Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures
Abstract
:1. Introduction
2. Results and Discussion
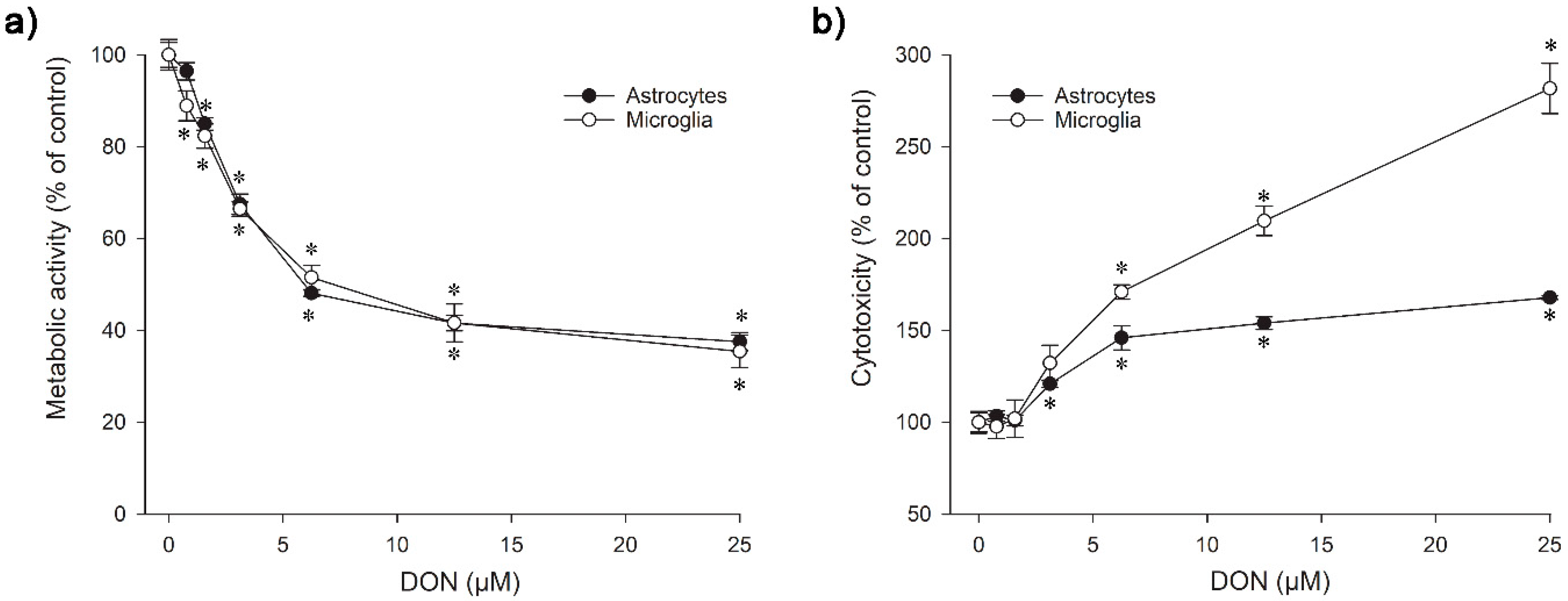
2.1. Viability of Mouse Astrocytes and Microglia
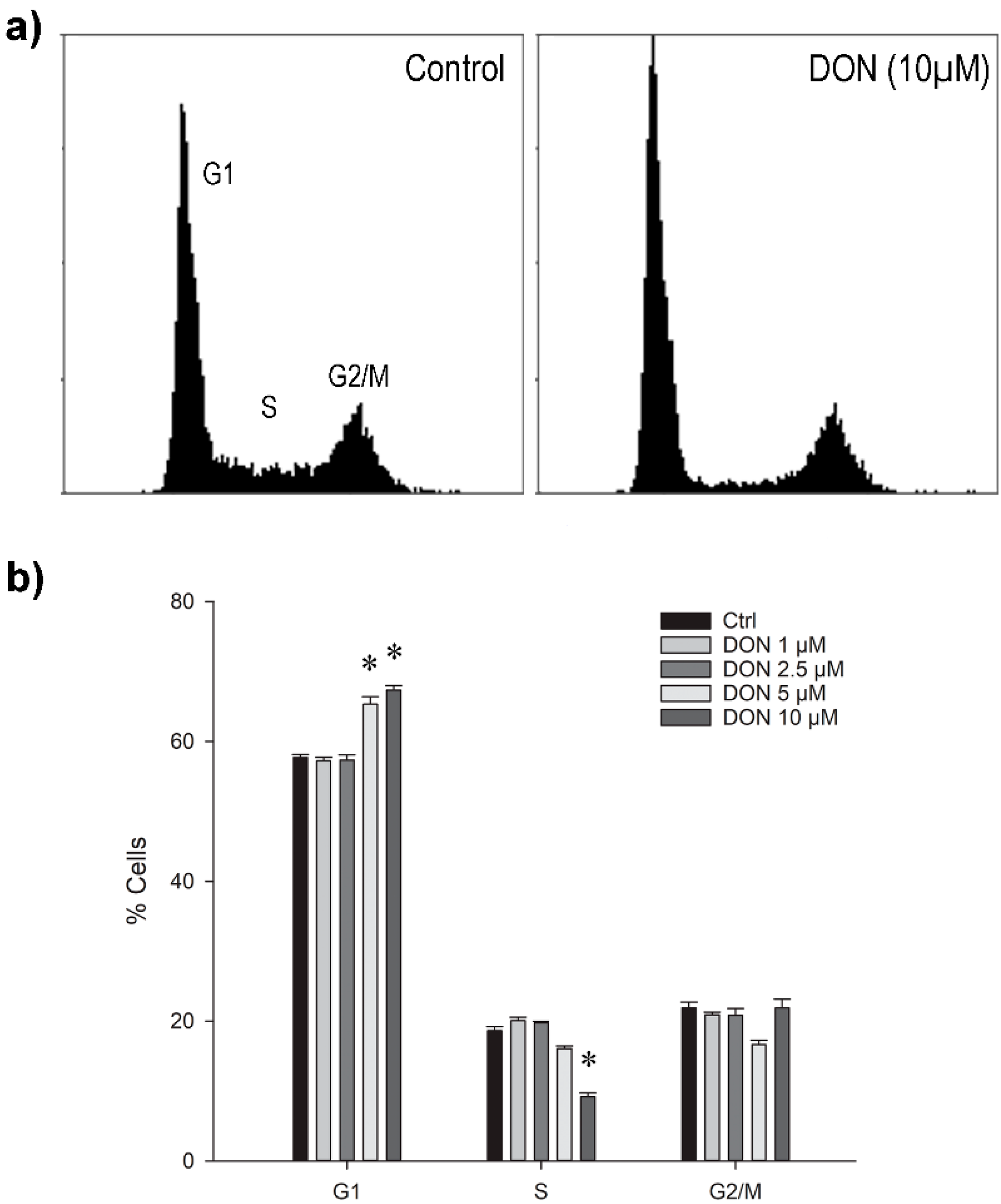
2.2. Cell Cycle Analysis in Astrocytes
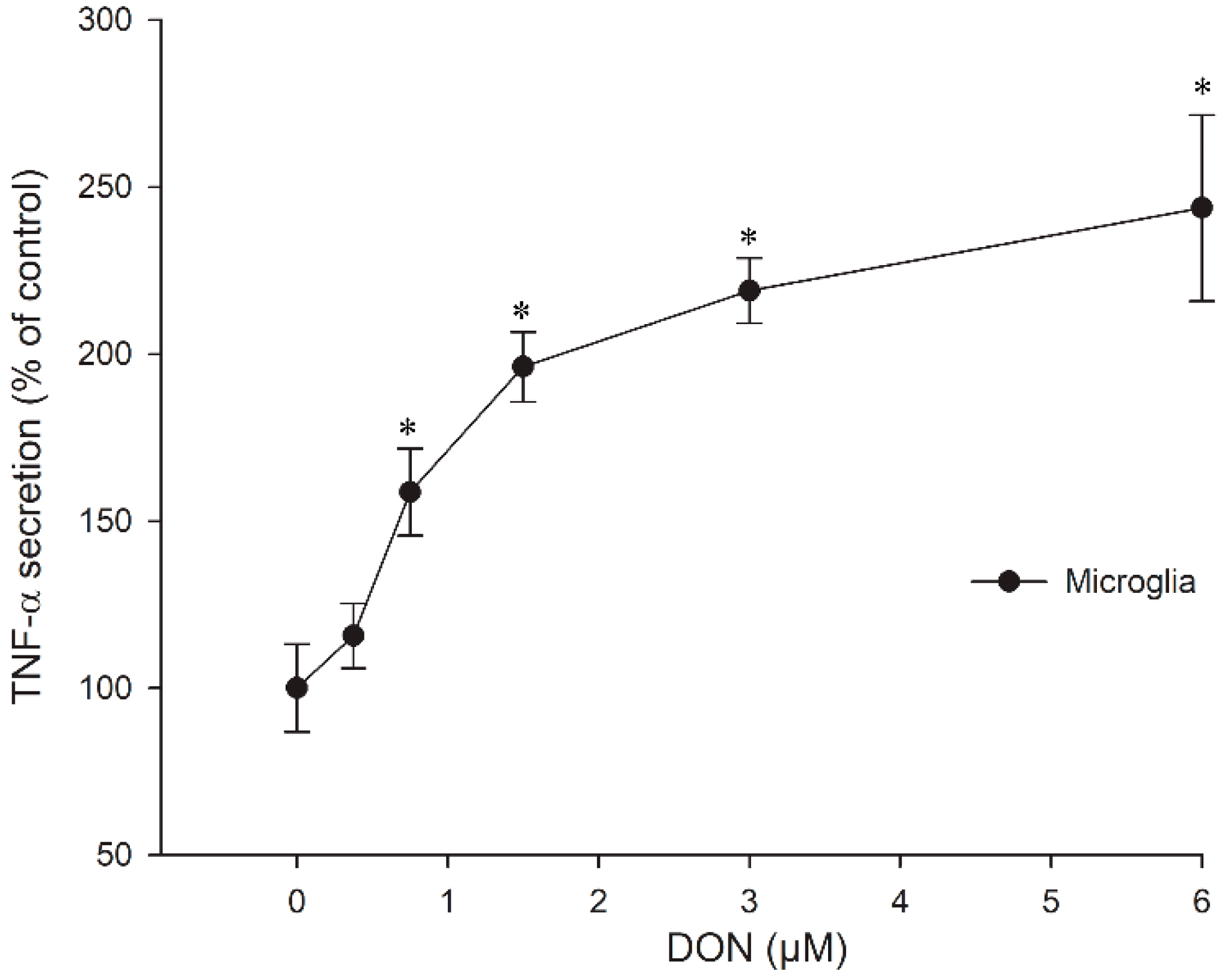
2.3. Cytokine Secretion in Microglia
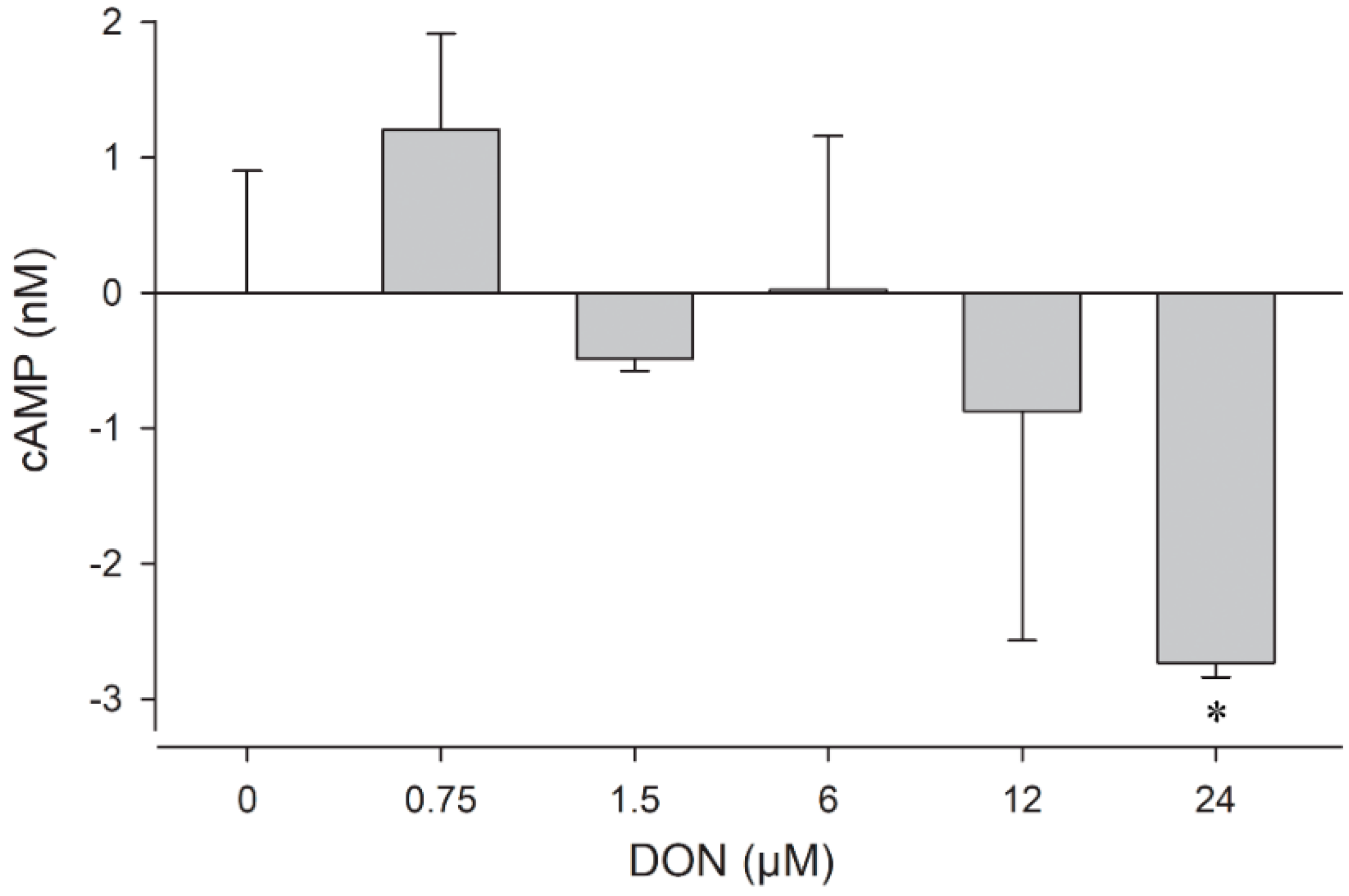
2.4. cAMP Levels
2.5. DON Impact on Glial Cells in Primary Hippocampal Cultures
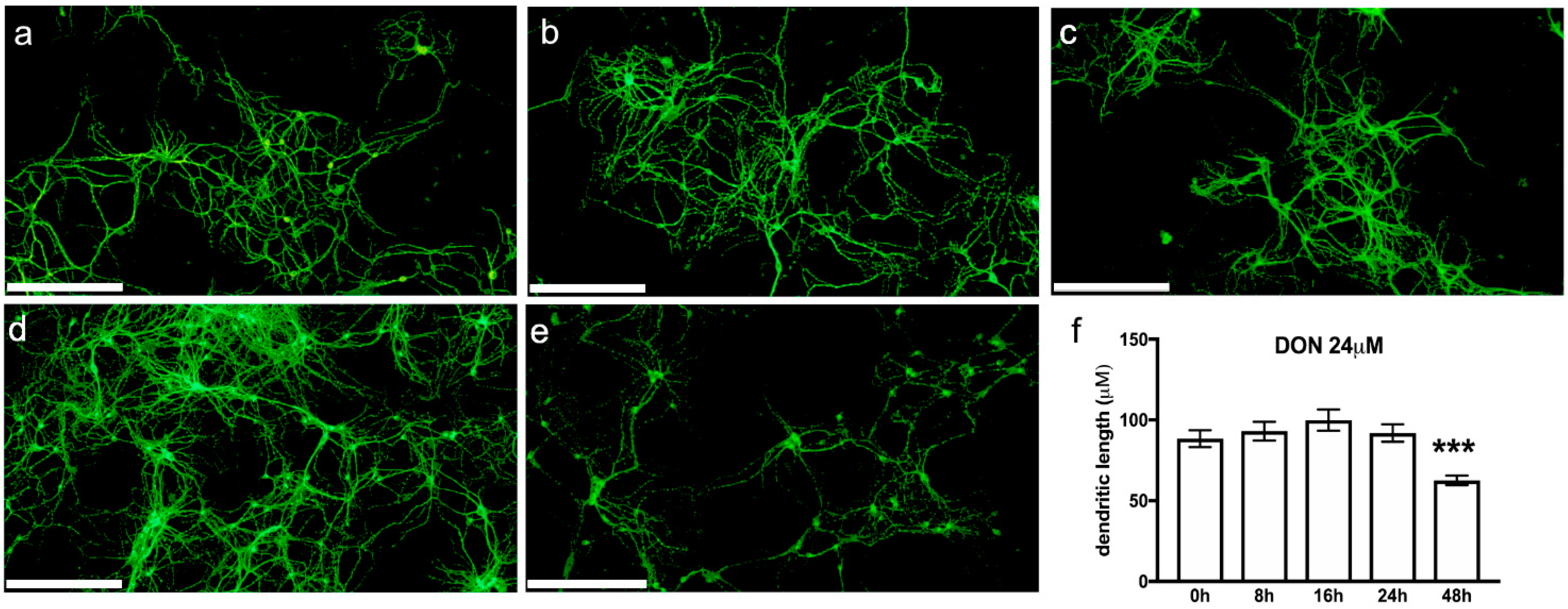
2.6. DON Impact on Neurons in Primary Hippocampal Cultures
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture Conditions
4.3. Metabolic Activity
4.4. Cytotoxicity
4.5. Expression of Cytokines
4.6. Cell Cycle Analysis
4.7. Determination of cAMP Levels
4.8. Primary Hippocampal Cultures from Mouse Pups
4.9. Determination of Neural and Glial Markers by Immunofluorescence
4.10. Image Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2020, 60, 1346–1374. [Google Scholar] [CrossRef]
- EU Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 22 June 2021).
- EU Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1440504898051&uri=CELEX:32006H0576 (accessed on 22 June 2021).
- EFSA. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar]
- Vidal, A.; Bouzaghnane, N.; De Saeger, S.; De Boevre, M. Human mycotoxin biomonitoring: Conclusive remarks on direct or indirect assessment of urinary deoxynivalenol. Toxins 2020, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; You, L.; Nepovimova, E.; Wang, X.; Musilek, K.; Wu, Q.; Wu, W.; Kuca, K. Biomarkers of deoxynivalenol (DON) and its modified form DON-3-glucoside (DON-3G) in humans. Trends Food Sci. Tech. 2021, 110, 551–558. [Google Scholar] [CrossRef]
