Assessment of Maize Hybrids Resistance to Aspergillus Ear Rot and Aflatoxin Production in Environmental Conditions in Serbia
Abstract
1. Introduction
2. Results
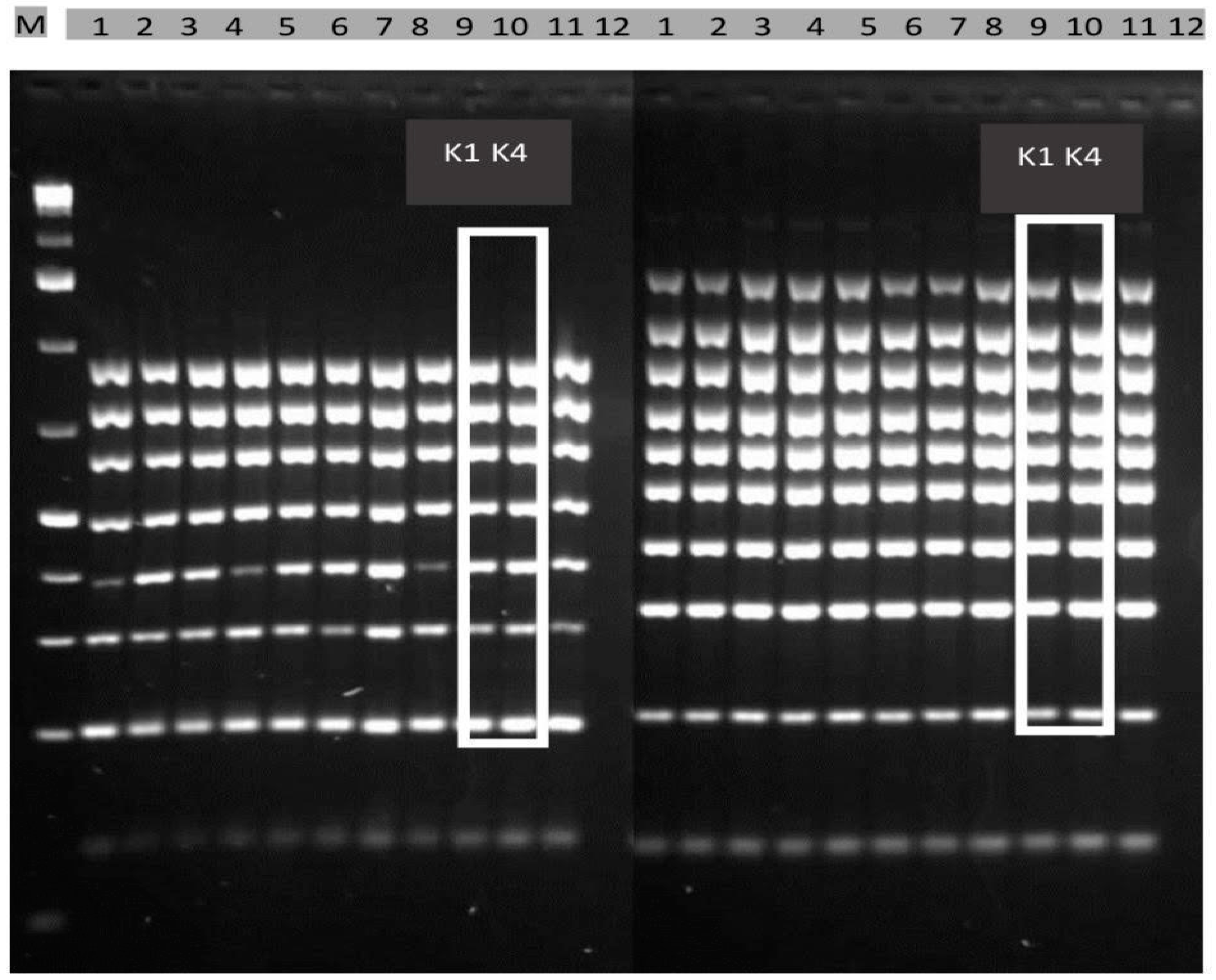
2.1. Selection of Toxigenic A. flavus Isolates
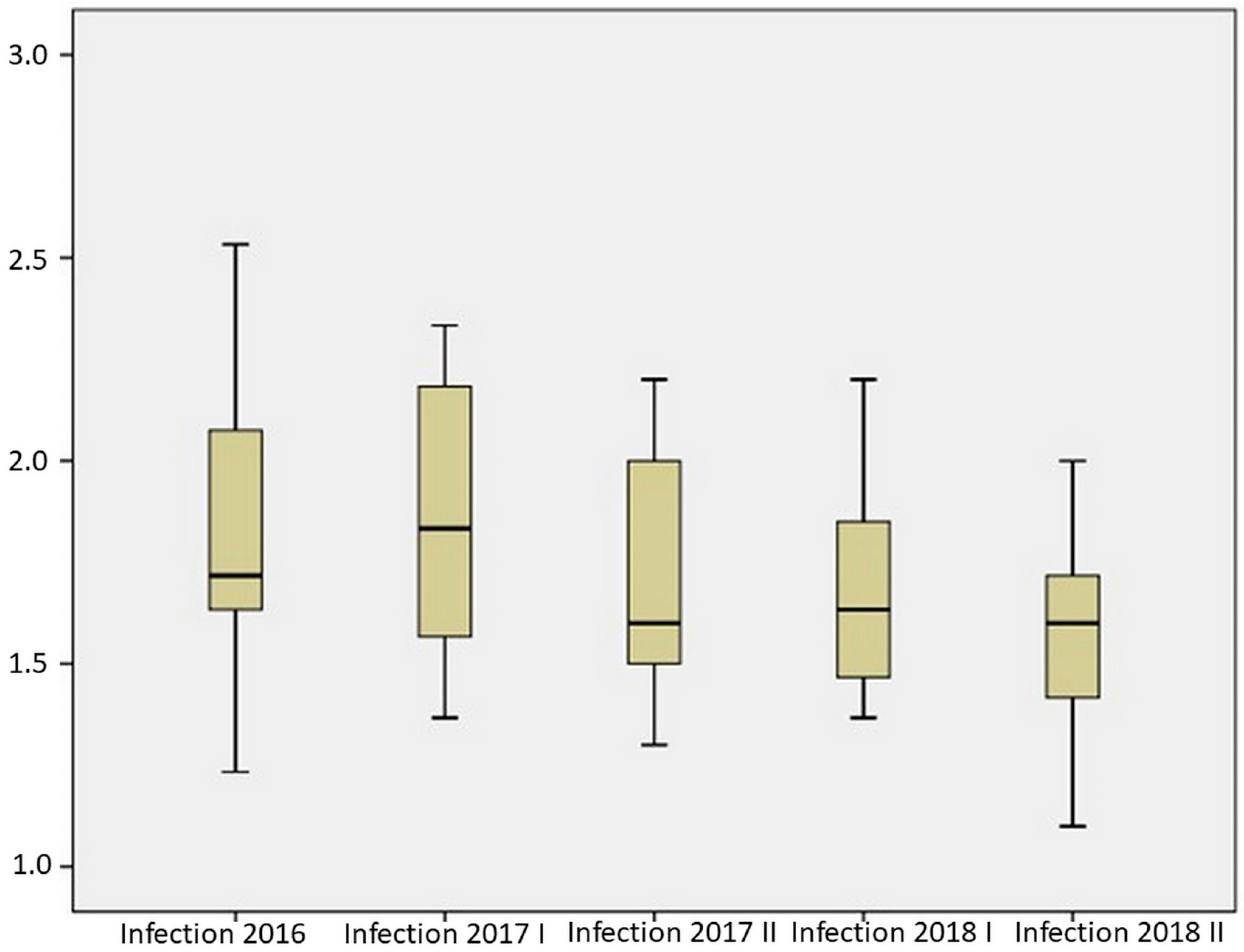
2.2. Resistance of Maize Hybrids to Aspergillus Ear Rot
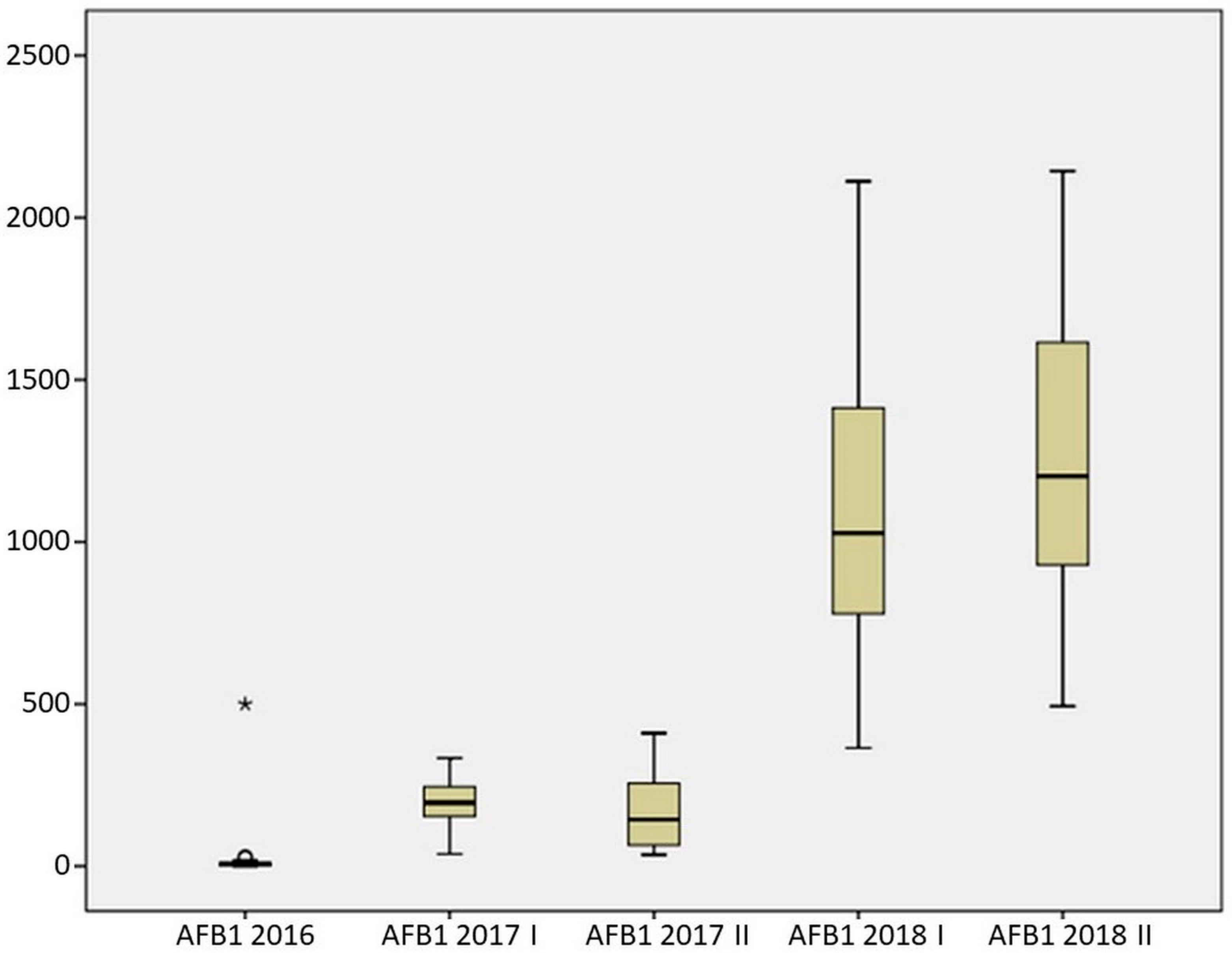
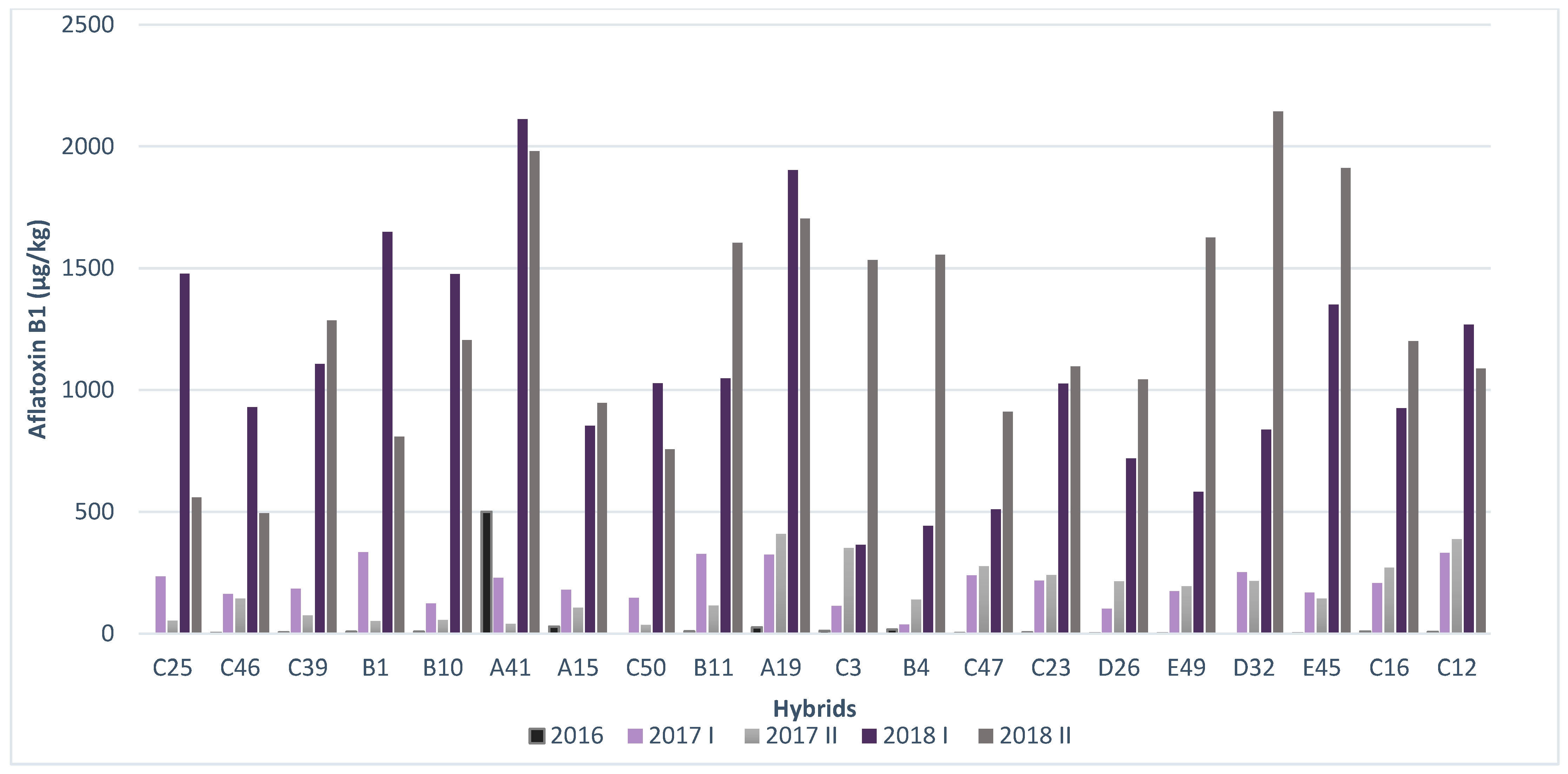
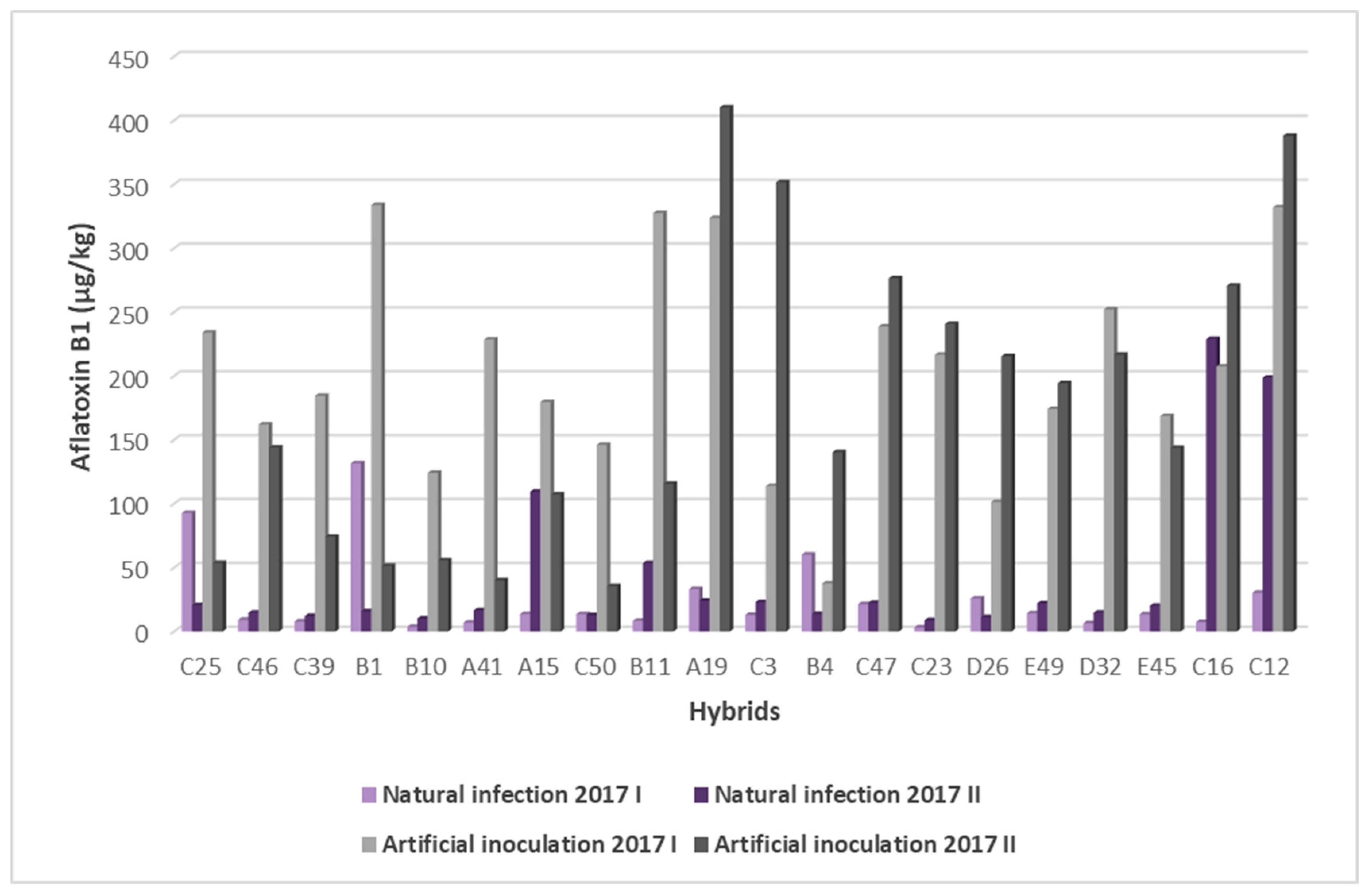
2.3. Resistance of Maize Hybrids to AFB1 Production
2.4. Relationship between Aspergillus Ear Rot and AFB1 Levels
2.5. Influence of Sowing Date on A. flavus Infection and AFB1 Content
2.6. Interactions between Aflatoxin B1 Production and Yield
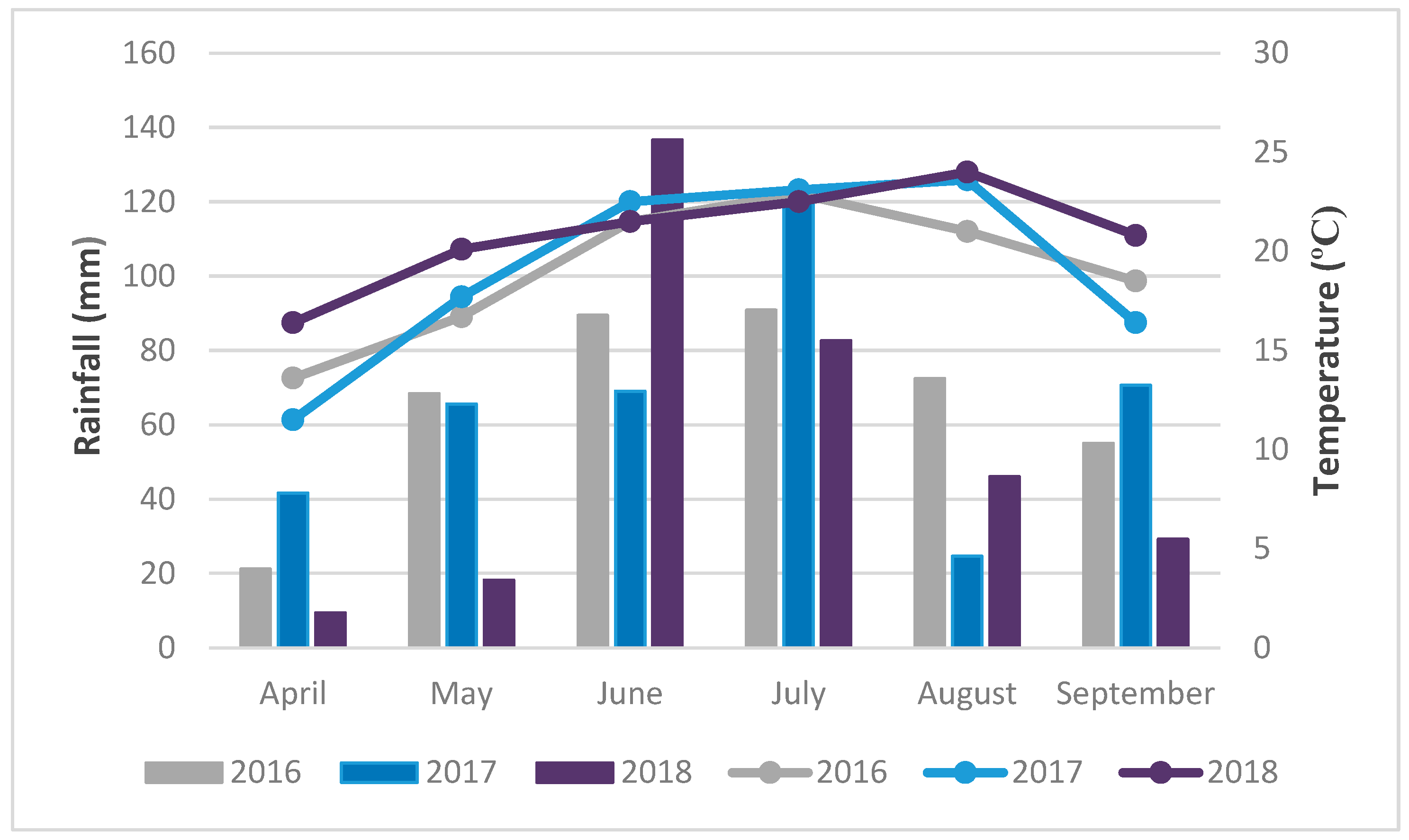
2.7. Meteorological Conditions
3. Discussion
3.1. Resistance of Maize Hybrids to Aspergillus Ear Rot and Aflatoxin Accumulation
3.2. Influence of Sowing Date on Aflatoxin B1 Content
3.3. Natural and Artificial Resistance Tests
3.4. Relationship between Yield and Aflatoxin B1 Concentration
4. Conclusions
5. Materials and Methods
5.1. Selection of A. flavus Isolates
5.2. Examination of Toxigenic Potential of A. flavus Isolates
5.3. Inoculum Preparation
5.4. Experimental Design and Artificial Inoculation of Maize Ears
5.5. Visual Evaluation of Disease
5.6. Harvest and Sampling
5.7. Aflatoxin B1 Measurement
5.8. Meteorological Conditions
5.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tandzi, L.N.; Mutengwa, C.S. Estimation of maize (Zea mays L.) yield per harvest area: Appropriate methods. Agronomy 2020, 10, 29. [Google Scholar] [CrossRef]
- Jocković, Đ.; Stojaković, M.; Ivanović, M.; Bekavac, G.; Popov, R.; Đalović, I. NS maize hybrids—Today and tomorrow. Ratar. Povrt. 2010, 47, 325–333. [Google Scholar]
- Liliane, T.N.; Charles, M.S. Factors Affecting Yield of Crops. In Agronomy—Climate Change & Food Security; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Obradović, A.; Krnjaja, V.; Nikolić, M.G.; Delibašić, G.; Filipović, M.; Stankovic, G.; Stankovic, S. Impacts of climatic conditions on aflatoxin B1 and fumonisins contamination of maize kernels and their co-occurrence. Biotechnol. Anim. Husb. 2018, 34, 469–480. [Google Scholar] [CrossRef]
- Lević, J.; Gošić-Dondo, S.; Ivanović, D.; Stanković, S.; Krnjaja, V.; Bočarov-Stančić, A.; Stepanić, A. An outbreak of Aspergillus species in Response to environmental conditions in Serbia. J. Pestic. Phytomedicine 2013, 28, 167–179. [Google Scholar] [CrossRef]
