaire, J.; Munaut, F.; Bande Castro, M.J.; Mansilla Vázquez, J.P. First report of Fusarium temperatum causing seedling blight and stalk rot on maize in Spain. Plant Dis. 2013, 97, 1252. [Google Scholar] [CrossRef]
- Boutigny, A.; Scauflaire, J.; Ballois, N.; Ioos, R. Fusarium temperatum isolated from maize in France. Eur. J. Plant Pathol. 2017, 148, 997–1001. [Google Scholar] [CrossRef]
- Nugroho, P.A.; Setyabudi, F.M.C.S.; Saleh, B.; Rahayu, E.S. Fumonisin-producing Fusarium from maize grains in Tretep, Indonesia. J. Food Sci. Eng. 2013, 3, 534–540. [Google Scholar]
- Fumero, M.V.; Reynoso, M.M.; Chulze, S. Fusarium temperatum and Fusarium subglutinans isolated from maize in Argentina. Int. J. Food Microbiol. 2015, 199, 86–92. [Google Scholar] [CrossRef]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. Fusarium temperatum as a new species causing ear rot on maize in Poland. Plant Dis. 2014, 98, 1001. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Kvas, M.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Steenkamp, E.T. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 2009, 34, 1–21. [Google Scholar]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar] [CrossRef] [Green Version]
- Laurence, M.H.; Summerell, B.A.; Liew, E.C.Y. Fusarium oxysporum f. sp. canariensis: Evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 2015, 64, 1068–1075. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Wit, M.; Leng, Y.; Du, Y.; Cegiełko, M.; Jabłońska, E.; Wakuliński, W.; Zhong, S. Genome sequence resources for the maize pathogen Fusarium temperatum isolated in Poland. Mol. Plant Microbe Interact. 2021, 34, 214–217. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef] [Green Version]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Meca, G.; Ruiz, M.J.; Soriano, J.M.; Ritieni, A.; Moretti, A.; Font, G.; Mañes, J. Isolation and purification of eniatins A, A1, B, B1, produced by Fusarium tricinctum in solid culture, and cytotoxicity effects on Caco-2 cells. Toxicon 2010, 56, 418–424. [Google Scholar] [CrossRef]
- Tamagno, S.; Greco, I.A.; Almeida, H.; Borrás, L. Physiological differences in yield related traits between flint and dent Argentinean commercial maize genotypes. Eur. J. Agron. 2015, 68, 50–56. [Google Scholar] [CrossRef]
- Unterseer, S.; Pophaly, S.D.; Peis, R.; Westermeier, P.; Mayer, M.; Seidel, M.A.; Haberer, G.; Mayer, K.F.X.; Ordas, B.; Pausch, H.; et al. A comprehensive study of the genomic differentiation between temperate Dent and Flint maize. Genome Biol. 2016, 17, 137. [Google Scholar] [CrossRef] [Green Version]
