Twenty-Five Years of PSP Toxicity in Galician (NW Spain) Bivalves: Spatial, Temporal, and Interspecific Variations
Abstract
1. Introduction
2. Results
2.1. General
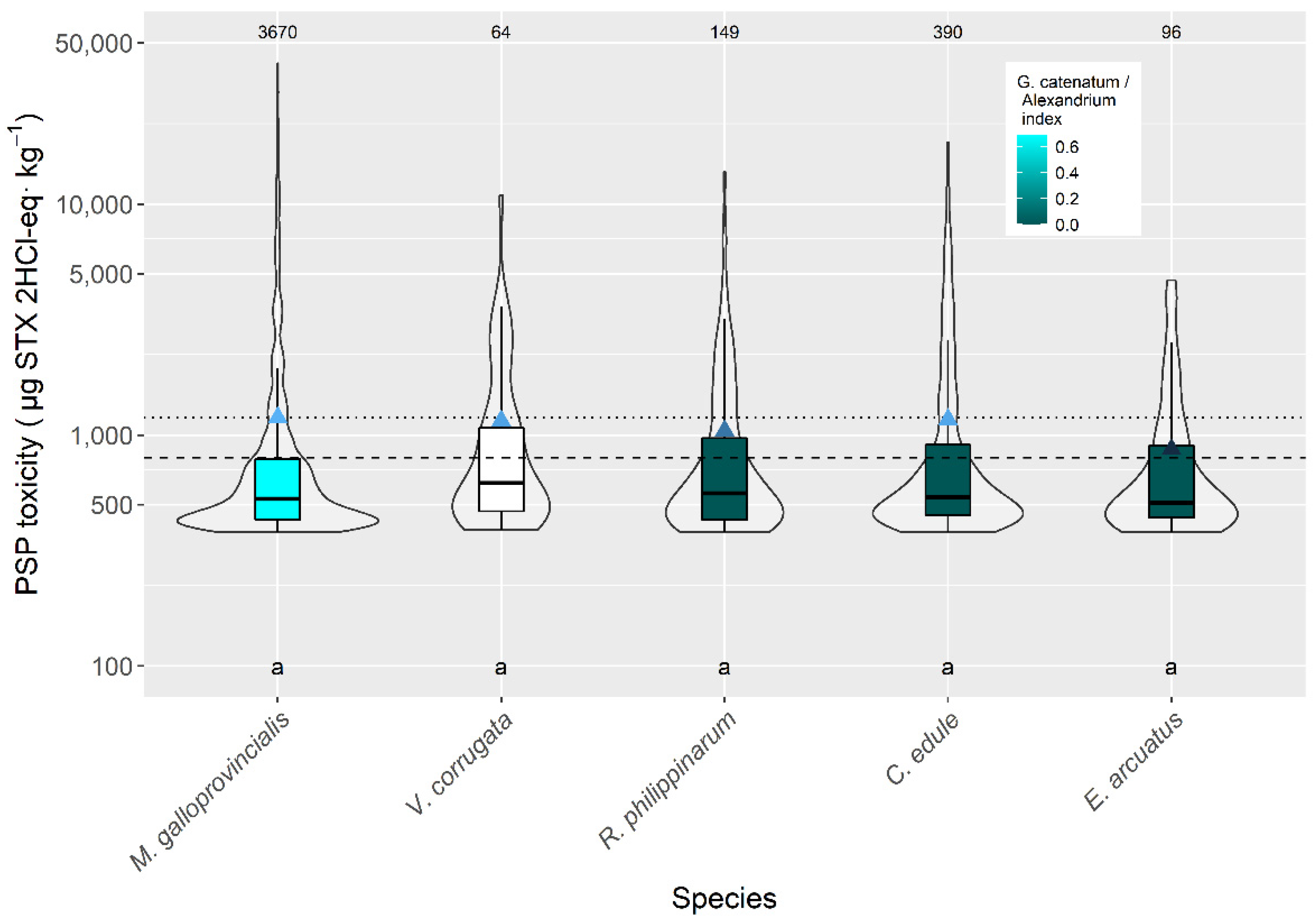
2.2. Interspecific Variation
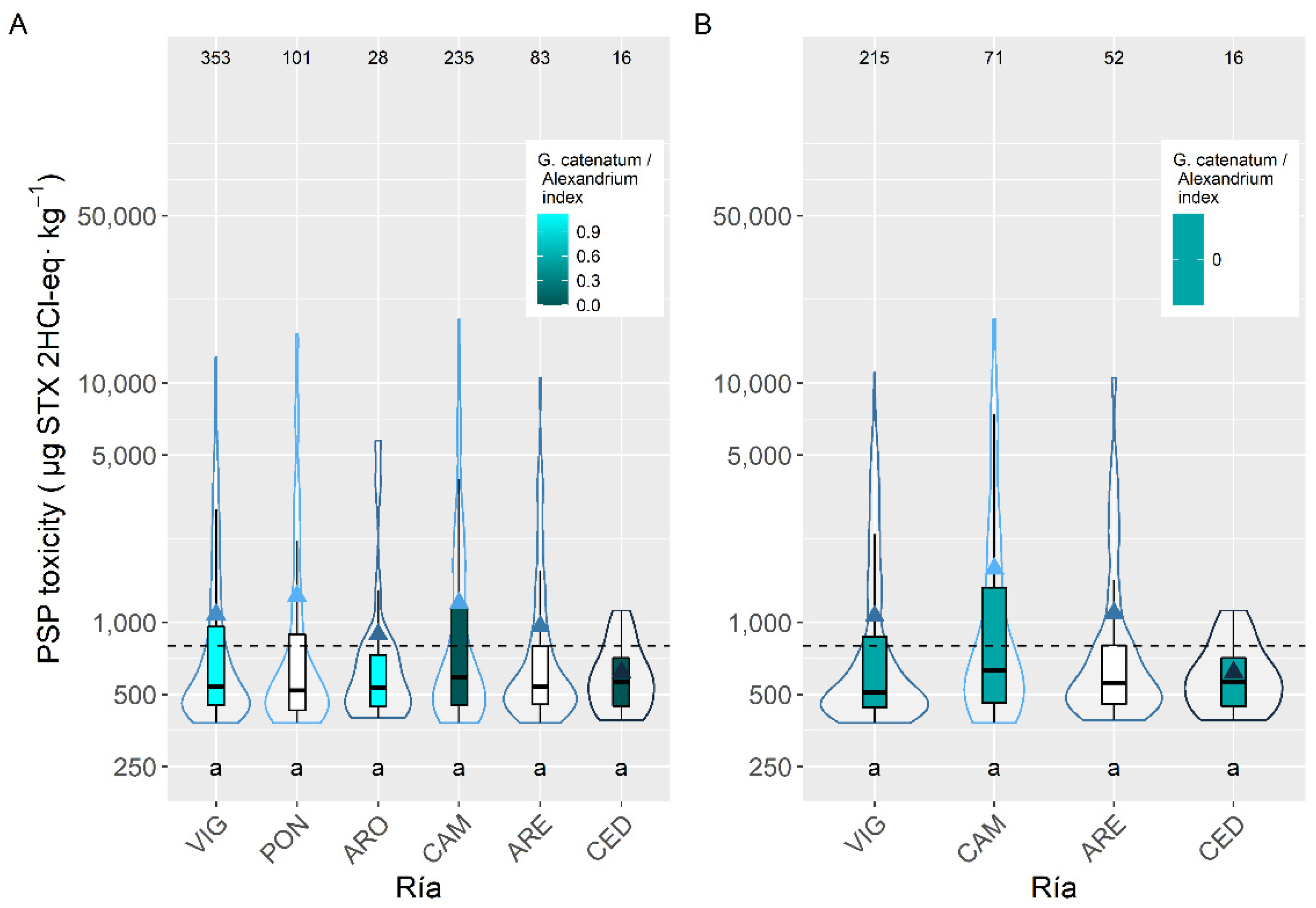
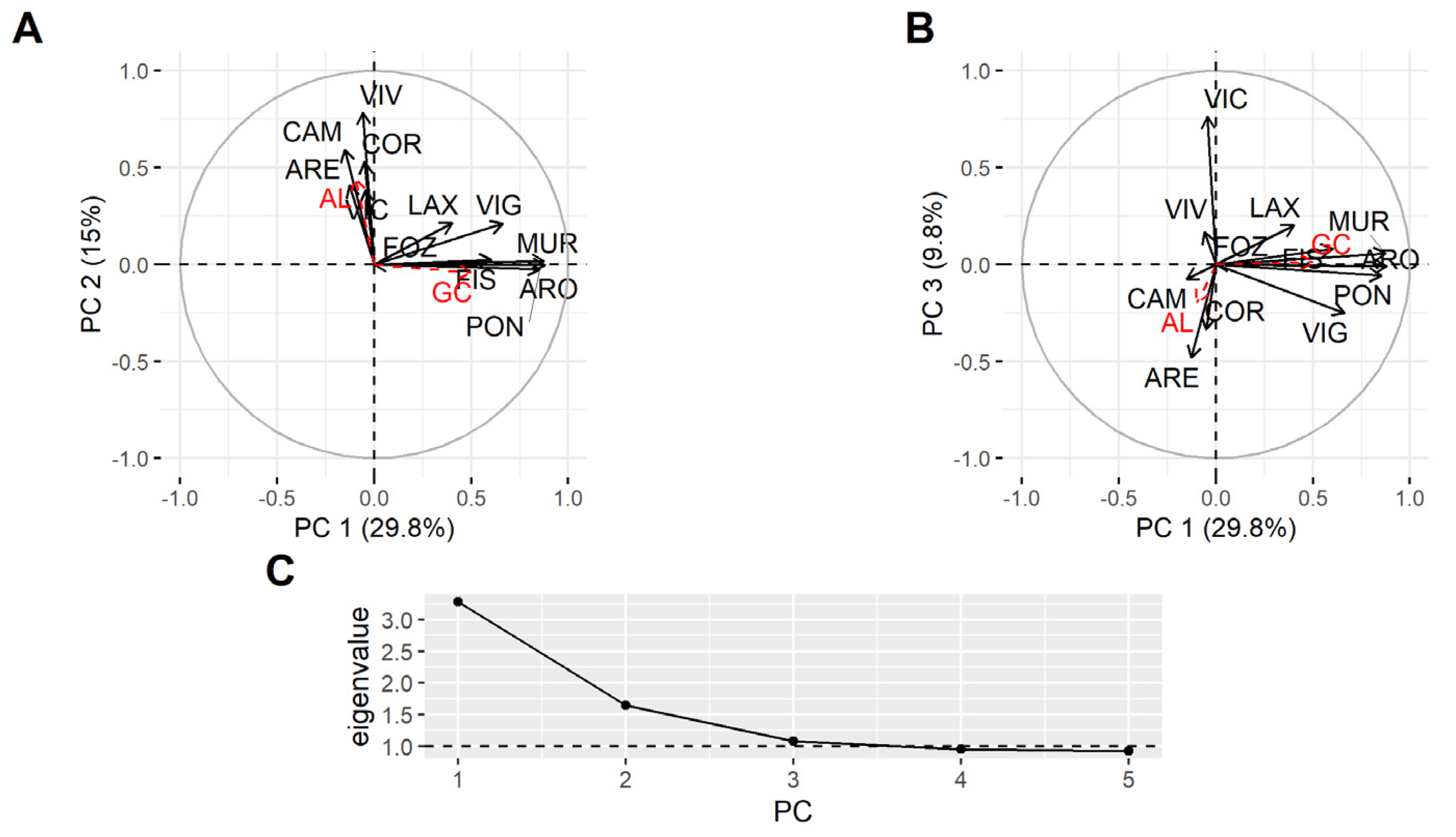
2.3. Spatial Variation
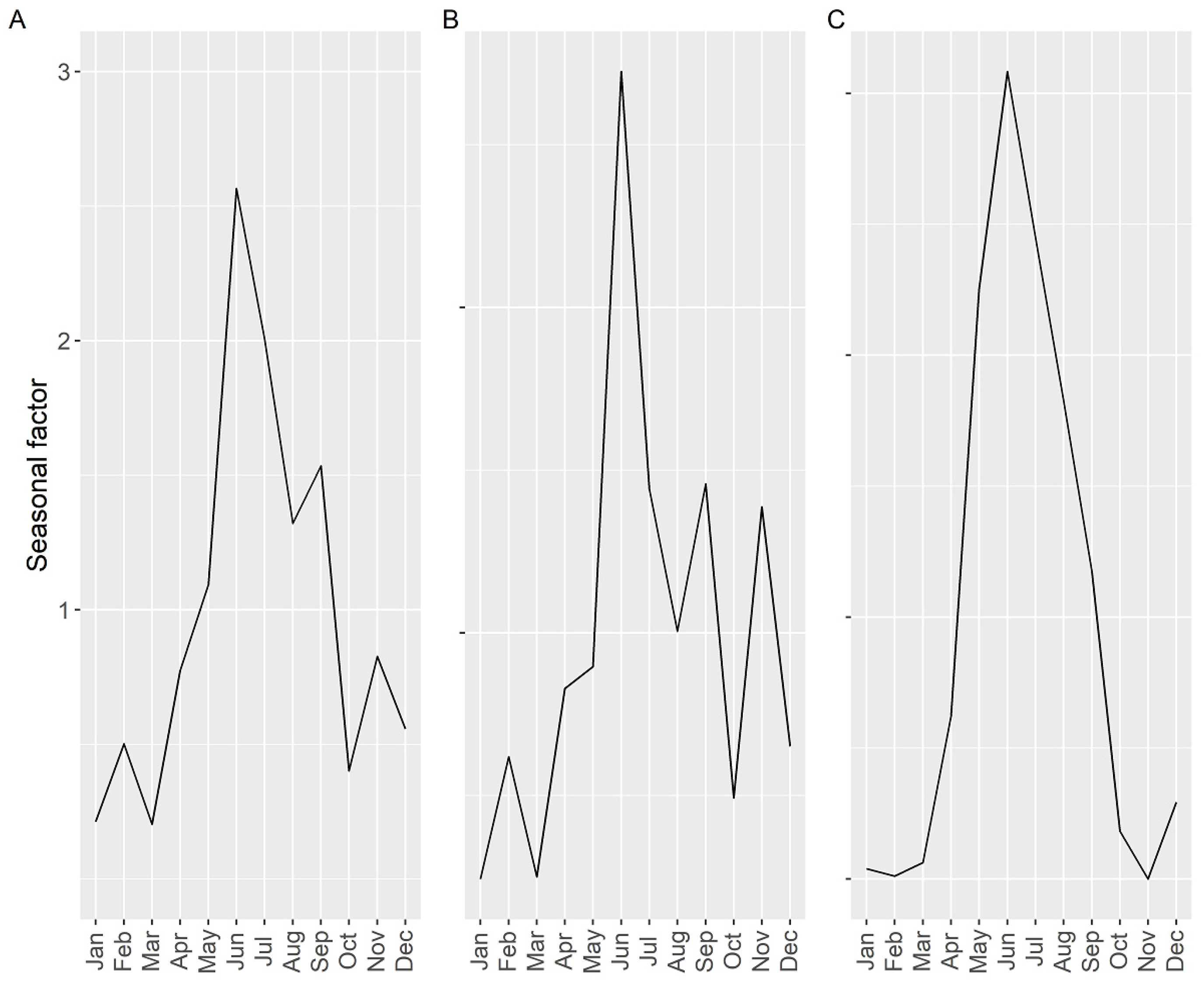
2.4. Seasonality