- Warburton, M.L.; Williams, W.P.; Windham, G.L.; Murray, S.C.; Xu, W.; Hawkins, L.K.; Duran, J.F. Phenotypic and genetic characterization of a maize association mapping panel developed for the identification of new sources of resistance to Aspergillus flavus and aflatoxin accumulation. Crop Sci. 2013, 53, 2374–2383. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Monograph on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82, pp. 171–300. [Google Scholar]
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu. Rev. Public Health 2008, 29, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of Aflatoxin-induced hepatocellular carcinoma. A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Peles, F.; Sipos, P.; Gyori, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef]
- Magan, N. Fungi in extreme environments. Chapter 6; In Environmental and Microbial Relationships, 2nd ed.; MYCOTA, I.V., Kubicek, C.P., Druzhinina, I.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 85–103. [Google Scholar]
- Abbas, H.K.; Williams, W.P.; Windham, G.L.; Pringle, H.C.; Xie, W.; Shier, W.T. Aflatoxin and fumonisin contamination of commercial corn (Zea mays) hybrids in Mississippi. J. Agric. Food Chem. 2002, 50, 5246–5254. [Google Scholar] [CrossRef]
- Moretti, A.; Logrieco, A.; Visconti, A.; Bottalico, A. An overview of mycotoxins and toxigenic fungi in Italy. In An Overview on Toxigenic Fungi and Mycotoxins in Europe; Springer: Berlin/Heidelberg, Germany, 2004; pp. 141–160. [Google Scholar]
- Battilani, P.; Formenti, S.; Ramponi, C.; Rossi, V. Dynamics of water activity in maize hybrids is crucial for fumonisin contamination in kernels. J. Cereal Chem. 2009, 54, 467–472. [Google Scholar] [CrossRef]
- Bruns, H.A. Controlling Aflatoxin and Fumonisin in Maize by Crop Management. J. Toxicol. 2003, 22, 153–173. [Google Scholar] [CrossRef]
- Widstrom, N.W.; Guo, B.Z.; Wilson, D.M. Integration of Crop Management and Genetics for Control of Preharvest Aflatoxin Contamination of Corn. J. Toxicol. 2003, 22, 195–223. [Google Scholar] [CrossRef]
- Munkvold, G. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Daves, C.A.; Windham, G.L.; Williams, W.P. Aflatoxin Accumulation in commercial corn Hybrids Artificially Inoculated with Aspergillus flavus in 2008 and 2009. Mississipi Agricultural and Forestry Experiment Station Research Report. 2010, 24, 6. [Google Scholar]