- Sundheim, L.; Lillegaard, I.T.; Fæste, C.K.; Brantsæter, A.L.; Brodal, G.; Eriksen, G.S. Deoxynivalenol exposure in Norway, risk assessments for different human age groups. Toxins 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Deoxynivalenol exposure assessment through a modelling approach of food intake and biomonitoring data–A contribution to the risk assessment of an enteropathogenic mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef] [PubMed]
- Tardivel, C.; Airault, C.; Djelloul, M.; Guillebaud, F.; Barbouche, R.; Troadec, J.D.; Gaigé, S.; Dallaporta, M. The foodborne mycotoxin deoxynivalenol induces low-grade inflammation in mice in the absence of observed-adverse effects. Toxicol. Lett. 2015, 232, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Fæste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and metabolomic changes from chronic dietary exposure to low-level deoxynivalenol reveal impact on mouse well-being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Csikós, V.; Varró, P.; Bódi, V.; Oláh, S.; Világi, I.; Dobolyi, A. The mycotoxin deoxynivalenol activates GABAergic neurons in the reward system and inhibits feeding and maternal behaviours. Arch. Toxicol. 2020, 94, 3297–3313. [Google Scholar] [CrossRef] [PubMed]
- Nordgreen, J.; Edwards, S.A.; Boyle, L.A.; Bolhuis, J.E.; Veit, C.; Sayyari, A.; Marin, D.E.; Dimitrov, I.; Janczak, A.M.; Valros, A. A proposed role for pro-inflammatory cytokines in damaging behavior in pigs. Front. Vet. Sci. 2020, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Chowdhury, S.R.; Oinas, N.; MacDonald, E.J.; Smith, T.K. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of laying hens, turkey poults, and broiler breeder hens. Poult. Sci. 2006, 85, 2117–2123. [Google Scholar] [CrossRef]
