Ciguatoxin Detection in Flesh and Liver of Relevant Fish Species from the Canary Islands
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Presence of CTX-like Activity by CBA
2.1.1. In Amberjack
2.1.2. In Dusky Grouper
2.1.3. In Black Moray Eel
2.1.4. In Common Two-Banded Seabream
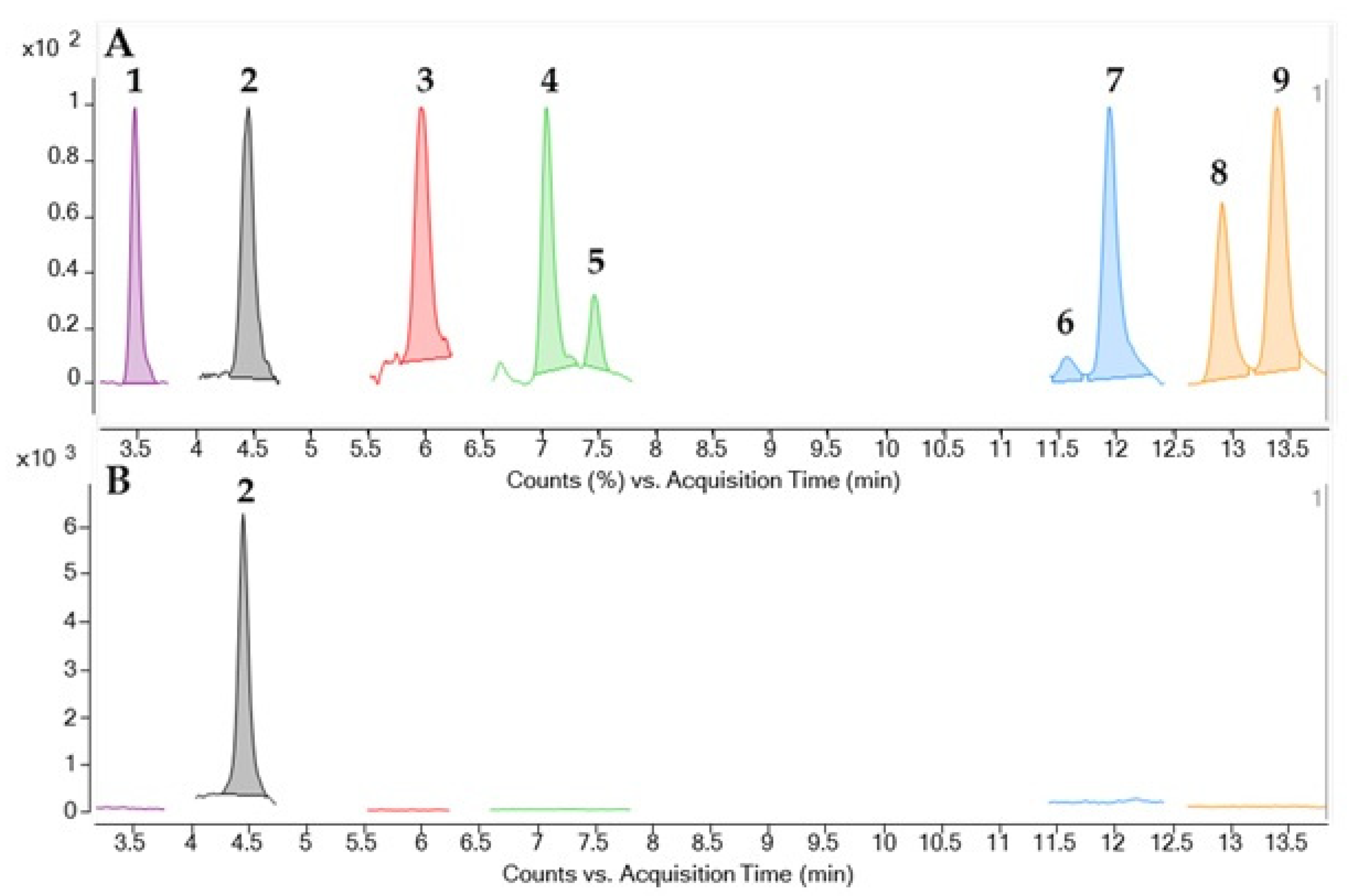
2.2. Identification and Quantification of CTX by LC-MS/MS
2.3. CTX-like Toxicity According to Fish Species by CBA
2.4. CTX-Like Toxicity According to Fish Weight and Length by CBA
2.4.1. In Amberjack
2.4.2. In Dusky Grouper
2.5. Influence of Liver Condition
3. Discussion
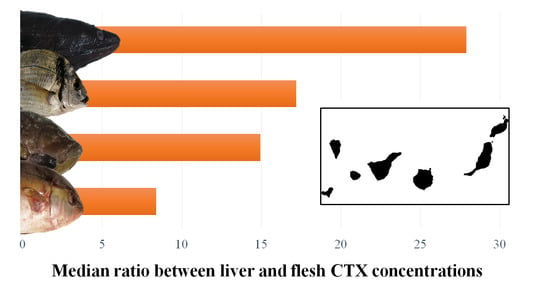
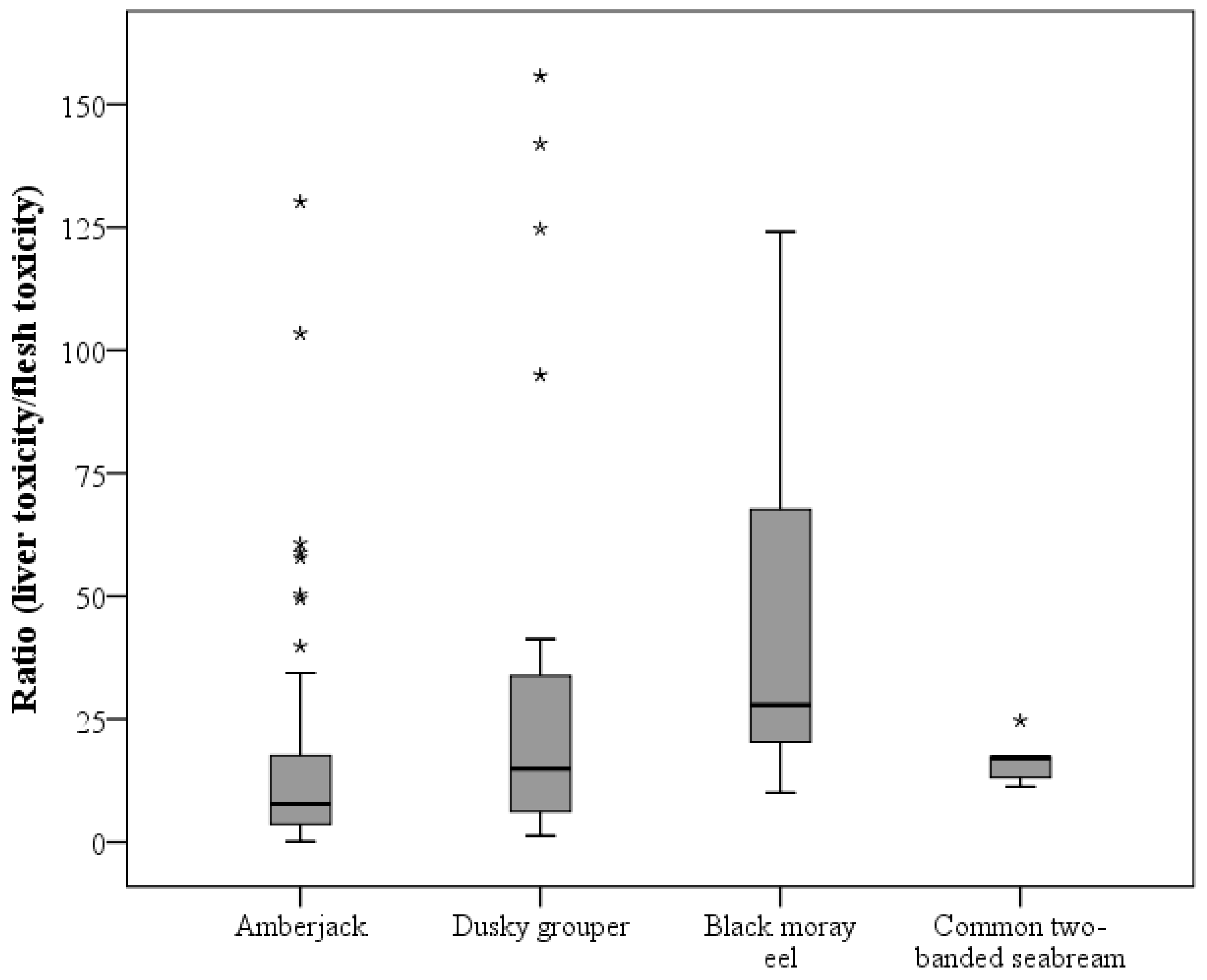
3.1. Differences in CTX Accumulation in Liver and Flesh
3.2. Toxicity Differences among Fish Species
4. Conclusions
5. Materials and Methods
5.1. Study Area
5.2. Fish Sample Collection
5.3. Muscle Sample Preparation and CTX Extraction
5.4. Liver Sample Preparation and Extraction of CTX
5.5. Cytotoxicity CBA (Neuro-2a-MTT)
5.6. CTX Identification by LC-MS/MS