- Cary, J.W.; Rajasekaran, K.; Brown, R.L.; Luo, M.; Chen, Z.-Y.; Bhatnagar, D. Developing resistance to aflatoxin in maize and cottonseed. Toxins 2011, 3, 678–696. [Google Scholar] [CrossRef]
- Warburton, M.L.; Tang, J.D.; Windham, G.L.; Hawkins, L.K.; Murray, S.C.; Xu, W.; Boykin, D.; Perkins, A.; Williams, W.P. Genome-Wide Association Mapping of Aspergillus flavus and aflatoxin accumulation resistance in maize. Crop Sci. 2015, 55, 1857–1867. [Google Scholar] [CrossRef]
- Wahl, N.; Murray, S.C.; Isakeit, T.; Krakowsky, M.; Windham, G.L.; Williams, W.P.; Guo, B.; Ni, N.; Knoll, J.; Xu, W.; et al. Identification of resistance to aflatoxin accumulation and yield potential in maize hybrids in the Southeast Regional Aflatoxin Trials (SERAT). Crop Sci. 2016, 57, 202–215. [Google Scholar] [CrossRef]
- Windham, G.L.; Williams, W.P.; Mylroie, J.E.; Reid, C.X.; Womack, E.D. A Histological Study of Aspergillus flavus Colonization of Wound Inoculated Maize Kernels of Resistant and Susceptible Maize Hybrids in the Field. Front. Microbiol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Mideros, S.X.; Warburton, M.L.; Jamann, T.M.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Quantitative trait loci for resistance to Aspergillus ear rot: Analysis by linkage mapping, characterization of near-isogenic lines and meta-analysis. Crop Sci. 2013, 54, 127–142. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Rajasekaran, K.; Brown, R.L.; Sayler, R.J.; Bhatnagar, D. Discovery and confirmation of genes/proteins associated with maize aflatoxin resistance. World Mycotoxin J. 2015, 8, 211–224. [Google Scholar] [CrossRef]
- Brown, R.L.; Chen, Z.Y.; Menkir, A.; Cleveland, T.E.; Cardwell, K.; Kling, J.; White, D.G. Resistance to aflatoxin accumulation in kernels of maize inbreds selected for ear rot resistance in west and central Africa. J. Food Prot. 2001, 64, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Mascagni, J.r.H.J.; Bruns, H.A.; Shier, W.T.; Damann, K.E. Effect of planting density, irrigation regimes, and maize hybrids with varying ear size on yield, and aflatoxin and fumonisin contamination levels. Am. J. Plant Sci. 2012, 3, 1341–1354. [Google Scholar] [CrossRef]
