Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors
Abstract
1. Introduction
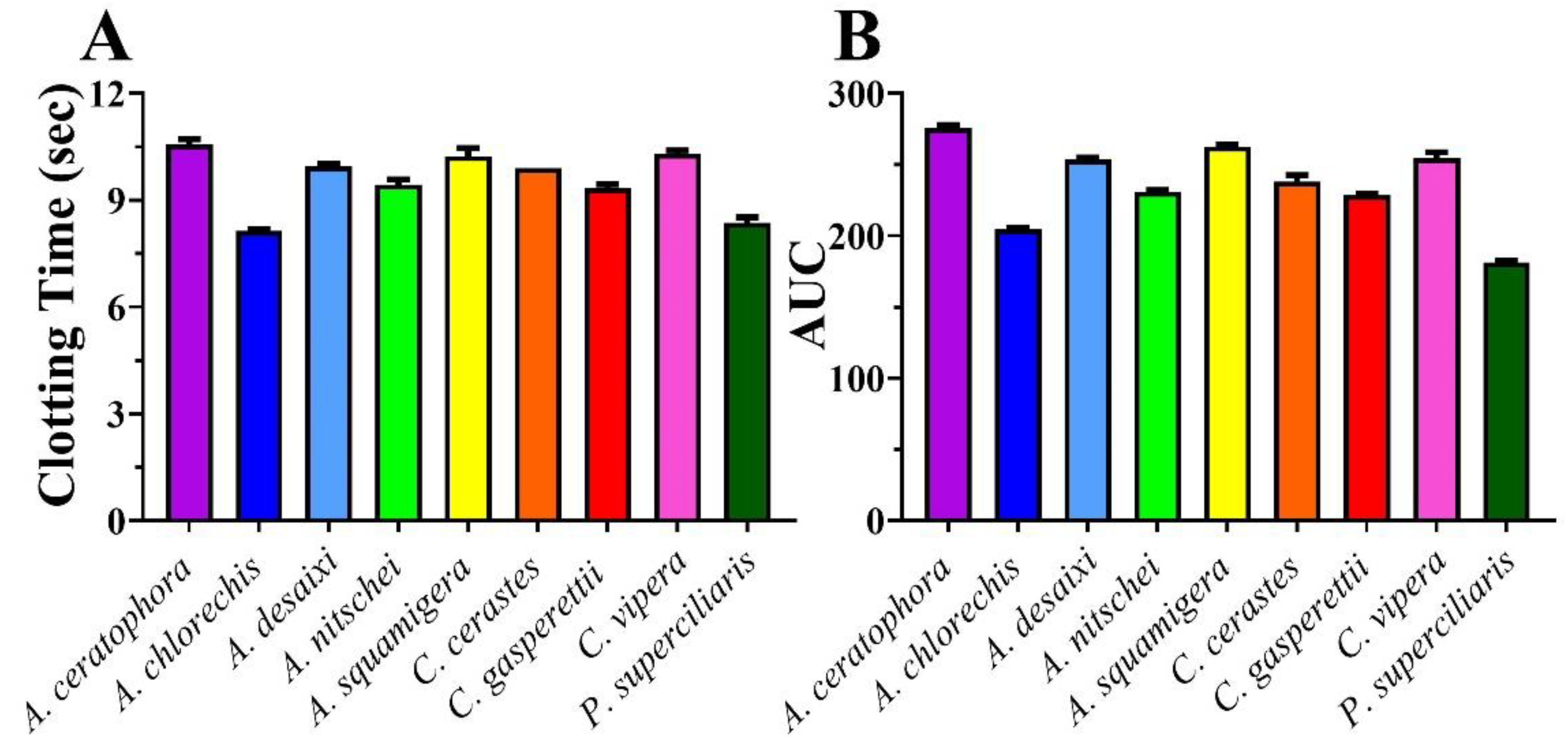
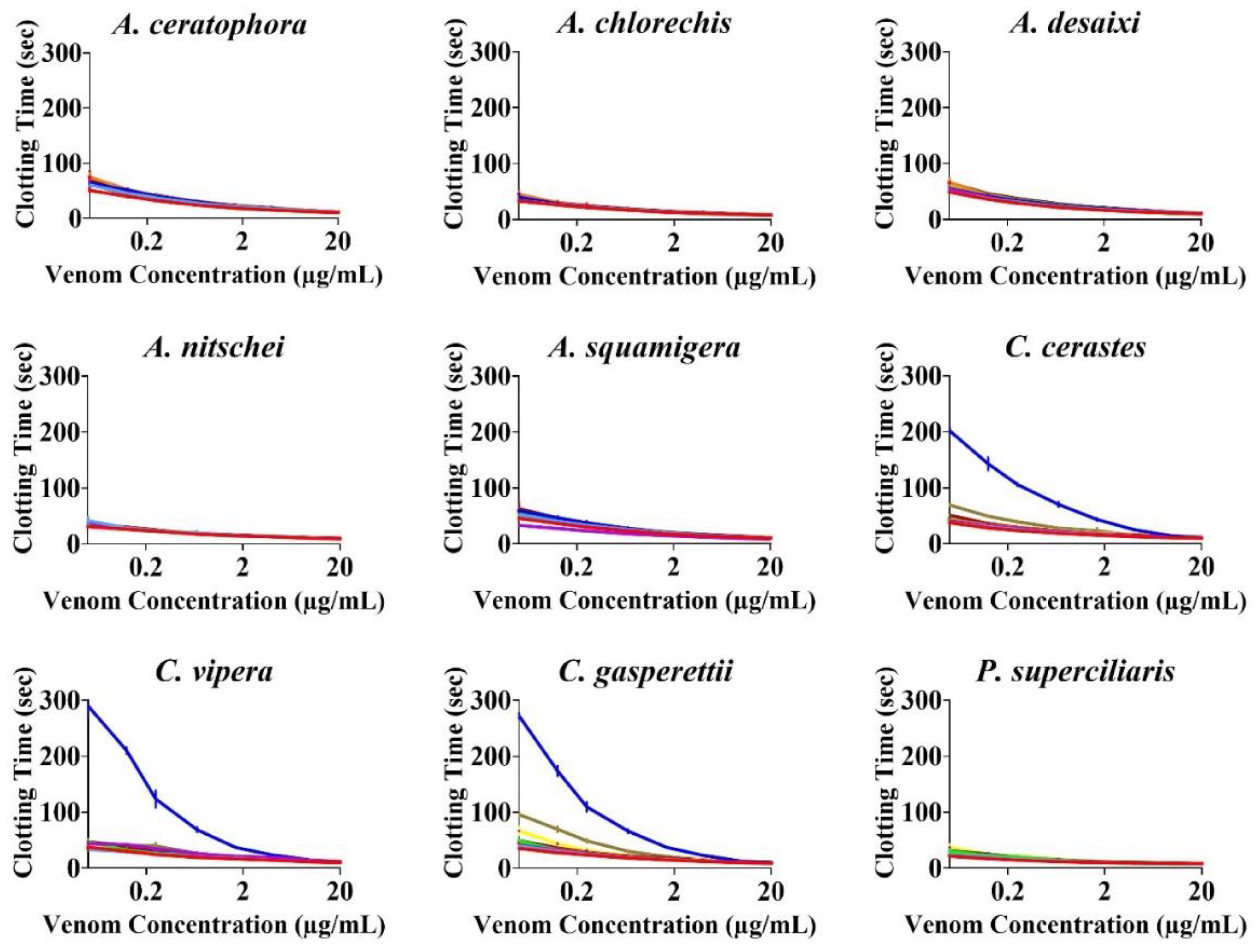
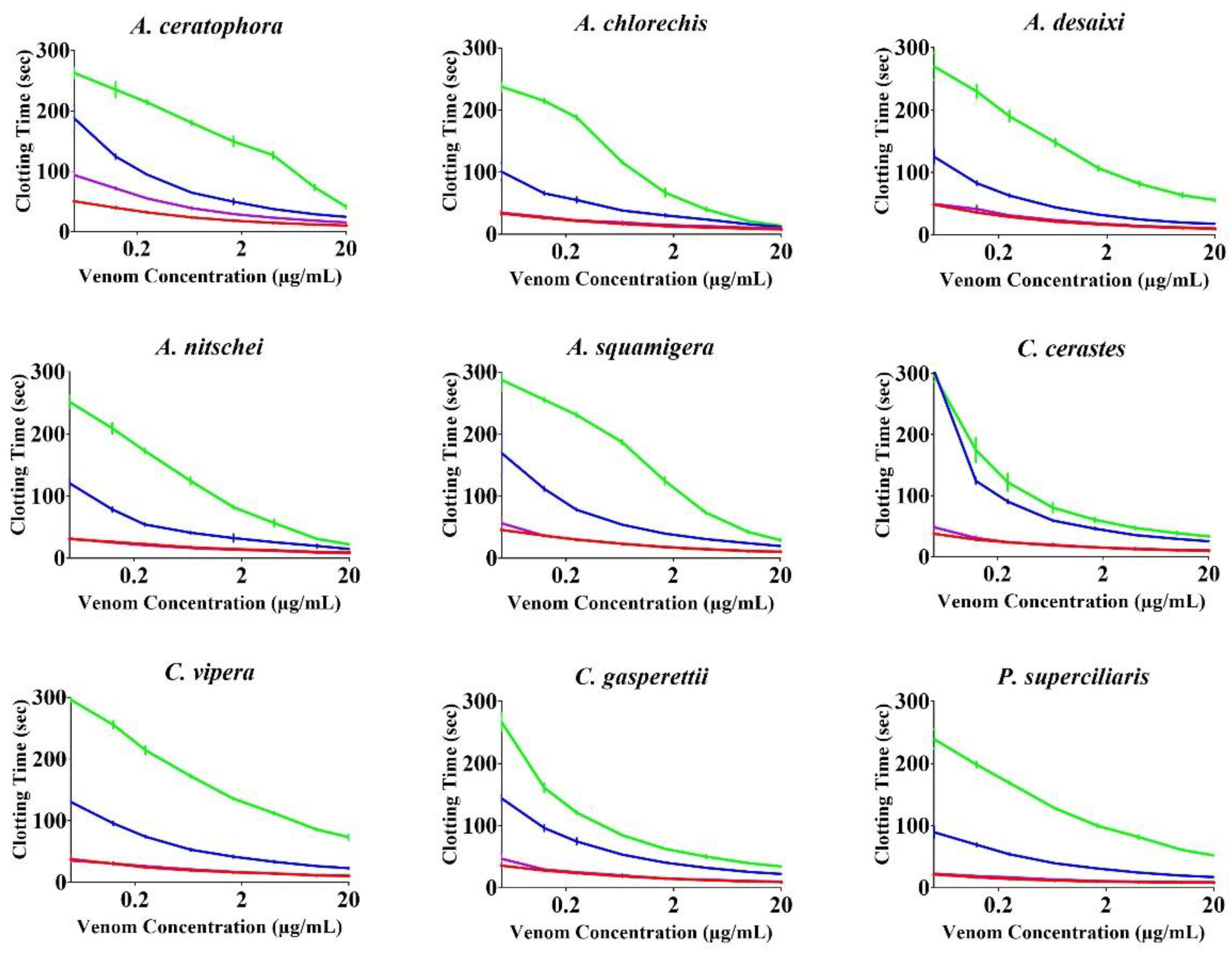
2. Results
3. Materials and Methods
3.1. Stock Preparation
3.1.1. Venoms
3.1.2. Plasma
3.1.3. Antivenom and Enzyme Inhibitors
3.2. Experimental Conditions
3.2.1. Coagulotoxicity Effects on Plasma
3.2.2. Antivenom and Enzyme-Inhibitor Efficacy
3.2.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, 1591–1604. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake Envenoming: A Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Fry, B.G. Snakebite: When the Human Touch Becomes a Bad Touch. Toxins 2018, 10, 170. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Debono, J.; Dobson, J.; Casewell, N.R.; Romilio, A.; Li, B.; Kurniawan, N.; Mardon, K.; Weisbecker, V.; Nouwens, A.; Kwok, H.F.; et al. Coagulating Colubrids: Evolutionary, Pathophysiological and Biodiscovery Implications of Venom Variations between Boomslang (Dispholidus Typus) and Twig Snake (Thelotornis Mossambicanus). Toxins 2017, 9, 171. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; den Brouw, B.O.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic Cobras: Clinical Implications of Strong Anticoagulant Actions of African Spitting Naja Venoms That Are Not Neutralised by Antivenom but Are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Zdenek, C.; Carter, R.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors against Anticoagulant Activities of African Spitting Cobra (Naja Species) Venoms. Front. Immunol. 2021, 12, 4215. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Dobson, J.S.; Zdenek, C.N.; den Brouw, B.O.; Naude, A.; Vonk, F.J.; Fry, B.G. Differential Destructive (Non-Clotting) Fibrinogenolytic Activity in Afro-Asian Elapid Snake Venoms and the Links to Defensive Hooding Behavior. Toxicol. Vitr. 2019, 60, 330–335. [Google Scholar] [CrossRef]