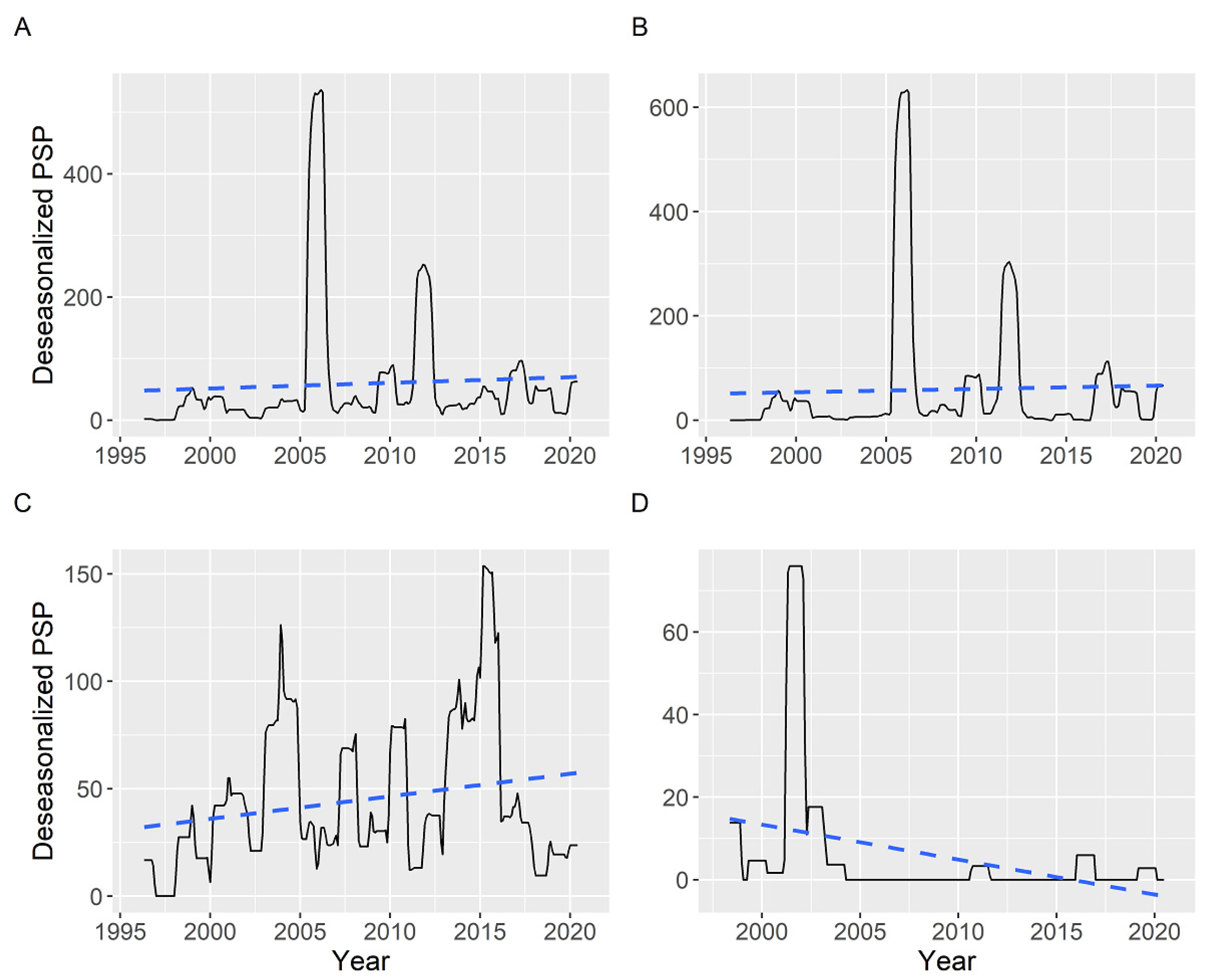
2.5. Time Course of the PSP Toxicity in Different Locations
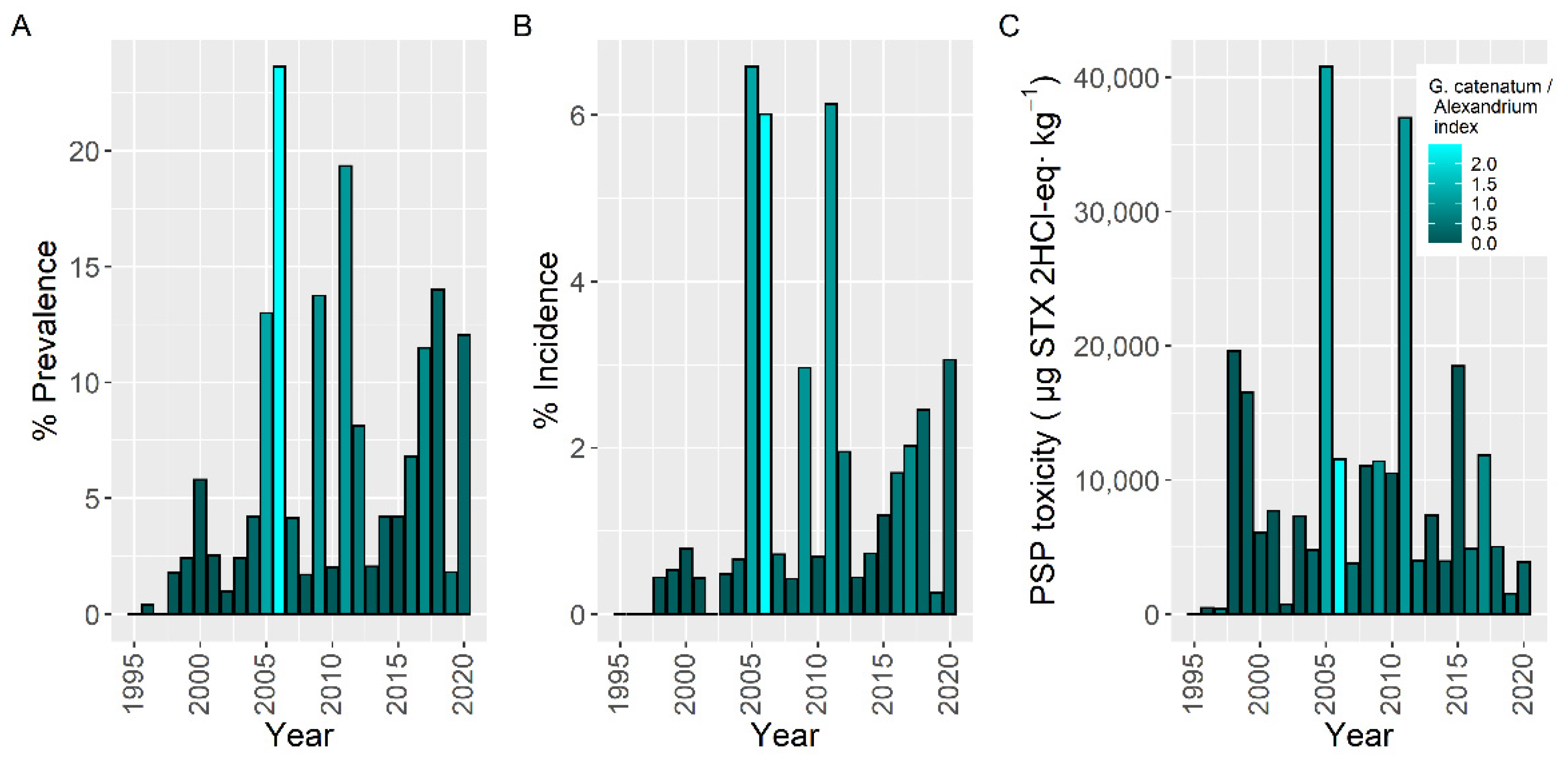
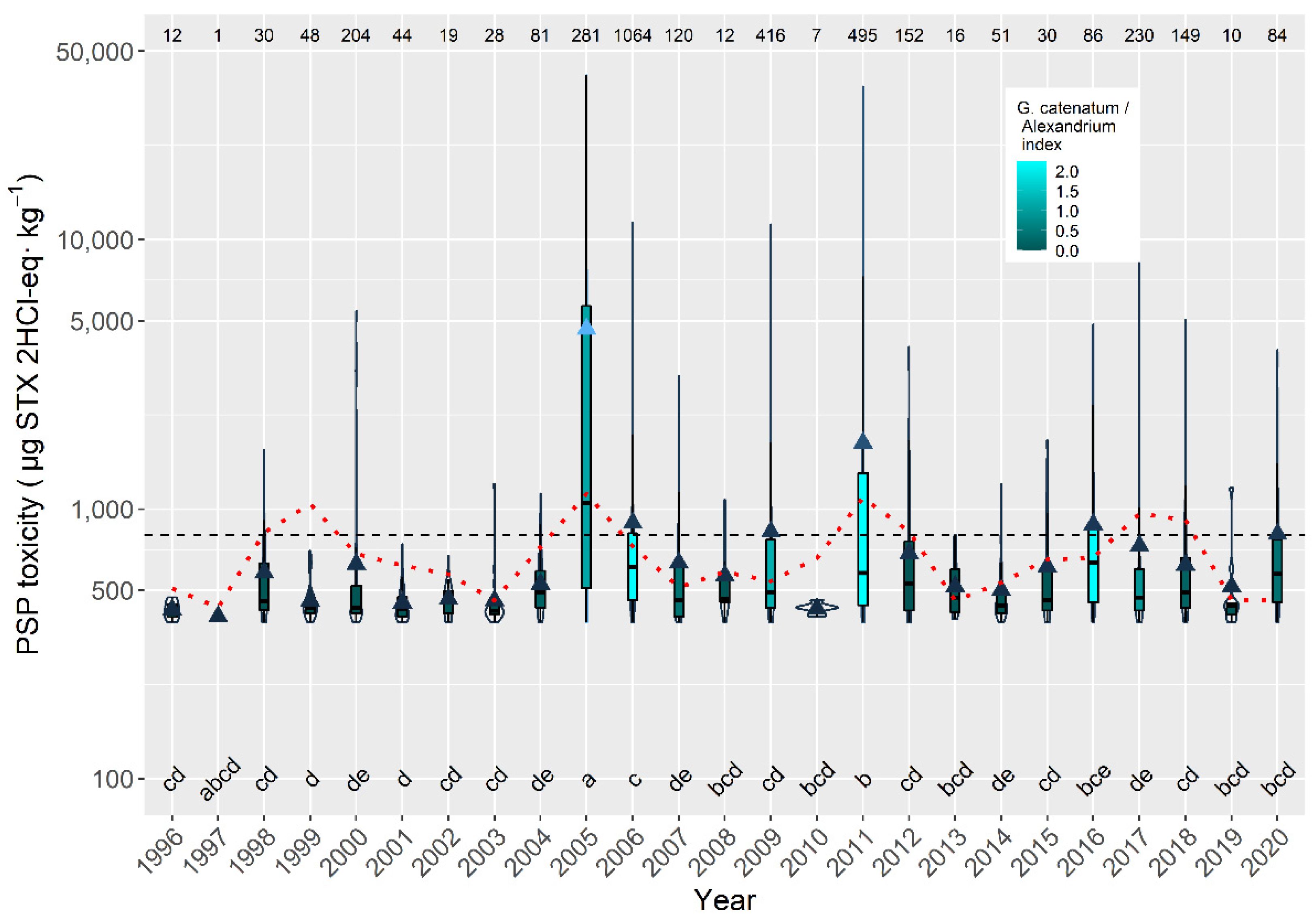
2.6. Interannual Differences
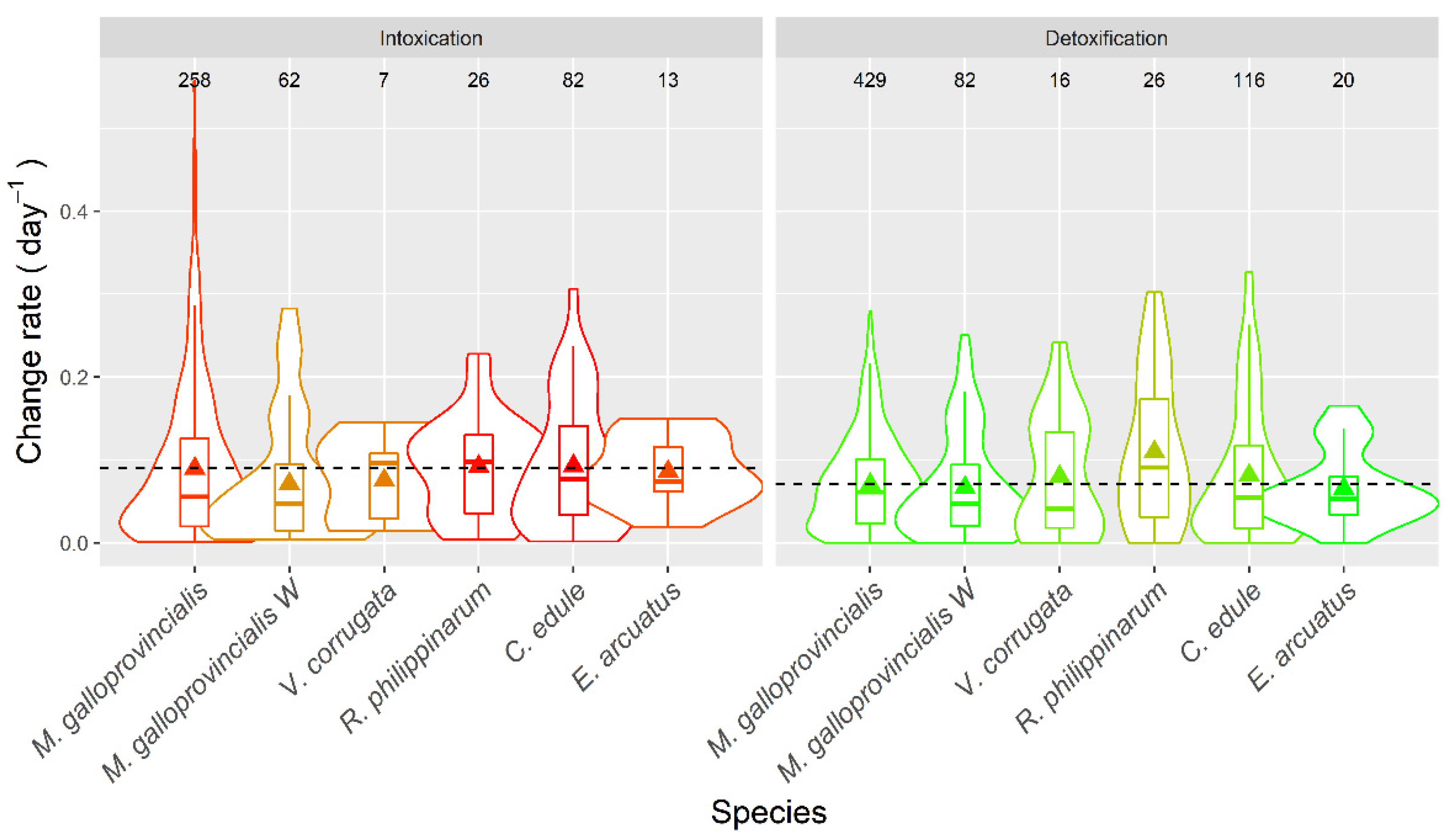
2.7. Apparent Intoxication and Detoxification
3. Discussion
3.1. General
3.2. Interspecific Variation
3.3. Spatial Variation