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2021, 102, 22. [Google Scholar] [CrossRef] [PubMed]
- Adachi, R.; Fukuyo, Y. Thecal Structure of a Marine Toxic Dinoflagellate Gambierdiscus-Toxicus Gen ET SP-NOV Collected in a Ciguatera-Endemic Area. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Gomez, F.; Qiu, D.J.; Lopes, R.M.; Lin, S.J. Fukuyoa paulensis gen. et sp nov., a New Genus for the Globular Species of the Dinoflagellate Gambierdiscus (Dinophyceae). PLoS ONE 2015, 10, e0119676. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Gatti, C.; Roué, M.; Darius, H. Ciguatera poisoning in French Polynesia: Insights into the novel trends of an ancient disease. New Microbes New Infect. 2019, 31, 100565. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, J.A.; Garcia-Alvarez, N.; Fernandez, A.; Saavedra, P.; Sergent, F.S.; Padilla, D.; Acosta-Hernandez, B.; Suarez, M.M.; Diogene, J.; Real, F. Predictive score and probability of CTX-like toxicity in fish samples from the official control of ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Tudo, A.; Gaiani, G.; Varela, M.R.; Tsumuraya, T.; Andree, K.B.; Fernandez-Tejedor, M.; Campas, M.; Diogene, J. Further Advance of Gambierdiscus Species in the Canary Islands, with the First Report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef]
- DG of Fisheries of the Canary Government. Official Control Protocol for CTX Detection of Fish Sampled at the Authorized First Sale Points, Implemented by the Canary Government. Available online: https://www.gobiernodecanarias.org/cmsgobcan/export/sites/pesca/galerias/doc/Veterinario/Protocolo-Ciguatoxina-Nov-2020.pdf (accessed on 15 January 2021).
- DG of Fisheries of the Canary Government. First-Sale-Points Fish Catch Statistics. Available online: https://www.gobiernodecanarias.org/agp/sgt/temas/estadistica/pesca/index.html (accessed on 29 October 2020).
- Papasissi, C.; Luna, S. FishBase. Available online: https://www.fishbase.in/Summary/SpeciesSummary.php?ID=1729&AT=black+moray (accessed on 21 September 2021).