- Oulion, B.; Dobson, J.S.; Zdenek, C.N.; Arbuckle, K.; Lister, C.; Coimbra, F.C.P.; den Brouw, B.O.; Debono, J.; Rogalski, A.; Violette, A.; et al. Factor X Activating Atractaspis Snake Venoms and the Relative Coagulotoxicity Neutralising Efficacy of African Antivenoms. Toxicol. Lett. 2018, 288, 119–128. [Google Scholar] [CrossRef]
- Rogalski, A.; Soerensen, C.; den Brouw, B.O.; Lister, C.; Dashvesky, D.; Arbuckle, K.; Gloria, A.; Zdenek, C.N.; Casewell, N.R.; Gutiérrez, J.M.; et al. Differential Procoagulant Effects of Saw-Scaled Viper (Serpentes: Viperidae: Echis) Snake Venoms on Human Plasma and the Narrow Taxonomic Ranges of Antivenom Efficacies. Toxicol. Lett. 2017, 280, 159–170. [Google Scholar] [CrossRef]
- Youngman, N.J.; Lewin, M.R.; Carter, R.; Naude, A.; Fry, B.G. Efficacy and Limitations of Chemically Diverse Small-Molecule Enzyme-Inhibitors against the Synergistic Coagulotoxic Activities of Bitis Viper Venoms. Molecules 2022, 27, 1733. [Google Scholar] [CrossRef]
- Youngman, N.J.; Harris, R.J.; Huynh, T.M.; Coster, K.; Sundman, E.; Braun, R.; Naude, A.; Hodgson, W.C.; Fry, B.G. Widespread and Differential Neurotoxicity in Venoms from the Bitis Genus of Viperid Snakes. Neurotox. Res. 2021, 1, 697–704. [Google Scholar] [CrossRef]
- Youngman, N.J.; Walker, A.; Naude, A.; Coster, K.; Sundman, E.; Fry, B.G. Varespladib (LY315920) Neutralises Phospholipase A 2 Mediated Prothrombinase-Inhibition Induced by Bitis Snake Venoms. Comp. Biochem. Physiol. Part C 2020, 236, 108818. [Google Scholar] [CrossRef]
- Coimbra, F.C.P.; Dobson, J.; Zdenek, C.N.; Op Den Brouw, B.; Hamilton, B.; Debono, J.; Masci, P.; Frank, N.; Ge, L.; Kwok, H.F.; et al. Does Size Matter? Venom Proteomic and Functional Comparison between Night Adder Species (Viperidae: Causus) with Short and Long Venom Glands. Comp. Biochem. Physiol. Part C 2018, 211, 7–14. [Google Scholar] [CrossRef]
- Yamada, D.; Sekiya, F.; Morita, T. Prothrombin and Factor X Activator Activities in the Venoms of Viperidae Snakes. Toxicon 1997, 35, 1581–1589. [Google Scholar] [CrossRef]
