Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador
Abstract
1. Introduction
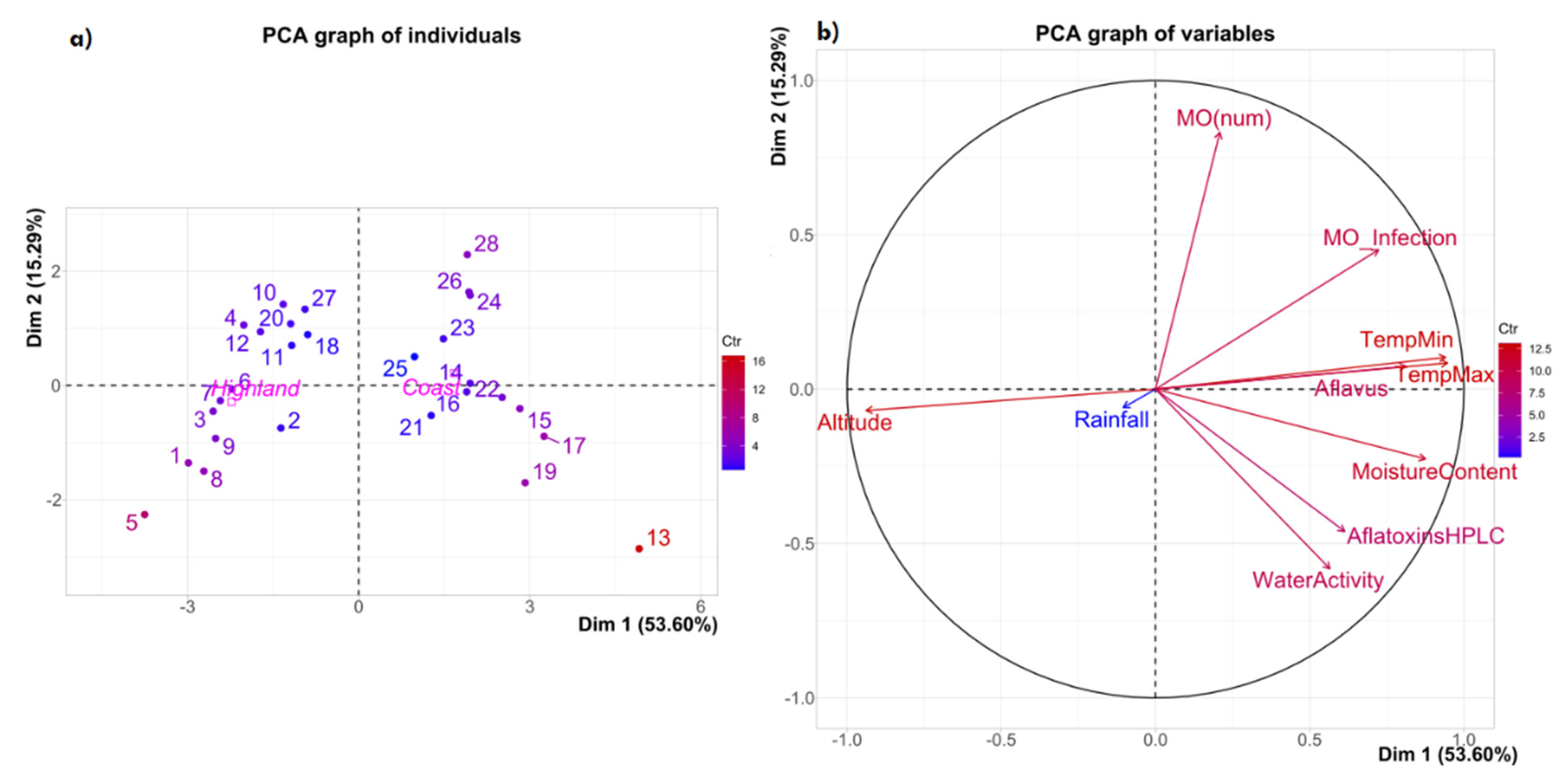
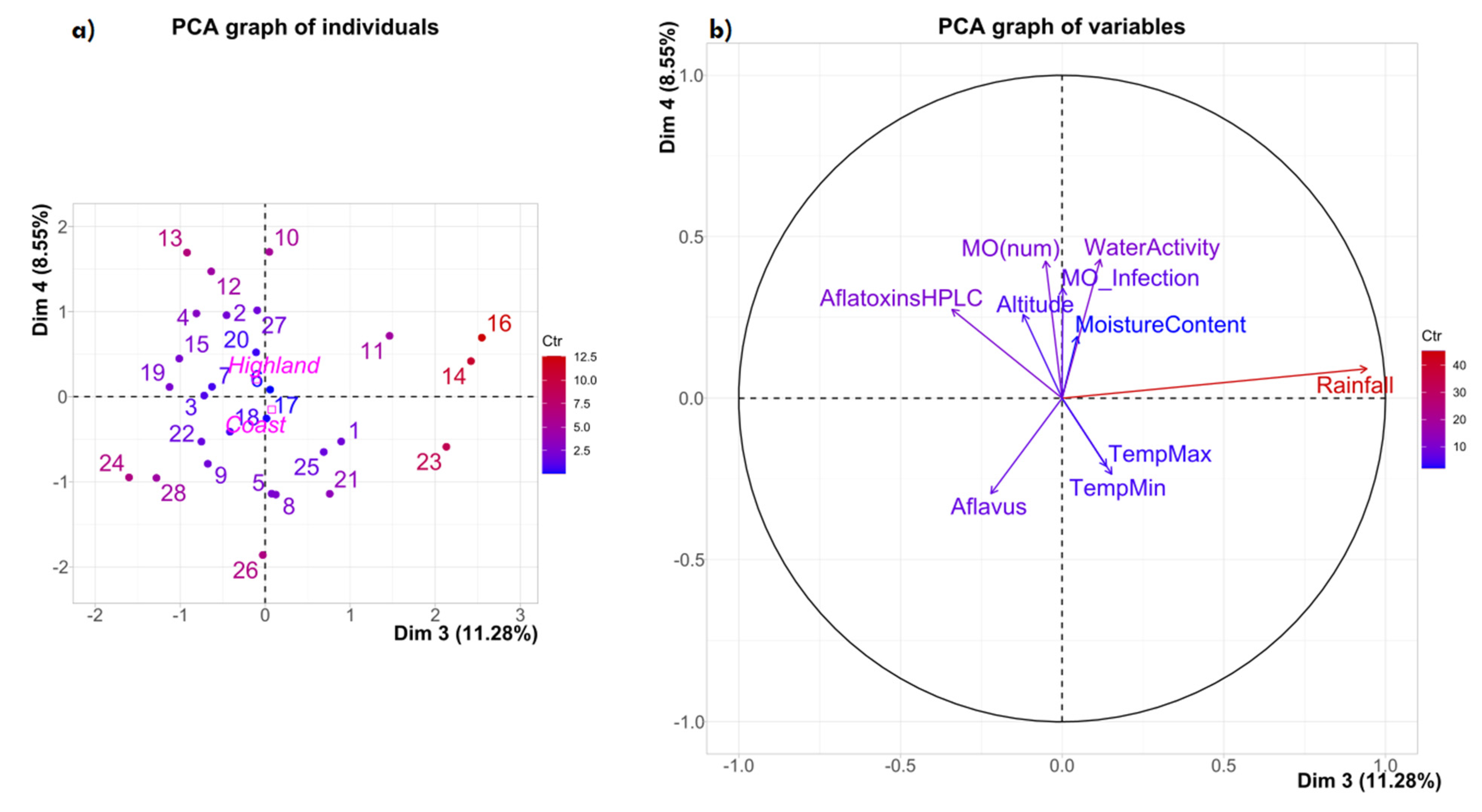
2. Results and Discussion
3. Conclusions
4. Materials and Methods
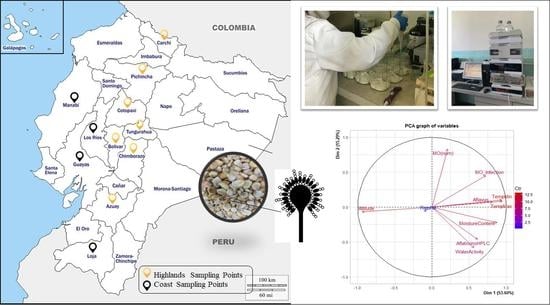
4.1. Samples
4.2. Humidity Content
4.3. Water Activity (WA)
4.4. Altitude, Temperature and Rainfall Data
4.5. Fungal Contamination and Identification
4.6. Aflatoxins Analyses
4.7. Principal Components Analysis (PCA)
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Instituto Nacional Autónomo de Investigaciones Agropecuarias (INIAP). Estación Experimental Santa Catalina, Programa de Maíz; Informe anual 2014; Instituto Nacional Autónomo de Investigaciones Agropecuarias: Quito, Ecuador, 2014.