- Farfan, I.D.B.; De La Fuente, G.N.; Murray, S.C.; Isakeit, T.; Huang, P.-C.; Warburton, M.; Williams, P.; Windham, G.L.; Kolomiets, M. Genome Wide Association Study for Drought, Aflatoxin Resistance, and Important Agronomic Traits of Maize Hybrids in the Sub-Tropics. PLoS ONE. 2015, 10, e0117737. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P.; Windham, G.l.; Buckley, P.M.; Perkins, J.M. Southwestern corn borer damage and aflatoxin accumulation in conventional and transgenic corn hybrids. Field Crops Res. 2005, 91, 329–336. [Google Scholar] [CrossRef]
- Abbas, H.K.; Cartwright, R.D.; Xie, W.; Shier, W.T. Aflatoxin and fumonisins contamination of corn hybrids in Arkansas. Crop Prot. 2006, 25, 1–9. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef]
- Warburton, M.L.; Williams, W.P. Aflatoxin resistance in maize: What have we learned lately? Adv. Bot. 2014, 2014, 352831. [Google Scholar] [CrossRef]
- Callicott, K.A.; Cotty, P.J. Method for monitoring deletions in the aflatoxin biosynthesis gene cluster of Aspergillus flavus with multiplex PCR. Lett. Appl. Microbiol. 2014, 60, 60–65. [Google Scholar] [CrossRef]
- Budakov, D. Faculty of Agriculture, Novi Sad, Serbia. 2016; Unpublished work. [Google Scholar]
- Brown, R.L.; Williams, W.P.; Windham, G.L.; Menkir, A.; Chen, Z.Y. Evaluation of African-bred maize germplasm lines for resistance to aflatoxin accumulation. Agronomy 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Campbell, K.W.; White, D.G. Evaluation of corn genotypes for resistance to Aspergillus ear rot, kernel infection, and aflatoxin production. Plant Dis. 1995, 79, 1039–1045. [Google Scholar] [CrossRef]
- Henry, W.B.; Williams, W.P.; Windham, G.L.; Hawkins, L.K. Evaluation of maize inbred lines for resistance to Aspergillus and Fusarium ear rot and mycotoxin accumulation. Agron. J. 2009, 101, 1219–1226. [Google Scholar] [CrossRef]
- Walker, R.D.; White, D.G. Inheritance of resistance to Aspergillus ear rot and aflatoxin production of corn from CI2. Plant Dis. 2001, 85, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Chiuraise, N.; Derera, J.; Yobo, K.S.; Magorokosho, C.; Nunkumar, A.; Qwabe, N.F.P. Progress in stacking aflatoxin and fumonisin contamination resistance genes in maize hybrids. Euphytica 2016, 207, 49–67. [Google Scholar] [CrossRef]