- Razok, A.; Shams, A.; Yousaf, Z. Cerastes Cerastes Snakebite Complicated by Coagulopathy and Cardiotoxicity with Electrocardiographic Changes. Toxicon 2020, 188, 1–4. [Google Scholar] [CrossRef]
- Labib, R.S.; Azab, M.H.; Farag, N.W. Effects of Cerastes Cerastes (Egyptian Sand Viper) and Cerastes Vipera (Sahara Sand Viper) Snake Venoms on Blood Coagulation: Separation of Coagulant and Anticoagulant Factors and Their Correlation with Arginine Esterase and Protease Activit. Toxicon 1981, 19, 85–94. [Google Scholar] [CrossRef]
- Mebs, D.; Holada, K.; Kornalík, F.; Simák, J.; Vanková, H.; Müller, D.; Schoenemann, H.; Lange, H.; Herrmann, H.W. Severe Coagulopathy after a Bite of a Green Bush Viper (Atheris Squamiger): Case Report and Biochemical Analysis of the Venom. Toxicon 1998, 36, 1333–1340. [Google Scholar] [CrossRef]
- Stach, V.J.; Vagenknechtová, Z.; Hoskovec, E.; Valenta, J.; Stach, Z.; Vagenknechtová, E.; Hoskovec, D. Splenic Rupture and Massive Hemoperitoneum Due to Coagulopathy after Atheris Viper Snakebite. Prague Med. Rep. 2021, 122, 216–221. [Google Scholar] [CrossRef]
- Valenta, J.; Hlavackova, A.; Stach, Z.; Stikarova, J.; Havlicek, M.; Michalek, P. Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study. Toxins 2022, 14, 538. [Google Scholar] [CrossRef]
- Valenta, J.; Stach, Z.; Fricova, D.; Zak, J.; Balik, M. Envenoming by the Viperid Snake Proatheris Superciliaris: A Case Report. Toxicon 2008, 52, 392–394. [Google Scholar] [CrossRef]
- Elkabbaj, D.; Hassani, K.; Jaoudi, R. El Acute Renal Failure Following the Saharan Horned Viper (Cerastes Cerastes) Bite. Arab J. Nephrol. Transplant. 2012, 5, 159–161. [Google Scholar] [CrossRef]
- Keyler, D.E. Envenomation by the Lowland Viper (Proatheris Superciliaris): Severe Case Profile Documentation. Toxicon 2008, 52, 836–841. [Google Scholar] [CrossRef]
- Hatten, B.W.; Bueso, A.; French, L.K.; Hendrickson, R.G.; Horowitz, B.Z. Envenomation by the Great Lakes Bush Viper (Atheris Nitschei). Clin. Toxicol. 2013, 51, 114–116. [Google Scholar] [CrossRef]