- Gonzalez, J.A.; Correia, S.; Jimenez, S.; Monteiro, C.A.; Delgado, J.; Pinho, M.R.; Lorenzo, J.M.; Gonzalez-Lorenzo, G. The fish family Muraenidae: An ideal group for testing at small-scale the coherency of Macaronesia as a biogeographic unit, with the first report on separate fishery statistics. Sci. Mar. 2021, 85, 157–167. [Google Scholar] [CrossRef]
- Capuli, E.; Luna, S. FishBase. Available online: https://fishbase.mnhn.fr/Summary/SpeciesSummary.php?ID=6470&AT=dusky+grouper (accessed on 21 September 2021).
- Andrello, M.; Mouillot, D.; Beuvier, J.; Albouy, C.; Thuiller, W.; Manel, S. Low Connectivity between Mediterranean Marine Protected Areas: A Biophysical Modeling Approach for the Dusky Grouper Epinephelus marginatus. PLoS ONE 2013, 8, e68564. [Google Scholar] [CrossRef] [Green Version]
- Cavaleiro, B.; Hermida, M.; Saraiva, A. Parasites of amberjacks from the archipelago of Madeira, Eastern Atlantic. Dis. Aquat. Org. 2018, 131, 133–142. [Google Scholar] [CrossRef]
- Papasissi, C.; Bailly, N. FishBase. Available online: https://fishbase.mnhn.fr/Summary/SpeciesSummary.php?ID=1006&AT=amberjack (accessed on 21 September 2021).
- Binohlan, C.; Reyes, R. FishBase. Available online: https://fishbase.mnhn.fr/summary/Diplodus-vulgaris.html (accessed on 21 September 2021).
- Mouine, N.; Ktari, M.H.; Chakroun-Marzouk, N. Age and growth of Diplodus vulgaris (Sparidae) in the Gulf of Tunis. Cybium 2010, 34, 37–45. [Google Scholar]
- Cengiz, O.; Parug, S.S.; Kizilayka, B. Maximum Length Record of Common Two-banded Seabream (Diplodus vulgaris Geoffroy Saint-Hilaire, 1817) for Aegean Sea with Turkish Waters. Alinteri J. Agric. Sci. 2019, 34, 160–163. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Regional Variations in the Risk and Severity of Ciguatera Caused by Eating Moray Eels. Toxins 2017, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.J.; Vernoux, J.-P.; Brereton, I.M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Vernoux, J.P.; Lewis, R.J. Isolation and characterisation of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar] [CrossRef]
- Sanchez-Henao, A.; Garcia-Alvarez, N.; Sergent, F.S.; Estevez, P.; Gago-Martinez, A.; Martin, F.; Ramos-Sosa, M.; Fernandez, A.; Diogene, J.; Real, F. Presence of CTXs in moray eels and dusky groupers in the marine environment of the Canary Islands. Aquat. Toxicol. 2020, 221, 9. [Google Scholar] [CrossRef] [PubMed]
- Bshary, R.; Hohner, A.; Ait-El-Djoudi, K.; Fricke, H. Interspecific communicative and coordinated hunting between groupers and giant moray eels in the Red Sea. PLoS. Biol. 2006, 4, 2393–2398. [Google Scholar] [CrossRef]
- Canary Government. C.H.S. Summary of Records from “El Sistema de Vigilancia Epidemiológica de la Intoxicación por Ciguatera en Canarias (SVEICC)”, 2008–2018. Available online: https://www3.gobiernodecanarias.org/sanidad/scs/content/f5edb564-6fde-11e9-800c-bbb933db73e3/Cuadrobrotes2008-2018.pdf (accessed on 15 October 2020).