3.4. Seasonality and Timing of the Episodes
3.5. Interannual Variability
3.6. Intoxication and Detoxification Rate
4. Conclusions
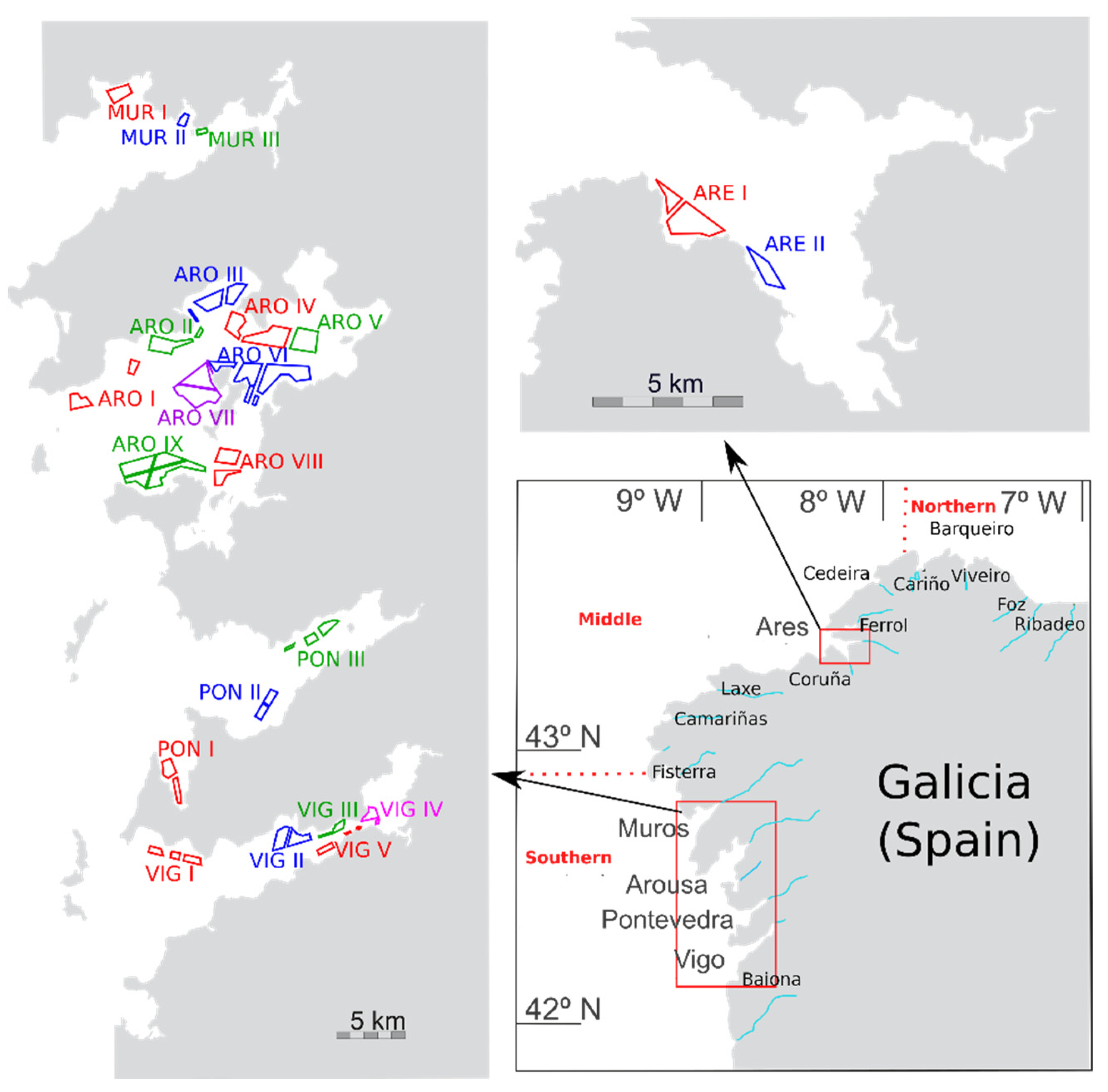
5. Materials and Methods
5.1. Sampling
5.2. Reagents and Reference Solutions
5.3. Toxin Extraction
5.4. PSP Toxicity Quantification
5.5. Data Processing
5.5.1. Monitoring PSP Toxicity in Bivalves
5.5.2. Characteristics of the Toxic Episodes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AOAC. Paralytic shellfish poison. Biological methods. Final action. In Official Methods of Analysis, 15th ed.; Hellrich, K., Ed.; Association of Official Analytic Chemists: Arlington, VA, USA, 1990; pp. 881–882. [Google Scholar]
- McFarren, E.F. Report on Collaborative Studies of the Bioassay for Paralytic Shellfish Poison. J. AOAC Int. 1959, 42, 263–271. [Google Scholar] [CrossRef]
- Sommer, H.; Meyer, K.F. Paralytic shellfish poisoning. Arch. Pathol. 1937, 24, 560–598. [Google Scholar]
- Australian Government. Australia New Zealand Food Standards Code—Schedule 19—Maximum Levels of Contaminants and Natural Toxicants. Available online: https://www.legislation.gov.au/Details/F2021C00628/Download (accessed on 25 October 2022).