- Warrell, D.A. Proatheris Superciliaris: The Deadly Venom of a Rare and Elusive Snake Revealed. Toxicon 2008, 52, 833–835. [Google Scholar] [CrossRef]
- Imperato, N.S.; Amaducci, A.M.; Abo, B.N.; Koons, A.L.; Fikse, D.J.; Katz, K.D. African Bush Viper Envenomation: A Case Report. Cureus 2022, 14, e28040. [Google Scholar] [CrossRef]
- Alencar, L.R.V.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in Vipers: Phylogenetic Relationships, Time of Divergence and Shifts in Speciation Rates. Mol. Phylogenet. Evol. 2016, 105, 50–62. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Frank, N. Role of Heme Modulation in Inhibition of Atheris, Atractaspis, Causus, Cerastes, Echis, and Macrovipera Hemotoxic Venom Activity. Hum. Exp. Toxicol. 2019, 38, 216–226. [Google Scholar] [CrossRef]
- Ainsworth, S.; Slagboom, J.; Alomran, N.; Pla, D.; Alhamdi, Y.; King, S.I.; Bolton, F.M.S.; Gutiérrez, J.M.; Vonk, F.J.; Toh, C.H.; et al. The Paraspecific Neutralisation of Snake Venom Induced Coagulopathy by Antivenoms. Commun. Biol. 2018, 1, 34. [Google Scholar] [CrossRef]
- Laing, G.D.; Compton, S.J.; Ramachandran, R.; Fuller, G.L.J.; Wilkinson, M.C.; Wagstaff, S.C.; Watson, S.P.; Kamiguti, A.S.; Theakston, R.D.G.; Senis, Y.A. Characterization of a Novel Protein from Proatheris Superciliaris Venom: Proatherocytin, a 34-KDa Platelet Receptor PAR1 Agonist. Toxicon 2005, 46, 490–499. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Reid, H.A. Development of Simple Standard Assay Procedures for the Characterization of Snake Venoms. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical Implications of Differential Procoagulant Toxicity of the Palearctic Viperid Genus Macrovipera, and the Relative Neutralization Efficacy of Antivenoms and Enzyme Inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Lewin, M.R.; Carter, R.; Jagar, T.; Ostanek, E.; Harjen, H.; Aldridge, M.; Soria, R.; Haw, G.; et al. Venom-Induced Blood Disturbances by Palearctic Viperid Snakes, and Their Relative Neutralization by Antivenoms and Enzyme-Inhibitors. Front. Immunol. 