- Tengey, T.K.; Kankam, F.; Ndela, D.N.; Frempong, D.; Appaw, W.O. Growth and Toxigenicity of A. flavus on Resistant and Susceptible Peanut Genotypes. Toxins 2022, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Toth, B.; Toth Toldine, E.; Varga, M.; Kovacs, N.; Varga, J.; Kocsube, S.; Palagyi, A.; Bagi, F.; Budakov, D.; et al. A new concept to secure food safety standards against Fusarium species and Aspergillus flavus and their toxins in maize. Toxins 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Mutiga, S.K.; Morales, L.; Angwenyi, S.; Wainaina, J.; Harvey, J.; Das, B.; Nelson, R.J. Association between agronomic traits and aflatoxin accumulation in diverse maize lines grown under two soil nitrogen levels in Eastern Kenya. Field Crops Res. 2017, 205, 124–134. [Google Scholar] [CrossRef]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Williams, W.P.; Krakowsky, M.D.; Scully, B.T.; Brown, R.L.; Menkir, A.; Warburton, M.L.; Windham, G.L. Identifying and developing maize germplasm with resistance to accumulation of aflatoxins. World Mycotoxin J. 2015, 8, 193–209. [Google Scholar] [CrossRef]
- Zuber, M.S.; Lillehoj, E.B. Status of the aflatoxin problem in corn. J. Environ. Qual. 1979, 8, 1–5. [Google Scholar] [CrossRef]
- Jones, R.K. The influence of cultural practices on minimizing the development of aflatoxin in field maize. In Aflatoxin in Maize. Proceedings of the Workshop; Zuber, M.S., Lillehoj, E.B., Renfro, B.L., Mexico, D.F., Eds.; CIMMYT: Veracruz, Mexico, 1987; pp. 136–144. [Google Scholar]
- Widstrom, N.W.; McMillian, W.W.; Beaver, R.W.; Wilson, D.M. Weather-associated changes in aflatoxin contamination of preharvest maize. J. Prod. Agric. 1990, 3, 196–199. [Google Scholar] [CrossRef]
- Damianidis, D.; Ortiz, B.V.; Bowen, K.L.; Windham, G.L.; Hoogenboom, G.; Hagan, A.; Knappenberger, T.; Abbas, H.K.; Scully, B.T.; Mourtzinis, S. Minimum Temperature, Rainfall, and Agronomic Management Impacts on Corn Grain Aflatoxin Contamination. Agron. J. 2018, 110, 1697–1708. [Google Scholar] [CrossRef]
- Löffler, M.; Miedaner, T.; Kessel, B.; Ouzunova, M. Mycotoxin accumulation and corresponding ear rot rating in three maturity groups of European maize inoculated by two Fusarium species. Euphytica 2010, 174, 153–164. [Google Scholar] [CrossRef]
- Windham, G.L.; Williams, W.P. Evaluation of corn inbreds and advanced breeding lines for resistance to aflatoxin contamination in the field. Plant Dis. 2001, 86, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.H.; Tevathan, L.E.; King, S.B.; Scott, G.E. Effect of four inoculation techniques on infection and aflatoxin concentration of resistant and susceptible corn hybrids inoculated with Aspergillus flavus. Phytopathology 1986, 76, 290–293. [Google Scholar] [CrossRef]