- Estevez, P.; Castro, D.; Leao, J.M.; Yasumoto, T.; Dickey, R.; Gago-Martinez, A. Implementation of liquid chromatography tandem mass spectrometry for the analysis of ciguatera fish poisoning in contaminated fish samples from Atlantic coasts. Food Chem. 2019, 280, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Boada, L.D.; Zumbado, M.; Luzardo, O.R.; Almeida-Gonzalez, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.E.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef]
- Agencia Española Seguridad Alimentaria y Nutrición (AESAN). EuroCigua Workshop 2020 English [Archivo de Video min 17:56]. Available online: https://www.youtube.com/watch?v=sglIrQIo_C0 (accessed on 15 February 2021).
- Estevez, P.; Castro, D.; Pequeno-Valtierra, A.; Leao, J.M.; Vilarino, O.; Diogene, J.; Gago-Martinez, A. An Attempt to Characterize the Ciguatoxin Profile in Seriola fasciata Causing Ciguatera Fish Poisoning in Macaronesia. Toxins 2019, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Ventura, D.; Bonhomme, V.; Colangelo, P.; Bonifazi, A.; Lasinio, G.J.; Ardizzone, G. Does morphology predict trophic niche differentiation? Relationship between feeding habits and body shape in four co-occurring juvenile species (Pisces: Perciformes, Sparidae). Estuar. Coast. Shelf Sci. 2017, 191, 84–95. [Google Scholar] [CrossRef]
- Bellassoued, K.; Hamza, A.; van Pelt, J.; Elfeki, A. Seasonal variation of Sarpa salpa fish toxicity, as related to phytoplankton consumption, accumulation of heavy metals, lipids peroxidation level in fish tissues and toxicity upon mice. Environ. Monit. Assess. 2013, 185, 1137–1150. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Ciguatera Fish Poisoning in East Asia and Southeast Asia. Marine Drugs 2015, 13, 3466–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, D.L.; Galbraith, R.M.; McMillan, J.P.; Higerd, T.B. Preliminary Immunological Studies of Ciguatera Poisoning. Arch. Intern. Med. 1983, 143, 1931–1933. [Google Scholar] [CrossRef] [PubMed]
- Morey, J.S.; Ryan, J.C.; Dechraoui, M.Y.B.; Rezvani, A.H.; Levin, E.D.; Gordon, C.J.; Ramsdell, J.S.; Van Dolah, F.M. Liver genomic responses to ciguatoxin: Evidence for activation of phase I and phase II detoxification pathways following an acute hypothermic response in mice. Toxicol. Sci. 2008, 103, 298–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernoux, J.P.; Lahlou, N.; Elandaloussi, S.A.; Riyeche, N.; Magras, L.P. A Study of the Distribution of Ciguatoxin in Individual Caribbean Fish. Acta Trop. 1985, 42, 225–233. [Google Scholar]
- Hokama, Y.; Banner, A.H.; Boylan, D.B. Radioimmunoassay for Detection of Ciguatoxin. Toxicon 1977, 15, 317–325. [Google Scholar] [CrossRef]
- Rayner, M.; Kosaki, T.; Fellmeth, E. Ciguatoxin: More than an Anticholinesterase. Science 1968, 80, 70–71. [Google Scholar] [CrossRef]
- Yasumoto, T.; Scheuer, P. Marine Toxins of the Pacific Part 8 Ciguatoxin from Moray Eel Livers. Toxicon 1969, 7, 273–276. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; de Mitcheson, Y.S.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.H.; Xiao, C.G.; Chen, Y.M.; Shen, J.C.; Wang, Q.; Ruan, Y.F.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, T.; Laczka, O.; Murray, S.A. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1B, in Spanish Mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Chateau-Degat, M.L.; Huin-Blondey, M.O.; Chinain, M.; Darius, T.; Legrand, A.M.; Nguyen, N.L.; Laudon, F.; Chansin, R.; Dewailly, E. Prevalence of chronic symptoms of Ciguatera disease in french polynesian adults. Am. J. Trop. Med. Hyg. 2007, 77, 842–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnis, R. Concerning a fatal case of Ciguatera Poisoning in the Tuamotu Islands. Clin. Toxicol. 1970, 3, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. The role of free radicals in toxicity and disease. J. Basic Clin. Physiol. Pharmacol. 1995, 6, 205–228. [Google Scholar] [CrossRef]