2021, 12, 2251. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Coimbra, F.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Basal but Divergent: Clinical Implications of Differential Coagulotoxicity in a Clade of Asian Vipers. Toxicol. Vitr. 2019, 58, 195–206. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Do, M.S.; Fry, B.G. Clinical Implications of Coagulotoxic Variations in Mamushi (Viperidae: Gloydius) Snake Venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108567. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical Implications of Differential Antivenom Efficacy in Neutralising Coagulotoxicity Produced by Venoms from Species within the Arboreal Viperid Snake Genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Nouwens, A.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Habu Coagulotoxicity: Clinical Implications of the Functional Diversification of Protobothrops Snake Venoms upon Blood Clotting Factors. Toxicol. Vitr. 2019, 55, 62–74. [Google Scholar] [CrossRef]
- Dobson, J.; Yang, D.C.; den Brouw, B.O.; Cochran, C.; Huynh, T.; Kurrupu, S.; Sánchez, E.E.; Massey, D.J.; Baumann, K.; Jackson, T.N.W.; et al. Rattling the Border Wall: Pathophysiological Implications of Functional and Proteomic Venom Variation between Mexican and US Subspecies of the Desert Rattlesnake Crotalus Scutulatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 62–69. [Google Scholar] [CrossRef]
- Lister, C.; Arbuckle, K.; Jackson, T.N.W.; Debono, J.; Zdenek, C.N.; Dashevsky, D.; Dunstan, N.; Allen, L.; Hay, C.; Bush, B.; et al. Catch a Tiger Snake by Its Tail: Differential Toxicity, Co-Factor Dependence and Antivenom Efficacy in a Procoagulant Clade of Australian Venomous Snakes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 202, 39–54. [Google Scholar] [CrossRef]
- Op Den Brouw, B.; Coimbra, F.C.P.; Bourke, L.A.; Huynh, T.M.; Vlecken, D.H.W.; Ghezellou, P.; Visser, J.C.; Dobson, J.S.; Fernandez-Rojo, M.A.; Ikonomopoulou, M.P.; et al. Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture. Toxins 2021, 13, 112. [Google Scholar] [CrossRef]