- Windham, G.L.; Williams, W.P.; Buckley, P.M.; Abbas, H.K. Inoculation techniques used to quantify aflatoxin resistance in corn. J. Toxicology 2003, 22, 313–325. [Google Scholar] [CrossRef]
- Božović, D.; Popović, D.; Popović, V.; Živanović, T.; Ljubičić, N.; Ćosić, M.; Spahić, A.; Simić, D.; Filipović, V. Economical Productivity of Maize Genotypes under Different Herbicides Application in Two Contrasting Climatic Conditions. Sustainability 2022, 14, 5629. [Google Scholar] [CrossRef]
- Mangini, G.; Gadaleta, A.; Colasuonno, P.; Marcotuli, I.; Signorile, A.M.; Simeone, R.; De Vita, P.; Mastrangelo, A.M.; Laidò, G.; Pecchioni, N.; et al. Genetic dissection of the relationships between grain yield components by genome-wide association mapping in a collection of tetraploid wheats. PLoS ONE 2018, 13, e0190162. [Google Scholar] [CrossRef]
- Betran, F.J.; Bhatnagar, S.; Isakeit, T.; Odvody, G.; Mayfield, K. Aflatoxin accumulation and associated traits in QPM maize inbreds and their testcrosses. Euphytica 2006, 152, 247–257. [Google Scholar] [CrossRef]
- Barošević, T.; Bagi, F.; Budakov, D.; Kocsubé, S.; Varga, J.; Grahovac, M.; Stojšin, V. Molecular and morphological identification of Aspergillus species on corn seeds. In Proceedings of the III International Congress Food Technology, Quality and Safety, Novi Sad, Serbia, 25–27 October 2016; University of Novi Sad, Institute of Food Technology: Novi Sad, Serbia, 2016; pp. 365–371. [Google Scholar]
- Reid, L.M.; Hamilton, R.E.; Mather, D.E. Screening Maize for Resistance to Gibberella Ear Rot; Technical Bulletin, Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1996; p. 62. [Google Scholar]
| Maize Hybrids | 2016 | 2017 I | 2017 II | 2018 I | 2018 II |
|---|---|---|---|---|---|
| C 25 | 1.733333 | 1.366667 | 1.33333333 | 1.866667 | 1.500000 |
| C 46 | 2.233333 | 1.433333 | 1.3 | 1.433333 | 1.233333 |
| C 39 | 2.033333 | 1.433333 | 1.5 | 1.566667 | 1.366667 |
| B 1 | 1.233333 | 1.466667 | 1.43333333 | 1.433333 | 1.366667 |
| B 10 | 1.600000 | 1.566667 | 1.5 | 1.833333 | 1.300000 |
| A 41 | 2.050000 | 1.566667 | 1.5 | 1.933333 | 1.566667 |
| A 15 | 1.650000 | 1.600000 | 1.33333333 | 1.366667 | 1.633333 |