- Baca, A. La Producción de Maíz Amarillo en el Ecuador y su Relación con la Soberanía Alimentaria; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2016. [Google Scholar]
- Blancas, M.B. Manejo de granos en almacenamiento, causas de deterioro y prevención. Arch. Latinoam. Prod. Anim. 2007, 15, 184. [Google Scholar]
- Nelson, P.E. Taxonomy and biology of Fusarium moniliforme. Mycopathologia 1992, 117, 29–36. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Scussel, V.M. Aflatoxin and food safety: Recent south American perspectives. J. Toxicol.-Toxin Rev. 2004, 23, 179–216. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorología e Hidrología—INAMHI. Available online: http://www.inamhi.gob.ec/ (accessed on 30 November 2021).
- Vásquez, F.M.F.F.; Vásquez, F.M.F.F. La prevalencia de Aspergillus spp. y aflatoxinas en Zea mays L.(maí z) almacenado en silos, en Ecuador. Rev. Publicando 2016, 3, 189–202. [Google Scholar]
- López, V.; José, M. María Determinación de Aflatoxinas B1, B2, G1 y G2 Presentes en Harina de maíz del sector Tumbaco Mediante el uso de Columnas de Inmunoafinidad (IAC) y Cromatografía Líquida de Alta Eficiencia (HPLC). 2012. Available online: http://repositorio.espe.edu.ec/handle/21000/5013 (accessed on 30 November 2021).
- Navarro, Y.M.C.; Salazar, L.M.B.; Giraldo, J.D.R.; González, J.P.P.; Osorio, J.I.T. Optimización del protocolo para la extracción y la cuantificación de proteínas totales en semillas germinadas de maíz (Zea mays L.). Rev. Fac. Cienc. Básicas 2017, 13, 65–68. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and food spoilage. Fungi Food Spoilage 2009, 1–519. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.A.M.P.; Kuijpers, A.F.A.; Frank, J.M.; Frisvad, J.C. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004, 50, 45–61. [Google Scholar]
- Gasperini, A.M.; Garcia-Cela, E.; Sulyok, M.; Medina, A.; Magan, N. Fungal diversity and metabolomic profiles in GM and isogenic non-GM maize cultivars from Brazil. Mycotoxin Res. 2021, 37, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, K.; Kasturi, K.; KRS, S.R. Kiran Lakkireddy; Kasturi Kondapalli; Krs Sambasiva Rao Aflatoxins in Food and Feed: The Science of Safe Food. Practice 2014, 3, 5. [Google Scholar]
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic Fungi of Concern in Foods and Feeds: A Review. J. Food Prot. 1995, 58, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Jayaratne, W.M.S.C.; Abeyratne, A.H.M.A.K.; De Zoysa, H.K.S.; Dissanayake, D.M.R.B.N.; Bamunuarachchige, T.C.; Waisundara, V.Y.; Chang, S. Detection and quantification of Aflatoxin B1 in corn and corn-grown soils in the district of Anuradhapura, Sri Lanka. Heliyon 2020, 6, e05319. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Resolução No° 7, de 18 de Fevereiro de 2011(*). Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0007_18_02_2011_rep.html (accessed on 30 November 2021).
- The Ministry of Health of the People’s Republic of China. Maximum Residue Level of Mycotoxin in Foodnational Regulations for Food Safety. National Standard No. 2761-2011. 2011. Available online: https://www.chinesestandard.net/PDF.aspx/GB2762-2017 (accessed on 30 November 2021).
- Commission Regulation (EC) No 466/2001 of 8 March 2001 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2001. Available online: https://op.europa.eu/en/publication-detail/-/publication/52b2484d-39e0-4aa9-ba19-4b13a887bb1c (accessed on 30 November 2021).
- FDA. FDA Compliance Program Guidance Manual Mycotoxins in Domestic and Imported Foods FY 15/16; FDA: Silver Spring, MD, USA, 2015.
- Biomin World Mycotoxin Survey: Impact 2020|Biomin. Available online: https://www.biomin.net/br/science-hub/pesquisa-mundial-de-micotoxinas-impacto-em-2020/ (accessed on 30 November 2021).
- Kaaya, A.N.; Kyamuhangire, W.; Kyamanywa, S. Factors affecting aflatoxin contamination of harvested maize in the three agroecological zones of Uganda. J. Appl. Sci. 2006, 6, 2401–2407. [Google Scholar] [CrossRef][Green Version]
- INEN Instituto Ecuatoriano de Normalización. Norma Técnica Ecuatoriana 1 233:95 Granos y Cereales. Muestreo. 1995. Available online: https://www.normalizacion.gob.ec/buzon/normas/nte_inen_1000-1.pdf (accessed on 30 November 2021).