- Seneci, L.; Zdenek, C.N.; Chowdhury, A.; Rodrigues, C.F.B.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Fry, B.G. A Clot Twist: Extreme Variation in Coagulotoxicity Mechanisms in Mexican Neotropical Rattlesnake Venoms. Front. Immunol. 2021, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Chowdhury, A.; Zdenek, C.N.; Coster, K.; Sundman, E.; Braun, R.; Fry, B.G. Utilising Venom Activity to Infer Dietary Composition of the Kenyan Horned Viper (Bitis Worthingtoni). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108921. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; den Brouw, B.O.; Dashevsky, D.; Gloria, A.; Youngman, N.J.; Watson, E.; Green, P.; Hay, C.; Dunstan, N.; Allen, L.; et al. Clinical Implications of Convergent Procoagulant Toxicity and Differential Antivenom Efficacy in Australian Elapid Snake Venoms. Toxicol. Lett. 2019, 316, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; Hay, C.; Arbuckle, K.; Jackson, T.N.W.; Bos, M.H.A.; den Brouw, B.O.; Debono, J.; Allen, L.; Dunstan, N.; Morley, T.; et al. Coagulotoxic Effects by Brown Snake (Pseudonaja) and Taipan (Oxyuranus) Venoms, and the Efficacy of a New Antivenom. Toxicol. Vitr. 2019, 58, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; den Brouw, B.O.; Coimbra, F.C.P.; Gillett, A.; Del-Rei, T.H.M.; de Chalkidis, H.M.; Sant’Anna, S.; Teixeira-Da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Mallow, D.; David, L.; Nilson, G. True Vipers: Natural History and Toxinology of Old World Vipers; Krieger Publishing Company: Malabar, FL, USA, 2003. [Google Scholar]
- Dobiey, M.; Gernot, V. Venomous Snakes of Africa; Edition Chimaira: Frankfurt, Germany, 2007; ISBN 9783899733655. [Google Scholar]
- Phelps, T. Old World Vipers: A Natural History of the Azemiopinae and Viperinae; Edition Chimaira: Frankfurt, Germany, 2010; ISBN 389973470X. [Google Scholar]