- Alvarez Prado, S.; López, C.G.; Senior, M.L.; Borrás, L. The genetic architecture of maize (Zea mays L.) kernel weight determination. G3 Genes Genomes Genet. 2014, 4, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.L.; Scheben, A.; Edwards, D.; Spillane, C.; Ortiz, R. Assessing and exploiting functional diversity in germplasm pools to enhance abiotic stress adaptation and yield in cereals and food legumes. Front. Plant Sci. 2017, 8, 1461. [Google Scholar] [CrossRef] [PubMed]
- Camus-Kulandaivelu, L.; Veyrieras, J.B.; Madur, D.; Combes, V.; Fourmann, M.; Barraud, S.; Dubreuil, P.; Gouesnard, B.; Manicacci, D.; Charcosset, A. Maize adaptation to temperate climate: Relationship between population structure and polymorphism in the Dwarf8 gene. Genetics 2006, 172, 2449–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, C.X.; Wang, B.B.; Sun, F.F.; Yang, Z.H.; Zhu, Z.D.; Wang, X.M. Occurrence of maize ear rot caused by Fusarium fujikuroi in China. Plant Dis. 2020, 104, 587. [Google Scholar] [CrossRef]
- Wotia, F.; Omukunda, E. Incidence of Maize Ear Rot and Stem Borer Participatory Rural Appraisal Efficacy Relationship by Farmers in Western Kenya. Plant 2021, 9, 10–15. [Google Scholar] [CrossRef]
- Stagnati, L.; Martino, M.; Battilani, P.; Busconi, M.; Lanubile, A.; Marocco, A. Development of early maturity maize hybrids for resistance to Fusarium and Aspergillus ear rots and their associated mycotoxins. World Mycotoxin J. 2020, 13, 459–471. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bissonnette, K.M.; Bradley, C.A.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 2020, 21, 238–247. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Toldine Toth, E.; Szel, S.; Varga, M.; Toth, B. Resistance of Maize Hybrids to Fusarium graminearum, F. culmorum, and F. verticillioides Ear Rots with Toothpick and Silk Channel Inoculation, as Well as Their Toxin Production. Agronomy 2020, 10, 1283. [Google Scholar] [CrossRef]
- Tran, T.M.; Ameye, M.; Landschoot, S.; Devlieghere, F.; de Saeger, S.; Eeckhout, M.; Audenaert, K. Molecular Insights into Defense Responses of Vietnamese Maize Varieties to Fusarium verticillioides Isolates. J. Fungi 2021, 7, 724. [Google Scholar] [CrossRef]
- Righetti, L.; Dall’Asta, C.; Lucini, L.; Battilani, P. Lipid Signaling Modulates the Response to Fumonisin Contamination and Its Source, Fusarium verticillioides, in Maize. Front. Plant Sci. 2021, 12, 701680. [Google Scholar] [CrossRef]
- Lanza, F.E.; Mayfield, D.A.; Munkvold, G.P. First report of Fusarium temperatum causing maize seedling blight and seed rot in North America. Plant Dis. 2016, 100, 1019. [Google Scholar] [CrossRef]