- Schlenk, D.; Celander, M.; Gallagher, E.; George, S.; James, M.; Kullman, S.; Van den Hurk, P.; Willet, K. Biotransformation in Fishes. In The Toxicology of Fishes; CRC Press, Taylor and Francis Group: London, UK, 2008; pp. 153–234. [Google Scholar]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.W.; Li, X.M.; Lam, P.K.S.; Cheng, S.H.; Schlenk, D.; de Mitcheson, Y.S.; Li, Y.; Gu, J.D.; Chan, L.L. Proteomic analysis of hepatic tissue of ciguatoxin (CTX) contaminated coral reef fish Cephalopholis argus and moray eel Gymnothorax undulatus. Harmful Algae 2012, 13, 65–71. [Google Scholar] [CrossRef]
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of Toxins Involved in Ciguatera Fish Poisoning in the Pacific by LC/MS. J. Aoac Int. 2014, 97, 398–402. [Google Scholar] [CrossRef]
- Ledreux, A.; Brand, H.; Chinain, M.; Bottein, M.Y.D.; Ramsdell, J.S. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Sanchez-Henao, A.; Garcia-Alvarez, N.; Padilla, D.; Ramos-Sosa, M.; Sergent, F.S.; Fernandez, A.; Estevez, P.; Gago-Martinez, A.; Diogene, J.; Real, F. Accumulation of C-CTX1 in Muscle Tissue of Goldfish (Carassius auratus) by Dietary Experience. Animals 2021, 11, 242. [Google Scholar] [CrossRef]
- Dierking, F.; Cawpora, C.E. Ciguatera in the Introduced Fish Cephalopholis argus (Serranidae) in Hawai’i and Implications for Fishery Management. Pac. Sci. 2009, 63, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.; Suárez, F.C.; Ramirez, A.; Acosta, F. Ciguatera, an emerging human poisoning in Europe. J. Aquac. Mar. Biol. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Review Article: Ciguatoxic Potential of Brown-Marbled Grouper in Relation to Fish Size and Geographical Origin. Am. J. Trop. Med. Hyg. 2015, 93, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Costa, P.R.; Estevez, P.; Castro, D.; Solino, L.; Gouveia, N.; Santos, C.; Rodrigues, S.M.; Leao, J.M.; Gago-Martinez, A. New Insights into the Occurrence and Toxin Profile of Ciguatoxins in Selvagens Islands (Madeira, Portugal). Toxins 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Estevez, P.; Sibat, M.; Leao-Martins, J.M.; Costa, P.R.; Gago-Martinez, A.; Hess, P. Liquid Chromatography Coupled to High-Resolution Mass Spectrometry for the Confirmation of Caribbean Ciguatoxin-1 as the Main Toxin Responsible for Ciguatera Poisoning Caused by Fish from European Atlantic Coasts. Toxins 2020, 12, 267. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Mahmoud, H. Feeding Biology of Diplodus sargus and Diplodus vulgaris (Teleostei, Sparidae) in Egyptian Mediterranean Waters. World J. Fish Mar. Sci. 2009, 1, 290–296. [Google Scholar]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish. Biol. Fish. 2001, 11, 217–254. [Google Scholar] [CrossRef]
- Andaloro, F.; Pipitone, C. Food and feeding habits of the amberjack, Seriola dumerili in the Central Mediterranean Sea during the spawning season. Cah. Biol. Mar. 1997, 38, 91–96. [Google Scholar]
- Gracia López, V.; Castelló i Orvay, F. Food habits of groupers Epinephelus marginatus (Lowe, 1834) and Epinephelus costae (Steindachner, 1878) in the Mediterranean Coast of Spain. Hidrobiológica 2005, 15, 27–34. [Google Scholar]
- Costa, P.R.; Estevez, P.; Solino, L.; Castro, D.; Rodrigues, S.M.; Timoteo, V.; Leao-Martins, J.M.; Santos, C.; Gouveia, N.; Diogene, J.; et al. An Update on Ciguatoxins and CTX-like Toxicity in Fish from Different Trophic Levels of the Selvagens Islands (NE Atlantic, Madeira, Portugal). Toxins 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Bas, C.; Castro, J.; Hernández-García, V.; Lorenzo, J.; Moreno, T.; Pajuelo, J.; Ramos, A.G. La Pesca en Canarias y Áreas de Influencia; Ediciones del Cabildo Insular de Gran Canaria: Las Palmas de Gran Canaria, Spain, 1995; p. 331. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Major Fishing Areas. Atlantic, Eastern Central (Major Fishing Area 34). CWP Data Collection. Available online: https://www.fao.org/fishery/en/area/34/en (accessed on 20 September 2020).