- Janković-Tomanić, M.; Petković, B.; Todorović, D.; Vranković, J.; Perić-Mataruga, V. Physiological and behavioral effects of the mycotoxin deoxynivalenol in Tenebrio molitor larvae. J. Stored Prod. Res. 2019, 83, 236–242. [Google Scholar] [CrossRef]
- Behrens, M.; Hüwel, S.; Galla, H.J.; Humpf, H.U. Blood-brain barrier effects of the Fusarium mycotoxins deoxynivalenol, 3-acetyldeoxynivalenol, and moniliformin and their transfer to the brain. PLoS ONE 2015, 10, e0143640. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins 2012, 4, 1120–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hazmi, M.A.; Waggas, A.M. Neurophysiological and behavioral effects of mycotoxin deoxynivalenol and fumonisin. Afri. Microbiol. Res. 2013, 7, 1371–1377. [Google Scholar]
- Fitzpatrick, D.W.; Boyd, K.E.; Wilson, L.M.; Wilson, J.R. Effect of the trichothecene deoxynivalenol on brain biogenic monoamines concentrations in rats and chickens. J. Environ. Sci. Health B 1988, 23, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Yeung, J.M.; Thompson, B.K.; Trenholm, H.I. Effect of deoxynivalenol on neurotransmitters in discrete regions of swine brain. Arch. Environ. Contam. Toxicol. 1992, 22, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Wanaverbecq, N.; Mounien, L.; et al. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS ONE 2011, 6, e26134. [Google Scholar] [CrossRef]
- Gaigé, S.; Bonnet, M.S.; Tardivel, C.; Pinton, P.; Trouslard, J.; Jean, A.; Guzylack, L.; Troadec, J.D.; Dallaporta, M. c-Fos immunoreactivity in the pig brain following deoxynivalenol intoxication: Focus on NUCB2/nesfatin-1 expressing neurons. Neuro Toxicol. 2013, 34, 135–149. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Ur Rahman, S.; Peng, C.; Feng, S.; et al. Mechanism of deoxynivalenol-induced neurotoxicity in weaned piglets is linked to lipid peroxidation, dampened neurotransmitter levels, and interference with calcium signaling. Ecotoxicol. Environ. Saf. 2020, 194, 110382. [Google Scholar] [CrossRef]