- Robles-Barrios, K.F.; Medina-Canales, M.G.; Rodríguez-Tovar, A.V.; Pérez, N.O. Morphological and molecular characterization, enzyme production and pathogenesis of Fusarium temperatum on corn in Mexico. Can. J. Plant Pathol. 2015, 37, 495–505. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, S.W.; Lee, H.G.; Lee, Y.S. Aggressiveness and Fumonisins Production of Fusarium Subglutinans and Fusarium Temperatum on Korean Maize Cultivars. Agronomy 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Wit, M.; Warzecha, R.; Mirzwa-Mróz, E.; Jabłońska, E.; Ochodzki, P.; Waśkiewicz, A.; Wakuliński, W. Susceptibility of flint and dent maize ears to Fusarium species. Phytopathologia 2011, 60, 35–45. [Google Scholar]
- Schaafsma, A.W.; Miller, J.D.; Savard, M.E.; Ewing, R.J. Ear rot development and mycotoxin production in corn in relation to inoculation method, corn hybrid, and species of Fusarium. Can. J. Plant Pathol. 1993, 15, 185–192. [Google Scholar] [CrossRef]
- Szabo, B.; Toth, B.; Toldine, E.T.; Varga, M.; Kovacs, N.; Varga, J.; Kocsube, S.; Palagyi, A.; Bagi, F.; Budakov, D.; et al. A New Concept to Secure Food Safety Standards against Fusarium Species and Aspergillus Flavus and Their Toxins in Maize. Toxins 2018, 10, 372. [Google Scholar] [CrossRef] [Green Version]
- Ritieni, A.; Moretti, A.; Logrieco, A.; Bottalico, A.; Randazzo, G.; Monti, S.M.; Ferracane, R.; Fogliano, V. Occurrence of fusaproliferin, fumonisin B1, and beauvericin in maize from Italy. J. Agric. Food Chem. 1997, 45, 4011–4016. [Google Scholar] [CrossRef]
- Gromadzka, K.; Błaszczyk, L.; Chełkowski, J.; Waśkiewicz, A. Occurrence of Mycotoxigenic Fusarium Species and Competitive Fungi on Preharvest Maize Ear Rot in Poland. Toxins 2019, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Snijders, C.H.A. Breeding for resistance to Fusarium in wheat and maize. In Mycotoxins in Grain Compounds Other than Aflatoxin; Miller, J.D., Trenholm, H.L., Eds.; Eagan Press: St. Paul, MN, USA, 1994; pp. 37–58. [Google Scholar]
- Shelby, R.A.; White, D.G.; Bauske, E.M. Differential fumonisin production in maize hybrids. Plant Dis. 1994, 78, 582–584. [Google Scholar] [CrossRef]
- Bluhm, B.H.; Woloshuk, C.P. Amylopectin induces fumonisin B1 production by Fusarium verticillioides during colonization of maize kernels. Mol. Plant Microbe Interact. 2005, 18, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Hennigen, M.; Valente Soares, L.M.; Sanchez, S.; Di Benedetto, N.M.; Longhi, A.; Eyherabide, G.; Torroba, J.; Zanelli, M. Fumonisin in corn hybrids grown in Argentina for two consecutive seasons. In Proceedings of the 10th International IUPAC Symposium on Mycotoxins and Phytotoxins, Guaruja, Brazil, 21–25 May 2000; de Koe, W.J., Samson, R.A., van Egmond, H.P., Gilbert, J., Sabino, M., Eds.; IUPAC: Research Triangle Park, NC, USA, 2000; pp. 331–339. [Google Scholar]
- Löffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays L.) inbred lines. Theor. Appl. Genet. 2010, 120, 1053–1062. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. (Eds.) Starch: Analysis of Quality. In Encyclopedia of Food Grains, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 198–207. [Google Scholar]
- Liu, X.; Xiao, X.; Liu, P.; Yu, L.; Li, M.; Zhou, S.; Xie, F. Shear degradation of corn starches with different amylose contents. Food Hydrocoll. 2017, 66, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Vittadini, E.; Fogliano, V. Designing food structure to slow down digestion in starch-rich products. Curr. Opin. Food Sci. 2020, 32, 50–57. [Google Scholar] [CrossRef]
- Reid, L.M.; Bolton, A.T.; Hamilton, R.I.; Woldemariam, T.; Mather, D.E. Effect of silk age on resistance of maize to Fusarium Graminearum. Can. J. Plant Pathol. 1992, 14, 293–298. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Iowa State University of Sciences and Technology Cooperative Extension Service: Ames, IA, USA, 1993. [Google Scholar]
- Chungu, C.; Mather, D.E.; Reid, L.M.; Hamilton, R.I. Comparison of techniques for inoculating maize silk, kernel, and cob tissues with Fusarium Graminearum. Plant Dis. 1996, 80, 81–84. [Google Scholar] [CrossRef]
- Wit, M.; Wakuliński, W.; Ochodzki, P.; Warzecha, R. Podatność wybranych genotypów kukurydzy na fuzariozę kolb w warunkach infekcji naturalnej/Susceptibility of selected lines to Fusarium cob rot in naturally infected corn. Prog. Plant Prot. Post. Ochr. Rośl. 2009, 49, 763–768. (In Polish) [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; p. 388. [Google Scholar]
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- Kwaśna, H.; Chełkowski, J.; Zajkowski, P. Grzyby (Mycota), Grzyby niedoskonałe (Deuteromycetes), Strzępczakowe (Hyphomycetales), Gruzełkowate (Tuberculariaceae), Sierpik (Fusarium); Instytut Botaniki PAN: Warszawa-Kraków, Poland, 1991; Volume 22, p. 136. (In Polish) [Google Scholar]
- Monti, S.M.; Fogliano, V.; Logrieco, A.; Ferracane, R.; Ritieni, A. Simultaneous determination of beauvericin, enniatins, and fusaproliferin by high performance liquid chromatography. J. Agric. Food Chem. 2000, 48, 3317–3320. [Google Scholar] [CrossRef]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Wit, M.; Golinski, P.; Chelkowski, J.; Warzecha, R.; Ochodzki, P.; Wakulinski, W. Kinetics of fumonisin B1 formation in maize ears inoculated with Fusarium Verticillioides. Food Addit. Contam. Part A 2012, 29, 1752–1761. [Google Scholar] [CrossRef]

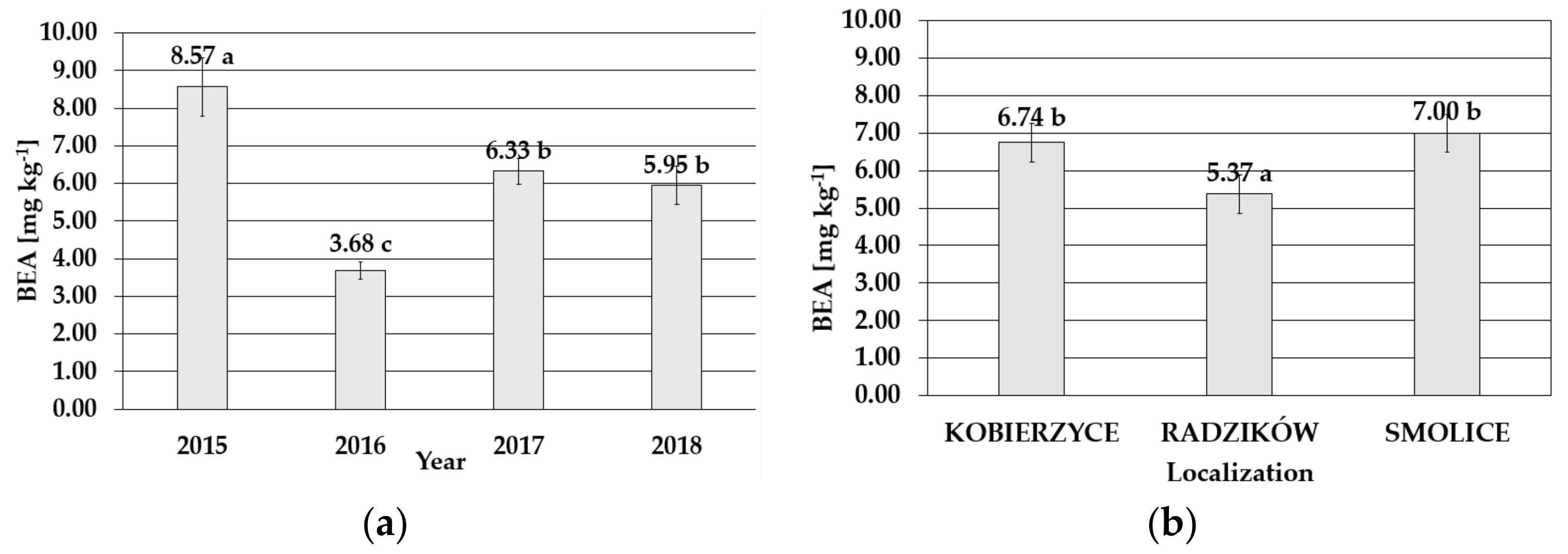
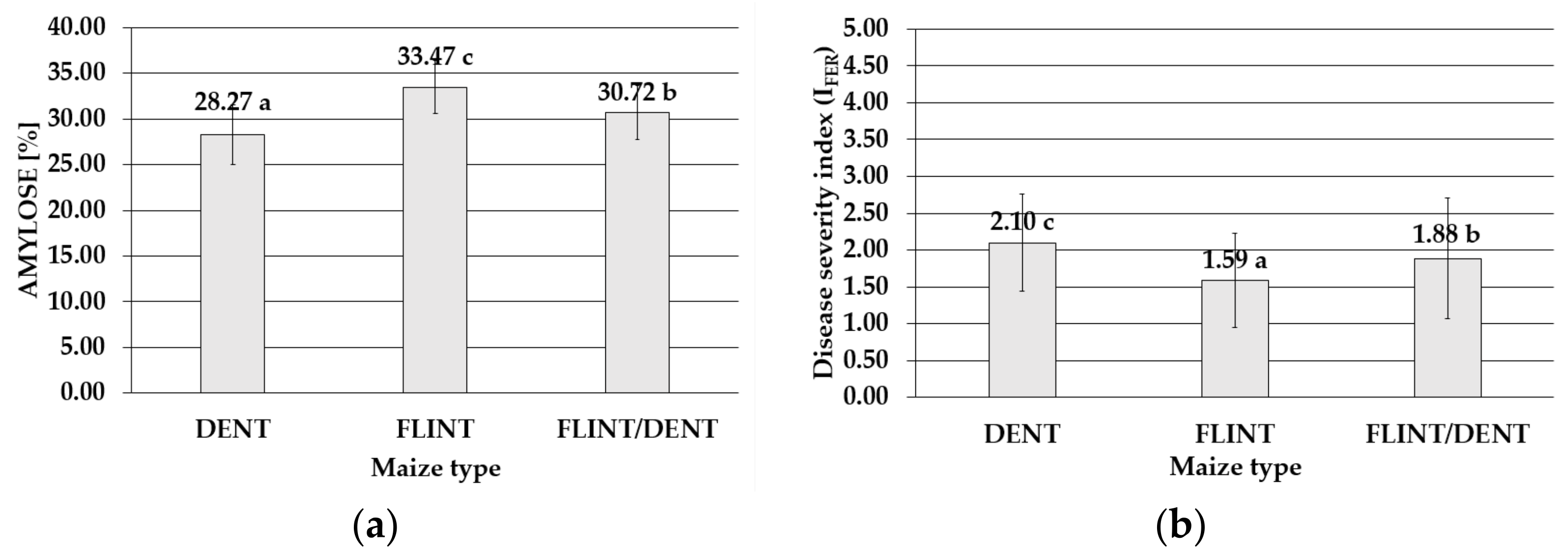