- den Brouw, B.O.; Coimbra, F.C.P.; Casewell, N.R.; Ali, S.A.; Vonk, F.J.; Fry, B.G. A Genus-Wide Bioactivity Analysis of Daboia (Viperinae: Viperidae) Viper Venoms Reveals Widespread Variation in Haemotoxic Properties. Int. J. Mol. Sci. 2021, 22, 13486. [Google Scholar] [CrossRef]
- Rodrigues, C.F.B.; Zdenek, C.N.; Bourke, L.A.; Seneci, L.; Chowdhury, A.; Freitas-de-Sousa, L.A.; de Alcantara Menezes, F.; Moura-da-Silva, A.M.; Tanaka-Azevedo, A.M.; Fry, B.G. Clinical Implications of Ontogenetic Differences in the Coagulotoxic Activity of Bothrops Jararacussu Venoms. Toxicol. Lett. 2021, 348, 59–72. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Carter, R.W.; Casewell, N.R.; Fry, B.G. Keel Venom: Rhabdophis Subminiatus (Red-Necked Keelback) Venom Pathophysiologically Affects Diverse Blood Clotting Pathways. Toxicon 2022, 218, 19–24. [Google Scholar] [CrossRef]
- Xie, B.; Dashevsky, D.; Rokyta, D.; Ghezellou, P.; Fathinia, B.; Shi, Q.; Richardson, M.K.; Fry, B.G. Dynamic Genetic Differentiation Drives the Widespread Structural and Functional Convergent Evolution of Snake Venom Proteinaceous Toxins. BMC Biol. 2022, 20, 4. [Google Scholar] [CrossRef]
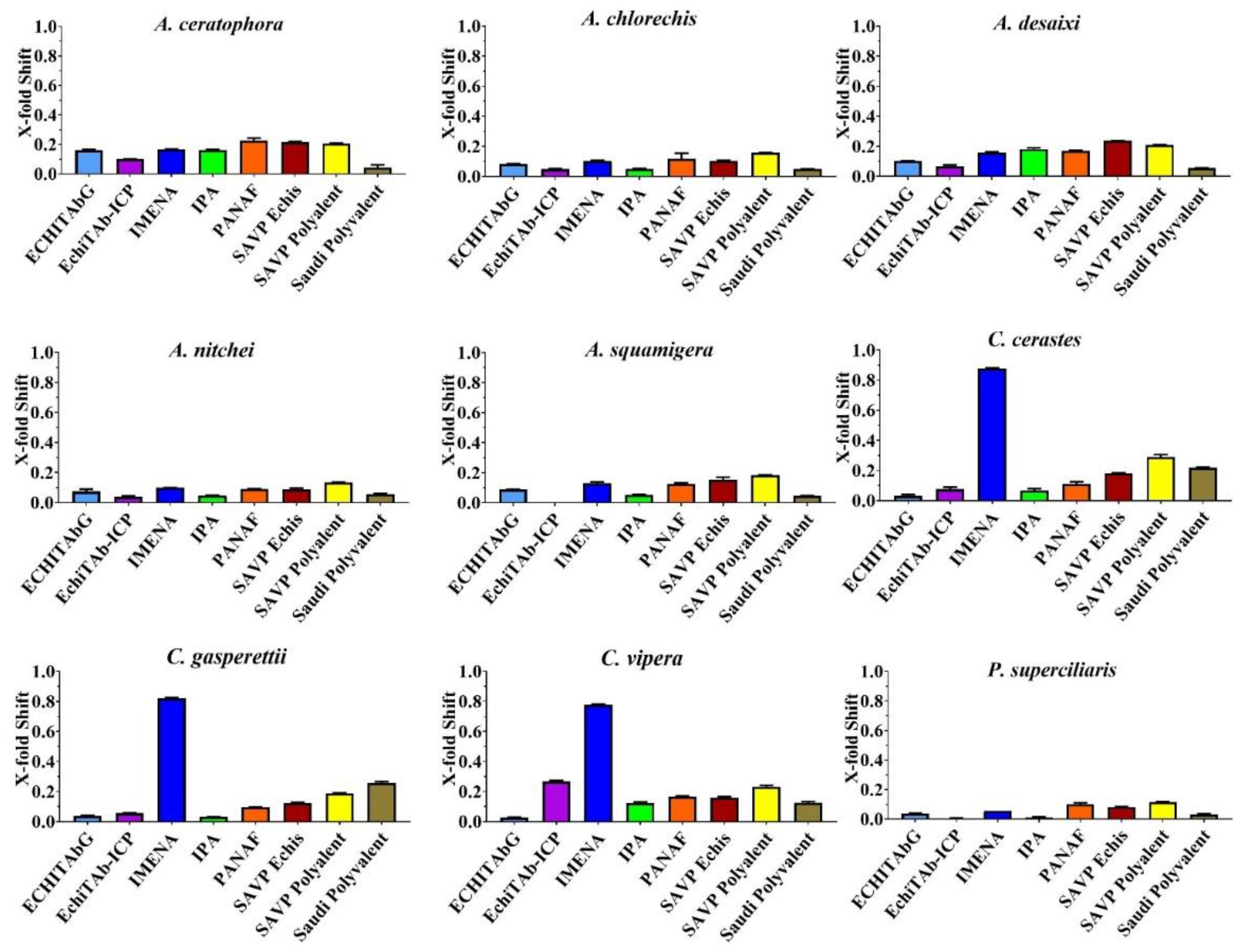

| Product Name | Company | Immunising Species | Codes Used in This Study |
|---|---|---|---|
| EchiTAbG | MicroPharm Ltd., Newcastle Emlyn, UK | Echis ocellatus | ECHITAbG |
| EchiTAb-Plus-ICP | Instituto Clodomiro picado Univerdad de Costa Rica | Bitis arietans, E. ocellatus, Naja nigricollis | EchiTAb-ICP |
| INOSERP MENA (Middle East and North Africa) | INOSAN Biopharma, Madrid, Spain | B. arietans, C. cerastes, C. gasperettii, C. vipera, Daboia deserti, D. mauritanica, D. palaestinae, E. carinatus sochureki, E. coloratus, E.s khosatzkii, E. leucogaster, E. megalocephalus, E. omanensis, E. pyramidum, Macrovipera lebetina obtusa, M. l. transmediterranea, M. l. turanica, Montivipera bornmuelleri, Montivipera raddei kurdistanica, Pseuocerastes fieldi, P. persicus, Vipera latastei, Naja haje, N. nubiae, N. pallida, Walterinnesia aegyptia | IMENA |