- Ospina Machado, J.E. Características Físico Mecánicas y Análisis de Calidad de Granos; Universidad Nacional de Colombia: Bogotá, Colombia, 2001; ISBN: 9587011821, 9789587011821. [Google Scholar]
- Agratonix MT-16 Operating Instructions Grain Moisture Tester. Available online: https://www.agratronix.com/wp-content/uploads/2016/11/DOCU-M0113_Manual_11-16.pdf (accessed on 30 November 2021).
- Klich, M.A.; Pitt, J.I. A Laboratory Guide to the Common Aspergillus Species and Their Teleomorphs; Commonwealth Scientific and Industrial Research Organization, Division of Food Processing: North Ryde, Australia, 1988.
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Introduction to Food- and Airborne Fungi, 7th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2004; ISBN 9789070351526. [Google Scholar]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjević; Kubátová, A.; et al. Polyphasic taxonomy of Aspergillus section Aspergillus (formerly Eurotium), and its occurrence in indoor environments and food. Stud. Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species, 3rd ed.; Commonwealth Scientific and Industrial Research Organization Division of Food Processing: North Ryde, Australia, 2000; ISBN 9780643048379.
- Maigua, S.; Rodrigo, I.; Mayorga, E.; Susana, H.; Linzán, V.; Paúl, J.; Ramos, O.; Efraín, B.; Mendoza, Z.; Luis, J. Evaluación de la contaminación por aflatoxinas B1, B2, G1 y G2 en maíz amarillo duro. Rev. Espamciencia 2018, 9, 13–21. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
| Sample | Town or Province | Aflatoxins (µg/kg) | WA * | FI (%) ** | Fungal Diversity (% of Ocurrence in the Sample) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| G2 | G1 | B2 | B1 | Total | ||||||
| Highlands | 1 | Pichincha | - | - | - | - | - | 0.685 | 8.34 | Cladosporium sp. (4.17); Dematiaceus (4.17) |
| 2 | Chimborazo | - | - | - | - | - | 0.746 | 100 | A. ruber (100); Fusarium sp. (12.5) | |
| 3 | Cotopaxi | - | - | - | - | - | 0.689 | 41.7 | Dematiaceus (20.8); Fusarium sp. (20.8); Mucor sp. (4.17) | |
| 4 | Cotopaxi | - | - | - | - | - | 0.654 | 83.4 | A. montevidensis (4.17); A. pseudoglaucus (20.9); A. restrictus (12.5); Dematiaceus (4.17); Fusarium sp. (37.5); | |
| 5 | Carchi | - | - | - | - | - | 0.665 | nd *** | - | |
| 6 | Azuay | - | - | - | - | - | 0.647 | 30.96 | A. pseudoglaucus (4.17); A. ruber (4.17); Dematiaceus (8.37); Fusarium sp. (4.17); | |
| 7 | Chimborazo | - | - | - | - | - | 0.681 | 54.2 | A. ruber (37.5); Fusarium sp. (8.34); P. raistricki (8.34) | |
| 8 | Azuay | - | - | - | - | - | 0.635 | 12.5 | Fusarium sp. (12.5) | |
| 9 | Tungurahua | - | - | - | - | - | 0.649 | 54.2 | Fusarium sp. (54.2) | |
| 10 | Azuay | - | - | - | - | - | 0.688 | 100 | A. chevalieri (4.17); A. pseudoglaucus (45.9); A. ruber (20.9); A. wentii (16.8); Dematiaceus (33.3); Fusarium sp. (4.17); | |
| 11 | Bolívar | - | - | - | - | - | 0.704 | 100 | A. niger complex (16.7); A. ruber (100); Dematiaceus (12.5); Mucor sp. (12.5) | |
| 12 | Chimborazo | - | - | - | - | - | 0.720 | 100 | A. pseudoglaucus (12.5); A. ruber (100); Dematiaceus (16.7); P. citrinum (20.8); Wallemia sp. (8.30) | |
| Coast | 13 | Balzar | - | 6.34 | 3.60 | 97.8 | 108 | 0.901 | 100 | A. Flavi *** (20.8); Fusarium sp. (79.2); P. aethiopicum (41.7) |
| 14 | Ventanas | - | - | - | - | - | 0.804 | 100 | A. Flavi (8.34); A. wentii (4.17); Fusarium sp. (58.4); P. citrinum (95.9) | |