- Codex Alimentarius. CXS 292-2008 Standard for Live and Raw Bivalve Molluscs. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 25 October 2022).
- Health Canada. Health Canada’s Maximum Levels for Chemical Contaminants in Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html#shr-pg0 (accessed on 25 October 2022).
- EU. Commission Delegated Regulation (EU) 2021/1374 of 12 April 2021 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council on specific hygiene requirements for food of animal origin. Off. J. Eur. Union 2021, 64, 1–15. [Google Scholar]
- Pescadegalicia. Estatísticas Publicacións. Available online: https://www.pescadegalicia.gal/gl/publicacions (accessed on 24 January 2022).
- Gestal Otero, J.J.; Hernández Cochón, J.M.; Bao Fernández, O.; Martínez-Risco López, L. Brote de mitilotoxismo en la provincia de La Coruña. Boletín Inst. Español Oceanogr. 1978, 4, 3–29. [Google Scholar]
- Luthy, J. Epidemic paralytic shellfish poisoning in western Europe, 1976. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier/North Holland: New York, NY, USA, 1979; pp. 15–22. [Google Scholar]
- Campos, M.J.; Fraga, S.; Mariño, J.; Sánchez, F.J. Red tide monitoring program on NW Spain. Report of 1977–1981. ICES. Biological Oceanography Committee. Ref. Marine Environmental Quality Committee, 1982; C.M. 1982/L:1927. [Google Scholar]
- Bricelj, V.M.; Shumway, S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Asakawa, M.; Beppu, R.; Ito, K.; Tsubota, M.; Takayama, H.; Miyazawa, K. Accumulation of paralytic shellfish poison (PSP) and biotransformation of its components in oysters Crassostrea gigas fed with the toxic dinoflagellate Alexandrium tamarense. J. Food Hyg. Soc. Jpn. 2006, 47, A28–A32. [Google Scholar] [CrossRef] [PubMed]
- Silvert, W.; Bricelj, M.; Cembella, A. Dynamic modelling of PSP toxicity in the surfclam (Spisula solidisssima): Multicompartmental kinetics and biotransformation. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and IOC of UNESCO: Santiago de Compostela, Spain, 1998; pp. 437–440. [Google Scholar]
- Sullivan, J.J.; Iwaoka, W.T.; Liston, J. Enzymatic Transformation of PSP toxins in the littleneck clam (Protothaca staminea). Biochem. Biophys. Res. Commun. 1983, 114, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.M.; Marsden, I.D.; Munro, M.H.G. Physiological Effects And Biotransformation Of PSP Toxins in the New Zealand Scallop, Pecten novaezelandiae. J. Shellfish. Res. 2012, 31, 1151–1159. [Google Scholar] [CrossRef]
- Raposo, M.I.C.; Gomes, M.; Botelho, M.J.; Rudnitskaya, A. Paralytic Shellfish Toxins (PST)-Transforming Enzymes: A Review. Toxins 2020, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.J.; Marques, F.; Freitas, R.; Pires, A.; Pereira, E.; Vale, C. Paralytic shellfish toxin profiles in mussel, cockle and razor shell under post-bloom natural conditions: Evidence of higher biotransformation in razor shells and cockles. Mar. Environ. Res. 2020, 154, 104839. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Reyero, M.I.; Franco, J.M. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis. Toxicon 2003, 42, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Cembella, A.D.; Ross, N.W. In Vitro Transformation of Paralytic Shellfish Toxins in the Clams Mya arenaria and Protothaca staminea. Harmful Algae 2006, 5, 79–90. [Google Scholar] [CrossRef]
- Oshima, Y. Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms. In Harmful Marine Algal Bloom; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Technique et Documentation-Lavoisier, Intercept Ltd.: Paris, France, 1993; pp. 475–480. [Google Scholar]
- Sato, S.; Kodama, M. Chemical and biological transformation of paralytic shellfish poisoning toxins. In Recent Advances in Marine Biotechnology, Volume 8: Bioremediation; Fingerman, M., Nagabhushanam, R., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2003; pp. 319–336. [Google Scholar]
- Marsden, I.D.; Contreras, A.M.; MacKenzie, L.; Munro, M.H.G. A comparison of the physiological responses, behaviour and biotransformation of paralytic shellfish poisoning toxins in a surf-clam (Paphies donacina) and the green-lipped mussel (Perna canaliculus). Mar. Freshw. Res. 2016, 67, 1163–1174. [Google Scholar] [CrossRef]
- Medina-Elizalde, J.; García-Mendoza, E.; Turner, A.D.; Sánchez-Bravo, Y.A.; Murillo-Martínez, R. Transformation and Depuration of Paralytic Shellfish Toxins in the Geoduck Clam Panopea globosa From the Northern Gulf of California. Front. Mar. Sci. 2018, 5, 335. [Google Scholar] [CrossRef]
- Anderson, D.M. Bloom dynamics of toxic Alexandrium species in the northeastern US. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Cembella, A.D.; Shumway, S.E.; Larocque, R. Sequestering and putative biotransformation of paralytic shellfish toxins by the sea scallop Placopecten magellanicus: Seasonal and spatial scales in natural populations. J. Exp. Mar. Biol. Ecol. 1994, 180, 1–22. [Google Scholar] [CrossRef]
- Brown, J.; Fernand, L.; Horsburgh, K.J.; Hill, A.E.; Read, J.W. Paralytic Shellfish Poisoning on the East Coast of the UK in Relation to Seasonal Density-driven Circulation. J. Plankton Res. 2001, 23, 105–116. [Google Scholar] [CrossRef][Green Version]
- Botelho, M.J.; Vale, C.; Ferreira, J.G. Seasonal and multi-annual trends of bivalve toxicity by PSTs in Portuguese marine waters. Sci. Total Environ. 2019, 664, 1095–1106. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Union L 2019, 131, 51–100. [Google Scholar]
- MacKenzie, A.L. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. N. Z. J. Mar. Freshw. Res. 2014, 48, 430–465. [Google Scholar] [CrossRef]
- IPMA. Available online: https://www.ipma.pt/pt/bivalves/biotox/ (accessed on 21 April 2022).