| Disease Severity Index (IFER) | YEAR | KOBIERZYCE | RADZIKÓW | SMOLICE | SUM | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breeding Lines | Breeding Lines | Breeding Lines | Breeding Lines | ||||||
| No | % | No | % | No | % | No | % | ||
| 0–1 | 2015 | 0 | 0.00 | 3 | 3.03 | 0 | 0.00 | 3 | 1.01 |
| 2016 | 10 | 20.41 | 34 | 33.33 | 6 | 11.54 | 50 | 21.76 | |
| 2017 | 1 | 2.00 | 4 | 3.92 | 1 | 1.92 | 6 | 2.61 | |
| 2018 | 6 | 11.76 | 23 | 22.77 | 6 | 12.00 | 35 | 15.51 | |
| 2015–2018 | 17 | 8.54 | 64 | 15.76 | 13 | 6.37 | 94 | 10.22 | |
| 1–2 | 2015 | 9 | 17.65 | 38 | 38.38 | 0 | 0.00 | 47 | 18.68 |
| 2016 | 28 | 57.14 | 58 | 56.86 | 39 | 75.00 | 125 | 63.00 | |
| 2017 | 19 | 38.00 | 54 | 52.94 | 32 | 61.54 | 105 | 50.83 | |
| 2018 | 36 | 70.59 | 73 | 72.28 | 37 | 74.00 | 146 | 72.29 | |
| 2015–2018 | 92 | 45.85 | 223 | 55.12 | 108 | 52.64 | 423 | 51.20 | |
| 2–3 | 2015 | 23 | 45.10 | 46 | 46.46 | 33 | 67.35 | 102 | 52.97 |
| 2016 | 10 | 20.41 | 10 | 9.80 | 7 | 13.46 | 27 | 14.56 | |
| 2017 | 24 | 48.00 | 38 | 37.25 | 18 | 34.62 | 80 | 39.96 | |
| 2018 | 5 | 9.80 | 4 | 3.96 | 6 | 12.00 | 15 | 8.59 | |
| 2015–2018 | 62 | 30.83 | 98 | 24.37 | 64 | 31.86 | 224 | 29.02 | |
| 3–4 | 2015 | 15 | 29.41 | 11 | 11.11 | 15 | 30.61 | 41 | 23.71 |
| 2016 | 1 | 2.04 | 0 | 0.00 | 0 | 0,00 | 1 | 0.68 | |
| 2017 | 5 | 10.00 | 6 | 5.88 | 1 | 1.92 | 12 | 5.93 | |
| 2018 | 4 | 7.84 | 1 | 0.99 | 1 | 2.00 | 6 | 3.61 | |
| 2015–2018 | 25 | 12.32 | 18 | 4.50 | 17 | 8.63 | 60 | 8.48 | |
| 4–5 | 2015 | 4 | 7.84 | 1 | 1.01 | 1 | 2.04 | 6 | 3.63 |
| 2016 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| 2017 | 1 | 2.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.67 | |
| 2018 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| 2015–2018 | 5 | 2.46 | 1 | 0.25 | 1 | 0.51 | 7 | 1.07 | |
| Estimated Variables | YEAR | IFER | ERG | BEA | AMYL |
|---|---|---|---|---|---|
| IFER | 2015 | 1.000 | 0.396 ** | 0.364 ** | −0.195 ** |
| 2016 | 1.000 | 0.441 ** | 0.430 ** | −0.180 * | |
| 2017 | 1.000 | 0.701 ** | 0.785 ** | −0.201 ** | |
| 2018 | 1.000 | 0.735 ** | 0.503 ** | −0.303 ** | |
| ERG | 2015 | 0.396 ** | 1.000 | 0.486 ** | −0.253 ** |
| 2016 | 0.441 ** | 1.000 | 0.787 ** | −0.028 | |
| 2017 | 0.701 ** | 1.000 | 0.911 ** | −0.175 * | |
| 2018 | 0.735 ** | 1.000 | 0.581 ** | −0.322 ** | |
| BEA | 2015 | 0.364 ** | 0.486 ** | 1.000 | −0.091 |
| 2016 | 0.430 ** | 0.787 ** | 1.000 | −0.086 | |
| 2017 | 0.785 ** | 0.911 ** | 1.000 | −0.160 * | |
| 2018 | 0.503 ** | 0.581 ** | 1.000 | −0.166 * | |
| AMYL | 2015 | −0.195 ** | −0.253 ** | −0.091 | 1.000 |
| 2016 | −0.180 * | −0.028 | −0.086 | 1.000 | |
| 2017 | −0.201 ** | −0.175 * | −0.160 * | 1.000 | |