- Lewis, R. Detection of toxins associated with ciguatera fish poisoning. In Manual on Harmful Marine Microalgae; UNESCO: Paris, France, 2003; Volume 11, pp. 267–277. [Google Scholar]
- Diogene, J.; Reverte, L.; Rambla-Alegre, M.; del Rio, V.; de la Iglesia, P.; Campas, M.; Palacios, O.; Flores, C.; Caixach, J.; Ralijaona, C.; et al. Identification of ciguatoxins in a shark involved in a fatal food poisoning in the Indian Ocean. Sci. Rep. 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS Analysis of Ciguatoxins Revealing Distinct Regional and Species Characteristics in Fish and Causative Alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Leao, J.M.; Yasumoto, T.; Dickey, R.W.; Gago-Martinez, A. Caribbean Ciguatoxin-1 stability under strongly acidic conditions: Characterisation of a new C-CTX1 methoxy congener. Food Addit. Contam. Part A-Chem. 2020, 37, 519–529. [Google Scholar] [CrossRef] [PubMed]


| Fish Studied | Amberjack (Seriola spp.) na = 60 | Dusky Grouper (E. marginatus) na = 27 | Black Moray Eel (M. helena) na = 11 | Common Two-Banded Seabream (D. vulgaris) na = 11 | Total na = 109 | |
|---|---|---|---|---|---|---|
| ng CTX1B Eq·(g flesh)−1 | nb | 57 | 22 | 7 | 7 | 93 |
| Mean ± SD | 0.165 ± 0.264 | 0.238 ± 0.325 | 0.096 ± 0.081 | 0.036 ± 0.011 | ||
| Median | 0.061 | 0.088 | 0.058 | 0.031 | ||
| Minimum | 0.011 | 0.013 | 0.026 | 0.024 | ||
| Maximum | 1.306 | 1.365 | 0.217 | 0.051 | ||
| ng CTX1B Eq·(g liver)−1 | nb | 59 | 27 | 11 | 10 | 107 |
| Mean ± SD | 0.953 ± 1.066 | 1.527 ± 1.317 | 1.949 ± 1.816 | 0.454 ± 0.223 | ||
| Median | 0.655 | 1.234 | 1.373 | 0.525 | ||
| Minimum | 0.069 | 0.020 | 0.361 | 0.084 | ||
| Maximum | 6.439 | 4.991 | 6.062 | 0.808 | ||
| Ratio (liver toxicity/flesh toxicity) | nb | 55 | 22 | 7 | 7 | 91 |
| Mean ± SD | 17.84 ± 25.05 | 35.51 ± 47.63 | 48.32 ± 41.55 | 16.32 ± 4.49 | ||
| Median | 8.39 | 14.94 | 27.91 | 17.19 | ||
| Minimum | 1.21 | 1.31 | 10.06 | 11.25 | ||
| Maximum | 130.15 | 155.70 | 124.09 | 24.69 |
| Fish Studied | Amberjack (Seriola spp.) n = 29 | Dusky Grouper (E. marginatus) n = 18 | Black Moray Eel (M. helena) n = 8 | Common Two-Banded Seabream (D. vulgaris) n = 7 | Total n = 62 | |
|---|---|---|---|---|---|---|
| ng·(g flesh)−1 by LC-MS/MS | C-CTX1 confirmed a | 8 | 15 | 2 | 5 | 30 |