| C 50 | 2.533333 | 1.633333 | 1.53333333 | 1.733333 | 1.466667 |
| B 11 | 1.633333 | 1.633333 | 1.5 | 1.366667 | 1.100000 |
| A 19 | 1.675000 | 1.700000 | 1.5 | 1.633333 | 1.500000 |
| C 3 | 1.500000 | 1.966667 | 1.96666667 | 1.766667 | 1.633333 |
| B 4 | 1.500000 | 1.966667 | 1.66666667 | 1.466667 | 1.633333 |
| C 47 | 2.233333 | 2.033333 | 2 | 1.933333 | 2.000000 |
| C 23 | 1.700000 | 2.033333 | 1.93333333 | 1.633333 | 1.866667 |
| D 26 | 1.733333 | 2.100000 | 2 | 1.466667 | 1.566667 |
| E 49 | 2.300000 | 2.266667 | 2 | 2.000000 | 1.966667 |
| D 32 | 1.900000 | 2.266667 | 2.16666667 | 1.600000 | 1.733333 |
| E 45 | 2.100000 | 2.300000 | 2.2 | 1.600000 | 1.700000 |
| C 16 | 1.666667 | 2.333333 | 1.93333333 | 1.700000 | 1.633333 |
| C 12 | 1.633333 | 2.333333 | 2.1 | 2.200000 | 1.766667 |
| Mean | 1.832 | 1.850 | 1.720 | 1.677 | 1.577 |
| Median | 1.717 | 1.833 | 1.600 | 1.633 | 1.600 |
| Standard deviation | 0.327 | 0.343 | 0.308 | 0.230 | 0.234 |
| Rank | 1.300 | 0.967 | 0.900 | 0.833 | 0.900 |
| Minimum | 1.233 | 1.367 | 1.300 | 1.367 | 1.100 |
| Maximum | 2.533 | 2.333 | 2.200 | 2.200 | 2.000 |
| Growing Seasons | |||||
|---|---|---|---|---|---|
| Maize Hybrid | 2016 | 2017 I | 2017 II | 2018 I | 2018 II |
| C 25 | 0 | 233.95 | 53.6175 | 1476.9125 | 559.3425 |
| C 46 | 2.48 | 161.9825 | 143.9425 | 930.215 | 493.68 |
| C 39 | 4.85 | 184.375 | 74.205 | 1107.78 | 1286.35 |
| B 1 | 8.08 | 333.7925 | 51.41 | 1648.055 | 809.14 |
| B 10 | 7.82 | 124.08 | 55.6975 | 1476.325 | 1205.1325 |
| A 41 | 500 | 228.4925 | 40.0525 | 2111.61 | 1979.7575 |
| A 15 | 28.99 | 179.51 | 107.3 | 853.2225 | 947.785 |
| C 50 | 0 | 146.0575 | 35.582 | 1027.83 | 757.095 |
| B 11 | 9.1 | 327.4225 | 115.635 | 1047.49 | 1604.42 |
| A 19 | 25.6 | 323.5975 | 410.1375 | 1902.5925 | 1703.31 |
| C 3 | 11.71 | 113.795 | 351.475 | 364.99 | 1533.66 |
| B 4 | 16.29 | 37.56 | 140.285 | 442.5625 | 1554.66 |
| C 47 | 2.28 | 238.665 | 276.39 | 509.4825 | 910.3525 |
| C 23 | 4.76 | 216.655 | 240.7575 | 1026.67 | 1096.56 |
| D 26 | 1.22 | 101.245 | 215.42 | 719.6175 | 1043.0775 |
| E 49 | 1.69 | 174.1225 | 194.2375 | 582.4 | 1626.24 |
| D 32 | 0 | 252.1225 | 216.74 | 838.0125 | 2143.7625 |
| E 45 | 1.31 | 168.5925 | 143.6325 | 1350.485 | 1910.97 |
| C 16 | 8.66 | 207.3725 | 270.575 | 925.6625 | 1200.4325 |
| C 12 | 7.17 | 331.885 | 387.9375 | 1269.0225 | 1089.01 |
| Mean | 32.101 | 204.264 | 176.252 | 1080.547 | 1272.737 |