| 2018 | −0.303 ** | −0.322 ** | −0.166 * | 1.000 |
| Year | Maize Type | No of Tested Breeding Lines | Disease Severity Index IFER | ERG (mg kg−1) | BEA (mg kg−1) | Amylose (%) |
|---|---|---|---|---|---|---|
| 2015 | D | 38 | 2.758 ± 0.576 b | 14,083 ± 12.68 a | 12,219 ± 8.83 b | 25.319 ± 2.38 a |
| F | 40 | 2.200 ± 0585 a | 6822 ± 11.71 ab | 4563 ± 5.41 a | 30.742 ± 2.37 c | |
| FD | 121 | 2.578 ± 0.790 b | 10,611 ± 15.32 b | 8780 ± 12.20 b | 28.065 ± 2.46 b | |
| 2016 | D | 40 | 1.692 ± 0.513 c | 17,630 ± 11.80 a | 3716 ± 2.40 a | 29.422 ± 2.82 a |
| F | 40 | 1.194 ± 0.439 a | 16,487 ± 15.25 a | 3419 ± 3.83 a | 33.758 ± 2.34 c | |
| FD | 123 | 1.432 ± 0.644 b | 16,616 ± 13.60 a | 3745 ± 3.30 a | 31.149 ± 2.13 b | |
| 2017 | D | 40 | 2.211 ± 0.426 b | 20,047 ± 11.01 b | 6987 ± 3.89 b | 29.779 ± 3.24 a |
| F | 40 | 1.679 ± 0.554 a | 12,042 ± 6.51 a | 4507 ± 2.86 a | 35.120 ± 2.14 c | |
| FD | 124 | 2.083 ± 0.680 b | 19,551 ± 15.32 b | 6702 ± 5.79 b | 32.552 ± 2.73 b | |
| 2018 | D | 40 | 1.767 ± 0.519 b | 19,665 ± 17.39 ab | 7024 ± 6.63 a | 28.413 ± 2.42 a |
| F | 40 | 1.278 ± 0.380 a | 12,189 ± 8.86 a | 4733 ± 3.06 a | 34.255 ± 2.65 c | |
| FD | 122 | 1.443 ± 0.531 a | 15,101 ± 16.00 b | 5949 ± 7.49 a | 31.082 ± 2.63 b | |
| 2015–2018 | D | 158 | 2.099 ± 0.659 c | 17,928 ± 13.54 b | 7401 ± 6.58 b | 28.270 ± 3.23 a |
| F | 160 | 1.588 ± 0.634 a | 11,885 ± 11.50 a | 4286 ± 3.95 a | 33.469 ± 2.88 c | |
| FD | 490 | 1.883 ± 0.820 b | 15,501 ± 15.38 ab | 6289 ± 8.06 b | 30.721 ± 2.98 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wit, M.; Ochodzki, P.; Warzecha, R.; Jabłońska, E.; Mirzwa-Mróz, E.; Mielniczuk, E.; Wakuliński, W. Influence of Endosperm Starch Composition on Maize Response to Fusarium temperatum Scaufl. & Munaut. Toxins 2022, 14, 200. https://doi.org/10.3390/toxins14030200
Wit M, Ochodzki P, Warzecha R, Jabłońska E, Mirzwa-Mróz E, Mielniczuk E, Wakuliński W. Influence of Endosperm Starch Composition on Maize Response to Fusarium temperatum Scaufl. & Munaut. Toxins. 2022; 14(3):200. https://doi.org/10.3390/toxins14030200
Chicago/Turabian StyleWit, Marcin, Piotr Ochodzki, Roman Warzecha, Emilia Jabłońska, Ewa Mirzwa-Mróz, Elżbieta Mielniczuk, and Wojciech Wakuliński. 2022. "Influence of Endosperm Starch Composition on Maize Response to Fusarium temperatum Scaufl. & Munaut" Toxins 14, no. 3: 200. https://doi.org/10.3390/toxins14030200
APA StyleWit, M., Ochodzki, P., Warzecha, R., Jabłońska, E., Mirzwa-Mróz, E., Mielniczuk, E., & Wakuliński, W. (2022). Influence of Endosperm Starch Composition on Maize Response to Fusarium temperatum Scaufl. & Munaut. Toxins, 14(3), 200. https://doi.org/10.3390/toxins14030200