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Morono, A.; Arevalo, F.; Correa, J.; Salgado, C.; Rossignoli, A.E.; Lamas, J.P. Twenty-Five Years of Domoic Acid Monitoring in Galicia (NW Spain): Spatial, Temporal, and Interspecific Variations. Toxins 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Arevalo, F.; Correa, J.; Morono, A. Lipophilic Toxins in Galicia (NW Spain) between 2014 and 2017: Incidence on the Main Molluscan Species and Analysis of the Monitoring Efficiency. Toxins 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gigirey, B.; Rossignoli, A.E.; Riobo, P.; Rodriguez, F. First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain. Toxins 2020, 12, 723. [Google Scholar] [CrossRef]
- Franco, J.M.; Fernández-Vila, P. Separation of paralytic shelfish toxins by reversed phase high performance liquid chromatography, with postcolumn reaction and fluorimetric detection. Chromatographia 1993, 35, 613–620. [Google Scholar] [CrossRef]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin Profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese Coast, as Determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef]
- Turner, A.D.; Stubbs, B.; Coates, L.; Dhanji-Rapkova, M.; Hatfield, R.G.; Lewis, A.M.; Rowland-Pilgrim, S.; O’Neil, A.; Stubbs, P.; Ross, S.; et al. Variability of paralytic shellfish toxin occurrence and profiles in bivalve molluscs from Great Britain from official control monitoring determined by pre-column oxidation liquid chromatography and implications for applying immunochemical tests. Harmful Algae 2014, 31, 87–99. [Google Scholar] [CrossRef]
- Molinet, C.; Niklitschek, E.; Seguel, M.; Díaz, P. Trends of natural accumulation and detoxification of paralytic shellfish poison in two bivalves from the Northwest Patagonian inland sea. Rev. Biol. Mar. Oceanogr. 2010, 45, 195–204. [Google Scholar] [CrossRef]
- Goya, A.B.; Tarnovius, S.; Hatfield, R.G.; Coates, L.; Lewis, A.M.; Turner, A.D. Paralytic shellfish toxins and associated toxin profiles in bivalve mollusc shellfish from Argentina. Harmful Algae 2020, 99, 101910. [Google Scholar] [CrossRef]
- Intergovernmental Oceanographic Commission of UNESCO. Harmful Algae Event Database (HAEDAT). Available online: http://haedat.iode.org (accessed on 15 May 2022).
- Taleb, H.; Vale, P.; Blaghen, M. Spatial and temporal evolution of PSP toxins along the Atlantic shore of Morocco. Toxicon 2003, 41, 199–205. [Google Scholar] [CrossRef]
- Trainer, V.L.; Eberhart, B.T.L.; Wekell, J.C.; Adams, N.G.; Hanson, L.; Cox, F.; Dowell, J. Paralytic shellfish toxins in Puget Sound, Washington State. J. Shellfish. Res. 2003, 22, 213–223. [Google Scholar]
- Bravo, I.; Fraga, S.; Figueroa, R.I.; Pazos, Y.; Massanet, A.; Ramilo, I. Bloom dynamics and life cycle strategies of two toxic dinoflagellates in a coastal upwelling system (NW Iberian Peninsula). Deep.-Sea Res. Part II-Top. Stud. Oceanogr. 2010, 57, 222–234. [Google Scholar] [CrossRef]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.d.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Artigas, M.; Amorim, A.; Vale, P.; Gomes, S.; Botelho, M.-J.; Rodrigues, S. Prolonged toxicity of Scrobicularia plana after PSP events and its relation to Gymnodinium catenatum cyst consumption and toxin depuration. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; Moestrup, O., Doucette, G., Enevoldsen, H., Godhe, A., Hallegraeff, G., Luckas, B., Lundholm, N., Lewis, J., Rengefors, K., Sellner, K., et al., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2008; pp. 273–275. [Google Scholar]
- Blanco, J. The distribution of dinoflagellate cysts along the Galician (NW Spain) coast. J. Plankton Res. 1995, 17, 283–302. [Google Scholar] [CrossRef]
- Blanco, J.; Mariño, J.; Campos, M.J. The first toxic bloom of Gonyaulax tamarensis detected in Spain (1984). In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; p. 561. [Google Scholar]
- Abouabdellah, R.; Taleb, H.; Bennouna, A.; Erler, K.; Chafik, A.; Moukrim, A. Paralytic shellfish poisoning toxin profile of mussels Perna perna from southern Atlantic coasts of Morocco. Toxicon 2008, 51, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Taleb, H.; Vale, P.; Jaime, E.; Blaghen, M. Study of paralytic shellfish poisoning toxin profile in shellfish from the Mediterranean shore of Morocco. Toxicon 2001, 39, 1855–1861. [Google Scholar] [CrossRef]
- Ayres, P.A.; Cullum, M. Paralytic shellfish poisoning. An account of the investigations into mussel toxicity in England 1968–1977; Fisheries Research Technical Report 40; MAFF Directorate of Fisheries Research: Lowestoft, UK, 1978; p. 23.
- Scientific Committee of the Food Safety Authority of Ireland. The Occurrence of Marine Biotoxins and Risk of Exposure to Seafood Consumers in Ireland; Scientific Committee of the Food Safety Authority of Ireland: Dublin, Ireland, 2016; p. 60. [Google Scholar]
- Belin, C.; Soudant, D.; Amzil, Z. Three decades of data on phytoplankton and phycotoxins on the French coast: Lessons from REPHY and REPHYTOX. Harmful Algae 2021, 102, 101733. [Google Scholar] [CrossRef] [PubMed]
- Guallar, C.; Bacher, C.; Chapelle, A. Global and local factors driving the phenology of Alexandrium minutum (Halim) blooms and its toxicity. Harmful Algae 2017, 67, 44–60. [Google Scholar] [CrossRef]
- Rodriguez-Cabo, T.; Morono, A.; Arevalo, F.; Correa, J.; Lamas, J.P.; Rossignoli, A.E.; Blanco, J. Paralytic Shellfish Poisoning (PSP) in Mussels from the Eastern Cantabrian Sea: Toxicity, Toxin Profile, and Co-Occurrence with Cyclic Imines. Toxins 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Neaud-Masson, N.; Gautier, E. Lieux et Périodes à Risque Pour les Toxines Paralysantes Dans les Coquillages des Côtes Françaises. Note d’Information-Données REPHYTOX-Ifremer, Banque Quadrige. 2021, p. 6. Available online: https://archimer.ifremer.fr/doc/00692/80445/ (accessed on 25 October 2022).