| Median | 6.010 | 195.874 | 143.787 | 1027.250 | 1202.782 |
| Standard deviation | 110.427 | 82.386 | 117.549 | 474.133 | 469.879 |
| Rank | 500.000 | 296.232 | 374.556 | 1746.620 | 1650.082 |
| Minimum | 0.000 | 37.560 | 35.582 | 364.990 | 493.680 |
| Maximum | 500.000 | 333.792 | 410.138 | 2111.610 | 2143.762 |
| Examination Period | Correlations | Infection Intensity | Yield | AFB1 |
|---|---|---|---|---|
| 2016 | Infection intensity | 1.000 | 0.787 ** 0.000 | −0.588 ** 0.006 |
| Yield | 0.787 ** 0.000 | 1.000 | −0.736 ** 0.000 | |
| AFB1 | −0.588 ** 0.006 | −0.736 ** 0.000 | 1.000 | |
| 2017 I | Infection intensity | 1.000 | 0.442 0.051 | −0.002 0.992 |
| Yield | 0.442 0.051 | 1.000 | 0.074 0.755 | |
| AFB1 | −0.002 0.992 | 0.074 0.755 | 1.000 | |
| 2017 II | Infection intensity | 1.000 | 0.692 ** 0.001 | 0.548 * 0.012 |
| Yield | 0.692 ** 0.001 | 1.000 | 0.225 0.340 | |
| AFB1 | 0.548 * 0.012 | 0.225 0.340 | 1.000 | |
| 2018 I | Infection intensity | 1.000 | 0.302 0.196 | 0.081 0.733 |
| Yield | 0.302 0.196 | 1.000 | 0.329 0.157 | |
| AFB1 | 0.081 0.733 | 0.329 0.157 | 1.000 | |
| 2018 II | Infection intensity | 1.000 | 0.086 0.718 | 0.233 0.323 |
| Yield | 0.086 0.718 | 1.000 | 0.140 0.556 | |
| AFB1 | 0.233 0.323 | 0.140 0.556 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barošević, T.; Bagi, F.; Savić, Z.; Ljubičić, N.; Ivanović, I. Assessment of Maize Hybrids Resistance to Aspergillus Ear Rot and Aflatoxin Production in Environmental Conditions in Serbia. Toxins 2022, 14, 887. https://doi.org/10.3390/toxins14120887
Barošević T, Bagi F, Savić Z, Ljubičić N, Ivanović I. Assessment of Maize Hybrids Resistance to Aspergillus Ear Rot and Aflatoxin Production in Environmental Conditions in Serbia. Toxins. 2022; 14(12):887. https://doi.org/10.3390/toxins14120887
Chicago/Turabian StyleBarošević, Tijana, Ferenc Bagi, Zagorka Savić, Nataša Ljubičić, and Ivana Ivanović. 2022. "Assessment of Maize Hybrids Resistance to Aspergillus Ear Rot and Aflatoxin Production in Environmental Conditions in Serbia" Toxins 14, no. 12: 887. https://doi.org/10.3390/toxins14120887
APA StyleBarošević, T., Bagi, F., Savić, Z., Ljubičić, N., & Ivanović, I. (2022). Assessment of Maize Hybrids Resistance to Aspergillus Ear Rot and Aflatoxin Production in Environmental Conditions in Serbia. Toxins, 14(12), 887. https://doi.org/10.3390/toxins14120887