| INOSERP PAN-AFRICA | INOSAN Biopharma, Madrid, Spain | B. arietans, B. gabonica, B. rhinoceros, Dendroaspis angusticeps, Dendroaspis jamesoni, D. polylepis, D. viridis, E. leucogaster, E. ocellatus, E. pyramidum, Naja haje, N. katiensis, N. melanoleuca, N. nigricollis, N. nivea, N. pallida | IPA |
| PANAF PREMIUM (Sub-Saharan Africa) | Premium Serums, Maharashtra, India | B arietans, B. gabonica, B. nasicornis, B. rhinoceros, Dendroaspis angusticeps, D. jamesoni, D. polylepis, D. viridis, Echis carinatus, E. leucogaster, E. ocellatus, Naja nigricollis, N. haje, N. melanoleuca | PANAF |
| SAVP Echis | South African Vaccine Producers | E. carinatus, E. ocellatus, E. coloratus, Cerastes spp. | SAVP Echis |
| SAVP Polyvalent | South African Vaccine Producers | B. arietans, B. gabonica, D. angusticeps, D. jamesoni, D. polylepis, Hemachatus haemachatus, N. annulifera, N. melanoleuca, N.mossambica, N. nivea | SAVP Polyvalent |
| Polyvalent Snake Antivenom | National Antivenom and Vaccine Production Centre, Saudi Arabia | B. arietans, C. cerastes, E. carinatus, E. coloratus, N. Haje, W. Aegyptia | Saudi Polyvalent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.; Lewin, M.R.; Carter, R.; Soria, R.; Aldridge, M.; Fry, B.G. Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors. Toxins 2022, 14, 836. https://doi.org/10.3390/toxins14120836
Chowdhury A, Lewin MR, Carter R, Soria R, Aldridge M, Fry BG. Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors. Toxins. 2022; 14(12):836. https://doi.org/10.3390/toxins14120836
Chicago/Turabian StyleChowdhury, Abhinandan, Matthew R. Lewin, Rebecca Carter, Raul Soria, Matt Aldridge, and Bryan G. Fry. 2022. "Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors" Toxins 14, no. 12: 836. https://doi.org/10.3390/toxins14120836
APA StyleChowdhury, A., Lewin, M. R., Carter, R., Soria, R., Aldridge, M., & Fry, B. G. (2022). Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors. Toxins, 14(12), 836. https://doi.org/10.3390/toxins14120836