- Figueiras, F.G.; Jones, K.J.; Mosquera, A.M.; Alvarez-Salgado, X.A.; Edwards, A.; MacDougall, N. Red tide assemblage formation in an estuarine upwelling ecosystem: Ria de Vigo. J. Plankton Res. 1994, 16, 857–878. [Google Scholar] [CrossRef]
- Nogueira, E.; Bravo, I.; Montero, P.; Díaz-Tapia, P.; Calvo, S.; Ben-Gigirey, B.; Figueroa, R.I.; Garrido, J.L.; Ramilo, I.; Lluch, N.; et al. HABs in coastal upwelling systems: Insights from an exceptional red tide of the toxigenic dinoflagellate Alexandrium minutum. Ecol. Indic. 2022, 137, 108790. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; McCausland, M.A.; Brown, R.K. Early warning of toxic dinoflagellate blooms of Gymnodinium catenatum in southern Tasmanian waters. J. Plankton Res. 1995, 17, 1163–1176. [Google Scholar] [CrossRef]
- Moore, S.; Mantua, N.; Hickey, B.; Trainer, V. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae 2009, 8, 463–477. [Google Scholar] [CrossRef]
- Blasco, D.; Levasseur, M.; Bonneau, E.; Gelinas, R.; Packard, T.T. Patterns of paralytic shellfish toxicity in the St. Lawrence region in relationship with the abundance and distribution of Alexandrium tamarense. Sci. Mar. 2003, 67, 261–278. [Google Scholar] [CrossRef]
- Vale, P. Can solar/geomagnetic activity restrict the occurrence of some shellfish poisoning outbreaks? The example of PSP caused by Gymnodinium catenatum at the Atlantic Portuguese coast. Biophysics 2013, 58, 554–567. [Google Scholar] [CrossRef]
- Lean, J.L. Cycles and trends in solar irradiance and climate. Wiley Interdiscip. Rev. Clim. Chang. 2010, 1, 111–122. [Google Scholar] [CrossRef]
- Bashmachnikov, I.; Belonenko, T.V.; Koldunov, A.V. Intra-annual and interannual non-stationary cycles of chlorophyll concentration in the Northeast Atlantic. Remote Sens. Environ. 2013, 137, 55–68. [Google Scholar] [CrossRef]
- Moroño, A.; Franco, J.; Miranda, M.; Reyero, M.I.; Blanco, J. The effect of mussel size, temperature, seston volume, food quality and volume-specific toxin concentration on the uptake rate of PSP toxins by mussels (Mytilus galloprovincialis LmK). J. Exp. Mar. Biol. Ecol. 2001, 257, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Gomes, S.S.; Botelho, M.J.; Rodrigues, S.M. Monitorização de PSP na costa portuguesa através de espécies-indicadoras. In Avances y Tendencias en Fitoplancton Tóxico y Biotoxinas: Actas de la IX Reunión Ibérica Sobre Fitoplancton Tóxico y Biotoxinas (Actas de la IX Reunión Ibérica Sobre Fitoplancton Tóxico y, Biotoxinas, Cartagena, 7–10 Mayo 2007); Gilabert, J., Ed.; Universidad Politécnica de Cartagena: Cartagena, Spain, 2008; pp. 177–180. [Google Scholar]
- Samsur, M.; Yamaguchi, Y.; Sagara, T.; Takatani, T.; Arakawa, O.; Noguchi, T. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella. Toxicon 2006, 48, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Samsur, M.; Takatani, T.; Yamaguchi, Y.; Sagara, T.; Noguchi, T.; Arakawa, O. Accumulation and Elimination Profiles of Paralytic Shellfish Poison in the Short-Necked Clam Tapes Japonica Fed with the Toxic Dinoflagellate Gymnodinium catenatum. J. Food Hyg. Soc. Jpn. 2007, 48, 13–18. [Google Scholar] [CrossRef]
- Blanco, J.; Moroño, A.; Franco, J.; Reyero, M.I. PSP detoxification kinetics in the mussel Mytilus galloprovincialis. One- and two-compartment models and the effect of some environmental variables. Mar. Ecol.-Prog. Ser. 1997, 158, 165–175. [Google Scholar] [CrossRef]
- Botelho, M.J.; Vale, C.; Mota, A.M.; Goncalves, M. Depuration kinetics of paralytic shellfish toxins in Mytilus galloprovincialis exposed to Gymnodinium catenatum: Laboratory and field experiments. J. Environ. Monit. 2010, 12, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.7. 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 25 October 2022).
- R. Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hyndman, R.J.; Khandakar, Y. Automatic time series forecasting: The forecast package for R. J. Stat. Softw. 2008, 26, 1–22. [Google Scholar]
- Trapletti, A.; Hornik, K. Tseries: Time Series Analysis and Computational Finance, R Package Version 0.10-11. 2019. Available online: https://cran.r-project.org/web/packages/tseries/tseries.pdf (accessed on 25 October 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.; Moroño, Á.; Arévalo, F.; Correa, J.; Salgado, C.; Pazos, Y.; Calvo, S.; Rossignoli, A.E. Twenty-Five Years of PSP Toxicity in Galician (NW Spain) Bivalves: Spatial, Temporal, and Interspecific Variations. Toxins 2022, 14, 837. https://doi.org/10.3390/toxins14120837
Blanco J, Moroño Á, Arévalo F, Correa J, Salgado C, Pazos Y, Calvo S, Rossignoli AE. Twenty-Five Years of PSP Toxicity in Galician (NW Spain) Bivalves: Spatial, Temporal, and Interspecific Variations. Toxins. 2022; 14(12):837. https://doi.org/10.3390/toxins14120837
Chicago/Turabian StyleBlanco, Juan, Ángeles Moroño, Fabiola Arévalo, Jorge Correa, Covadonga Salgado, Yolanda Pazos, Silvia Calvo, and Araceli Escudeiro Rossignoli. 2022. "Twenty-Five Years of PSP Toxicity in Galician (NW Spain) Bivalves: Spatial, Temporal, and Interspecific Variations" Toxins 14, no. 12: 837. https://doi.org/10.3390/toxins14120837
APA StyleBlanco, J., Moroño, Á., Arévalo, F., Correa, J., Salgado, C., Pazos, Y., Calvo, S., & Rossignoli, A. E. (2022). Twenty-Five Years of PSP Toxicity in Galician (NW Spain) Bivalves: Spatial, Temporal, and Interspecific Variations. Toxins, 14(12), 837. https://doi.org/10.3390/toxins14120837