- Rissato, D.F.; de Santi Rampazzo, A.P.; Borges, S.C.; Sousa, F.C.; Busso, C.; Buttow, N.C.; Natali, M.R.M. Chronic ingestion of deoxynivalenol-contaminated diet dose-dependently decreases the area of myenteric neurons and gliocytes of rats. Neurogastroenterol. Motil. 2020, 32, e13770. [Google Scholar] [CrossRef]
- Ivanova, L.; Fæste, C.K.; Solhaug, A. Role of P-glycoprotein in deoxynivalenol-mediated in vitro toxicity. Toxicol. Lett. 2018, 284, 21–28. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pan, X.; Whitten, D.A.; Wu, M.; Chan, C.; Wilkerson, C.G.; Pestka, J.J. Global protein phosphorylation dynamics during deoxynivalenol-induced ribotoxic stress response in the macrophage. Toxicol. Appl. Pharmacol. 2013, 268, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, W.; Fan, M.; Meng, T.; Chen, X.; Jiang, Y.; Zhu, D.; Hu, W.; Gong, J.; Feng, S.; et al. Deoxynivalenol induces apoptosis in PC12 cells via the mitochondrial pathway. Environ. Toxicol. Pharmacol. 2016, 43, 193–202. [Google Scholar] [CrossRef]
- Li, D.; Ye, Y.; Lin, S.; Deng, L.; Fan, X.; Zhang, Y.; Deng, X.; Li, Y.; Yan, H.; Ma, Y. Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: Cell-cycle arrest, oxidative stress, and apoptosis. Environ. Toxicol. Pharmacol. 2014, 37, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Schrickx, J.A.; Dänicke, S.; Fink-Gremmels, J. Deoxynivalenol-induced cytotoxicity, cytokines and related genes in unstimulated or lipopolysaccharide stimulated primary porcine macrophages. Toxicol. Lett. 2009, 184, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. In Vitro 2016, 36, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, Q.; Ihsan, A.; Huang, L.; Dai, M.; Hao, H.; Cheng, G.; Liu, Z.; Wang, Y.; Yuan, Z. JAK/STAT pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicol. Sci. 2012, 127, 412–424. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, H.R.; Pestka, J.J. Mechanisms for ribotoxin-induced ribosomal RNA cleavage. Toxicol. Appl. Pharmacol. 2012, 265, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waché, Y.J.; Hbabi-Haddioui, L.; Guzylack-Piriou, L.; Belkhelfa, H.; Roques, C.; Oswald, I.P. The mycotoxin deoxynivalenol inhibits the cell surface expression of activation markers in human macrophages. Toxicology 2009, 262, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.J.; Vidal, N.; Di Scala, C.; Varini, K.; Maresca, M. The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia 2011, 59, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Islam, Z.; Amuzie, C.J. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 2008, 178, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Teschemacher, A.G.; Kasparov, S. Neuroprotective potential of astroglia. J. Neurosci. Res. 2017, 95, 2126–2139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nasri, T.; Bosch, R.R.; Ten Voorde, S.; Fink-Gremmels, J. Differential induction of apoptosis by type A and B trichothecenes in Jurkat T-lymphocytes. Toxicol. In Vitro 2006, 20, 832–840. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar] [CrossRef] [Green Version]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J. Cerebr. Blood Flow Metabol. 2007, 27, 1766–1791. [Google Scholar] [CrossRef]
- Monnet-Tschudi, F.; Zurich, M.G.; Honegger, P. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Hum. Exp. Toxicol. 2007, 26, 339–346. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Ji, G.E.; Park, S.Y.; Wong, S.S.; Pestka, J.J. Modulation of nitric oxide, hydrogen peroxide and cytokine production in a clonal macrophage model by the trichothecene vomitoxin (deoxynivalenol). Toxicology 1998, 125, 203–214. [Google Scholar] [PubMed]
- Lukas, J.; Lukas, C.; Bartek, J. Mammalian cell cycle checkpoints: Signalling pathways and their organization in space and time. DNA Repair 2004, 3, 997–1007. [Google Scholar] [CrossRef]
- Marzocco, S.; Russo, R.; Bianco, G.; Autore, G.; Severino, L. Pro-apoptotic effects of nivalenol and deoxynivalenol trichothecenes in J774A. 1 murine macrophages. Toxicol. Lett. 2009, 189, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS ONE 2012, 7, e52051. [Google Scholar] [CrossRef]
- Tiemann, U.; Viergutz, T.; Jonas, L.; Schneider, F. Influence of the mycotoxins α-and β-zearalenol and deoxynivalenol on the cell cycle of cultured porcine endometrial cells. Reprod. Toxicol. 2003, 17, 209–218. [Google Scholar] [CrossRef]
- Juan-García, A.; Taroncher, M.; Font, G.; Ruiz, M.J. Micronucleus induction and cell cycle alterations produced by deoxynivalenol and its acetylated derivatives in individual and combined exposure on HepG2 cells. Food Chem. Toxicol. 2018, 118, 719–725. [Google Scholar] [CrossRef]
- Diesing, A.K.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H.-J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE 2011, 6, e17472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Chung, D.H.; Kim, Y.B.; Choi, Y.H.; Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology 2008, 243, 145–154. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, X.; Dong, J.; Yang, Q.; Wu, Y.; Gong, Z. Transcriptome Analysis of Caco-2 Cells upon the exposure of mycotoxin deoxynivalenol and its acetylated derivatives. Toxins 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Stanic, A.; Uhlig, S.; Solhaug, A.; Rise, F.; Wilkins, A.L.; Miles, C.O. Nucleophilic addition of thiols to deoxynivalenol. J. Agric. Food Chem. 2015, 63, 7556–7566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slotkin, T.A.; Seidler, F.J. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull. 2007, 72, 232–274. [Google Scholar] [CrossRef] [Green Version]
- Adornetto, A.; Pagliara, V.; Di Renzo, G.; Arcone, R. Polychlorinated biphenyls impair dibutyryl cAMP-induced astrocytic differentiation in rat C6 glial cell line. FEBS Open Biol. 2013, 3, 459–466. [Google Scholar] [CrossRef]
- Pahan, K.; Namboodiri, A.M.; Sheikh, F.G.; Smith, B.T.; Singh, I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 1997, 272, 7786–7791. [Google Scholar] [CrossRef] [Green Version]
- Karaçay, B.; Li, G.; Pantazis, N.J.; Bonthius, D.J. Stimulation of the cAMP pathway protects cultured cerebellar granule neurons against alcohol-induced cell death by activating the neuronal nitric oxide synthase (nNOS) gene. Brain Res. 2007, 1143, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.; Peterson, D.A.; Schinstine, M.; Gage, F.H. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc. Natl. Acad. Sci. USA 1993, 90, 3602–3606. [Google Scholar] [CrossRef] [Green Version]
- McKeon, A.; Benarroch, E.E. Glial fibrillary acid protein: Functions and involvement in disease. Neurology 2018, 90, 925–930. [Google Scholar] [CrossRef]
- Caceres, A.; Banker, G.; Steward, O.; Binder, L.; Payne, M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Dev. Brain Res. 1984, 13, 314–318. [Google Scholar] [CrossRef]