| 15 | Portoviejo | - | - | 1.81 | 23.6 | 25.4 | 0.839 | 100 | A. Flavi (12.5); A. niger complex (4.17); Fusarium sp. (37.5); P. citrinum (83.4) | |
| 16 | Mocache | - | - | - | - | - | 0.821 | 100 | A. Flavi (4.17); A. chevalieri (4.17); Fusarium sp. (95.9); P. corylophilum (4.17) | |
| 17 | Empalme | - | - | 1.73 | 25.0 | 26.8 | 0.845 | 95.9 | A. Flavi (25.0); Fusarium sp. (54.2); P. citrinum (25.0) | |
| 18 | Pindal | - | - | - | - | - | 0.586 | 75.1 | A. chevalieri (12.5); A. Flavi (12.5); A. ruber (12.5); Dematiaceus (16.6); Fusarium sp. (45.9) | |
| 19 | Tosagua | - | - | 1.73 | 37.8 | 39.6 | 0.861 | 95.9 | A. Flavi (12.5); Fusarium sp. (70.9) | |
| 20 | Pindal | - | - | - | - | - | 0.610 | 75.06 | A. chevalieri (4.17); A. niger complex (4.17); Dematiaceus (8.34); Fusarium sp. (54.2); T. rugulosum (4.17) | |
| 21 | Balzar | - | - | - | - | - | 0.723 | 75.06 | A. Flavi (4.17); Fusarium sp. (70.89); | |
| 22 | Tosagua | - | - | - | - | - | 0.808 | 100 | A. Flavi (16.7); Fusarium sp. (83.4); P. citrinum (8.34) | |
| 23 | Ventanas | - | - | - | 0.42 | 0.42 | 0.674 | 95.9 | A. chevalieri (33.4); A. Flavi (12.5); A. wentii (4.17); Fusarium sp. (54.2) | |
| 24 | Portoviejo | - | - | 1.87 | 29.0 | 30.8 | 0.573 | 100 | A. Flavi (16.7); A. niger complex (4.17); Dematiaceus (4.17); Fusarium sp. (70.9); P. citrinum (25.2) | |
| 25 | Mocache | - | - | - | - | - | 0.664 | 100 | A. chevalieri (8.34); Dematiaceus (8.34); Fusarium sp. (62.6) | |
| 26 | Empalme | - | - | - | 1.50 | 1.50 | 0.572 | 100 | A. Flavi (25.0); A. niger complex (4.17); Fusarium sp. (70.9) | |
| 27 | Pindal | - | - | - | - | - | 0.634 | 75.06 | A. chevalieri (4.17); A. niger complex (4.17); A. pseudoglaucus (4.17); Cladosporium sp. (8.34); Dematiaceus (8.34); Fusarium sp. (41.7); P. citrinum (4.17) | |
| 28 | Portoviejo | - | - | - | 3.43 | 3.43 | 0.624 | 100 | A. chevalieri (8.34); A. Flavi (20.8); A. wentii (4.17); Dematiaceus (16.7); Fusarium sp. (62.5); Rhizopus sp. (4.2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abel Palacios, H.; Stefanello, A.; García Gavilánez, M.S.; Castro Demera, D.A.; Garcia, M.V.; Vásquez Castillo, W.A.; Almeida Marcano, M.A.; Samaniego Maigua, I.R.; Copetti, M.V. Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador. Toxins 2022, 14, 196. https://doi.org/10.3390/toxins14030196
Abel Palacios H, Stefanello A, García Gavilánez MS, Castro Demera DA, Garcia MV, Vásquez Castillo WA, Almeida Marcano MA, Samaniego Maigua IR, Copetti MV. Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador. Toxins. 2022; 14(3):196. https://doi.org/10.3390/toxins14030196
Chicago/Turabian StyleAbel Palacios, Héctor, Andrieli Stefanello, Margarita Susana García Gavilánez, Dicke Alejandro Castro Demera, Marcelo Valle Garcia, Wilson Arturo Vásquez Castillo, Marcelo Alejandro Almeida Marcano, Iván Rodrigo Samaniego Maigua, and Marina Venturini Copetti. 2022. "Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador" Toxins 14, no. 3: 196. https://doi.org/10.3390/toxins14030196
APA StyleAbel Palacios, H., Stefanello, A., García Gavilánez, M. S., Castro Demera, D. A., Garcia, M. V., Vásquez Castillo, W. A., Almeida Marcano, M. A., Samaniego Maigua, I. R., & Copetti, M. V. (2022). Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador. Toxins, 14(3), 196. https://doi.org/10.3390/toxins14030196