| Mean ± SD | 0.109 ± 0.091 | 0.057 ± 0.059 | 0.035 ± 0.021 | 0.040 ± 0.017 | ||
| Median | 0.075 | 0.030 | 0.035 | 0.030 | ||
| Minimum | 0.020 | 0.018 | 0.020 | 0.030 | ||
| Maximum | 0.270 | 0.240 | 0.050 | 0.070 |
| Fish Studied | Amberjack n = 60 | Dusky Grouper n = 27 | Black Moray Eel n = 11 | Common Two-Banded Seabream n = 11 | |
|---|---|---|---|---|---|
| Individual weight a | Mean ± SD | 29.97 ± 12.41 | 19.53 ± 7.43 | 1.24 ± 0.86 | 0.46 ± 0.16 |
| Median | 28.50 | 21.30 | 0.98 | 0.48 | |
| Minimum | 3.53 | 3.80 | 0.41 | 0.15 | |
| Maximum | 73.00 | 33.00 | 2.81 | 0.71 | |
| Individual length b | Mean ± SD | 124.43 ± 42.33 | 92.32 ± 17.20 | 76.25 ± 17.24 | 28.17 ± 3.17 |
| Median | 132.00 | 98.50 | 74.00 | 29.50 | |
| Minimum | 66.00 | 58.00 | 56.90 | 21.00 | |
| Maximum | 190.00 | 110.00 | 110.00 | 32.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Sosa, M.J.; García-Álvarez, N.; Sanchez-Henao, A.; Silva Sergent, F.; Padilla, D.; Estévez, P.; Caballero, M.J.; Martín-Barrasa, J.L.; Gago-Martínez, A.; Diogène, J.; et al. Ciguatoxin Detection in Flesh and Liver of Relevant Fish Species from the Canary Islands. Toxins 2022, 14, 46. https://doi.org/10.3390/toxins14010046
Ramos-Sosa MJ, García-Álvarez N, Sanchez-Henao A, Silva Sergent F, Padilla D, Estévez P, Caballero MJ, Martín-Barrasa JL, Gago-Martínez A, Diogène J, et al. Ciguatoxin Detection in Flesh and Liver of Relevant Fish Species from the Canary Islands. Toxins. 2022; 14(1):46. https://doi.org/10.3390/toxins14010046
Chicago/Turabian StyleRamos-Sosa, María José, Natalia García-Álvarez, Andres Sanchez-Henao, Freddy Silva Sergent, Daniel Padilla, Pablo Estévez, María José Caballero, José Luís Martín-Barrasa, Ana Gago-Martínez, Jorge Diogène, and et al. 2022. "Ciguatoxin Detection in Flesh and Liver of Relevant Fish Species from the Canary Islands" Toxins 14, no. 1: 46. https://doi.org/10.3390/toxins14010046
APA StyleRamos-Sosa, M. J., García-Álvarez, N., Sanchez-Henao, A., Silva Sergent, F., Padilla, D., Estévez, P., Caballero, M. J., Martín-Barrasa, J. L., Gago-Martínez, A., Diogène, J., & Real, F. (2022). Ciguatoxin Detection in Flesh and Liver of Relevant Fish Species from the Canary Islands. Toxins, 14(1), 46. https://doi.org/10.3390/toxins14010046