- Furlong, R.M.; O’Keeffe, G.W.; O’Neill, C.; Sullivan, A.M. Alterations in α-synuclein and PINK1 expression reduce neurite length and induce mitochondrial fission and Golgi fragmentation in midbrain neurons. Neurosci. Lett. 2020, 720, 134777. [Google Scholar] [CrossRef]
- Burak, K.; Lamoureux, L.; Boese, A.; Majer, A.; Saba, R.; Niu, Y.; Frost, K.; Booth, S.A. MicroRNA-16 targets mRNA involved in neurite extension and branching in hippocampal neurons during presymptomatic prion disease. Neurobiol. Dis. 2018, 112, 1–13. [Google Scholar] [CrossRef]
- Derouiche, L.; Ugur, M.; Pierre, F.; Mann, A.; Doridot, S.; Ory, S.; Schulz, S.; Massotte, D. Heteromerization of endogenous mu and delta opioid receptors tunes mu opioid receptor signaling and trafficking. bioRxiv 2018, 455147. [Google Scholar] [CrossRef]
- Pemberton, K.; Mersman, B.; Xu, F. Using ImageJ to assess neurite outgrowth in mammalian cell cultures: Research data quantification exercises in undergraduate neuroscience lab. J. Undergrad. Neurosci. Educ. 2018, 16, A186–A194. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fæste, C.K.; Solhaug, A.; Gaborit, M.; Pierre, F.; Massotte, D. Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures. Toxins 2022, 14, 48. https://doi.org/10.3390/toxins14010048
Fæste CK, Solhaug A, Gaborit M, Pierre F, Massotte D. Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures. Toxins. 2022; 14(1):48. https://doi.org/10.3390/toxins14010048
Chicago/Turabian StyleFæste, Christiane Kruse, Anita Solhaug, Marion Gaborit, Florian Pierre, and Dominique Massotte. 2022. "Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures" Toxins 14, no. 1: 48. https://doi.org/10.3390/toxins14010048
APA StyleFæste, C. K., Solhaug, A., Gaborit, M., Pierre, F., & Massotte, D. (2022). Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures. Toxins, 14(1), 48. https://doi.org/10.3390/toxins14010048






