Update of the Planktonic Diatom Genus Pseudo-nitzschia in Aotearoa New Zealand Coastal Waters: Genetic Diversity and Toxin Production
Abstract
:1. Introduction
2. Results
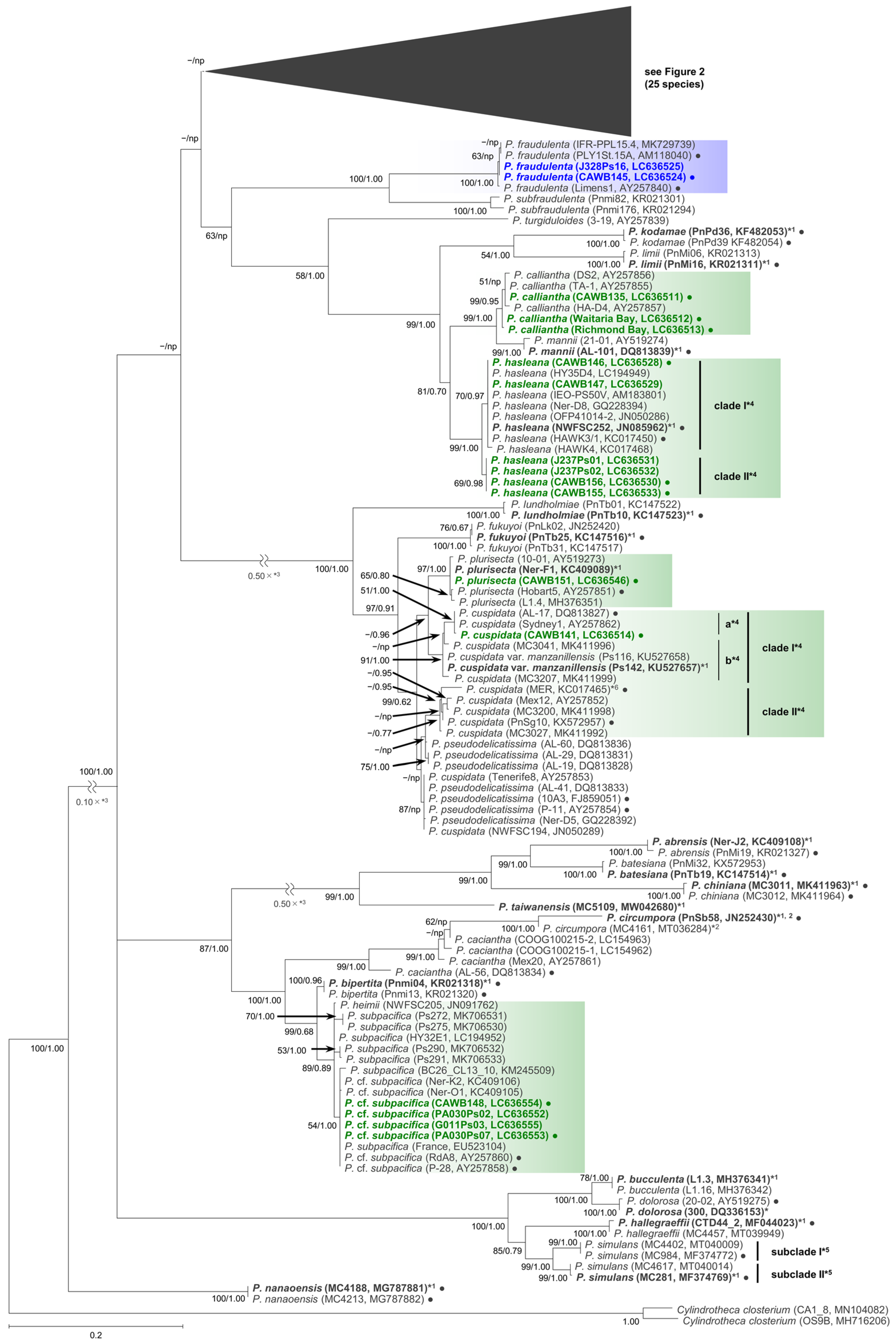
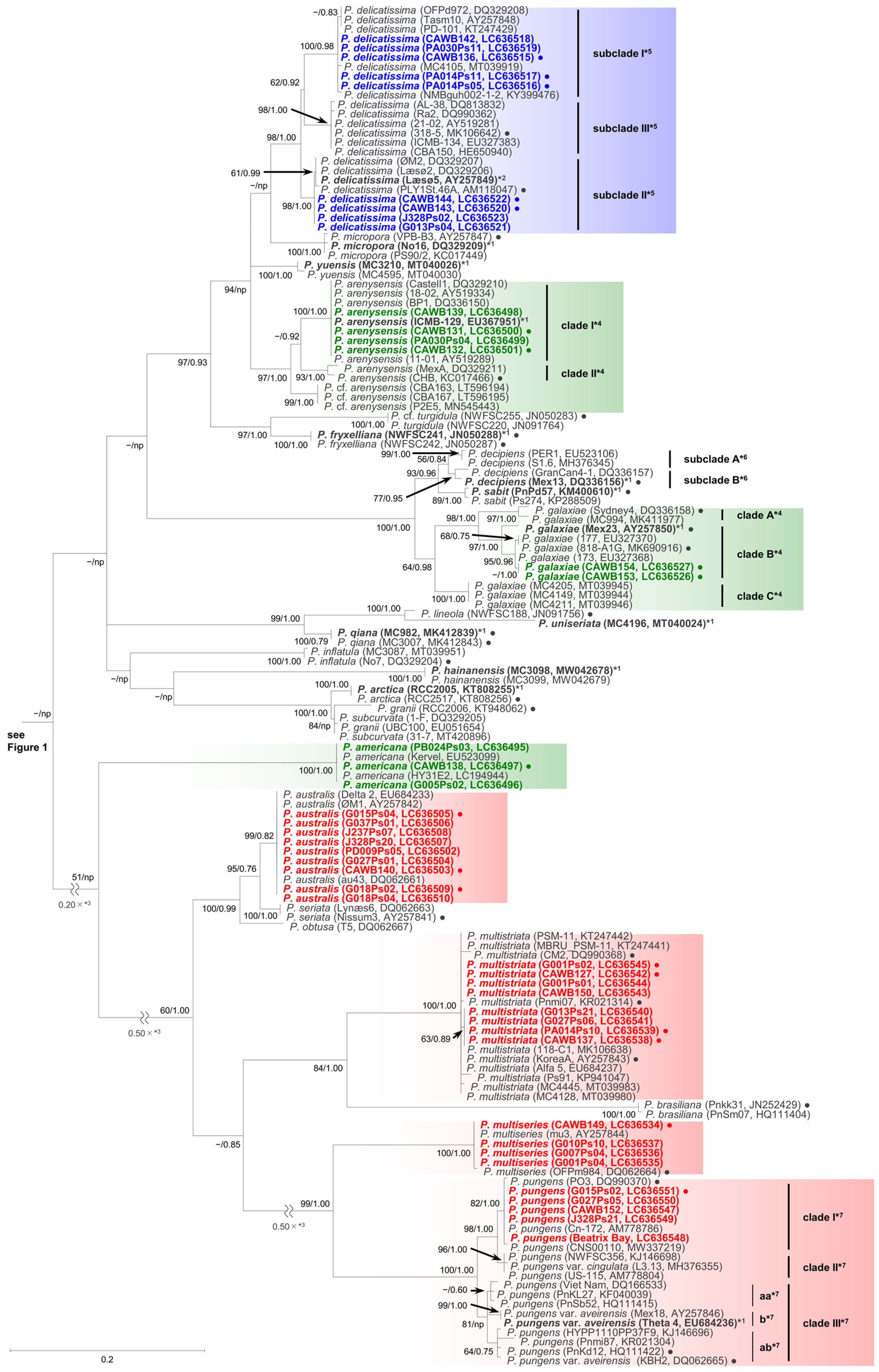
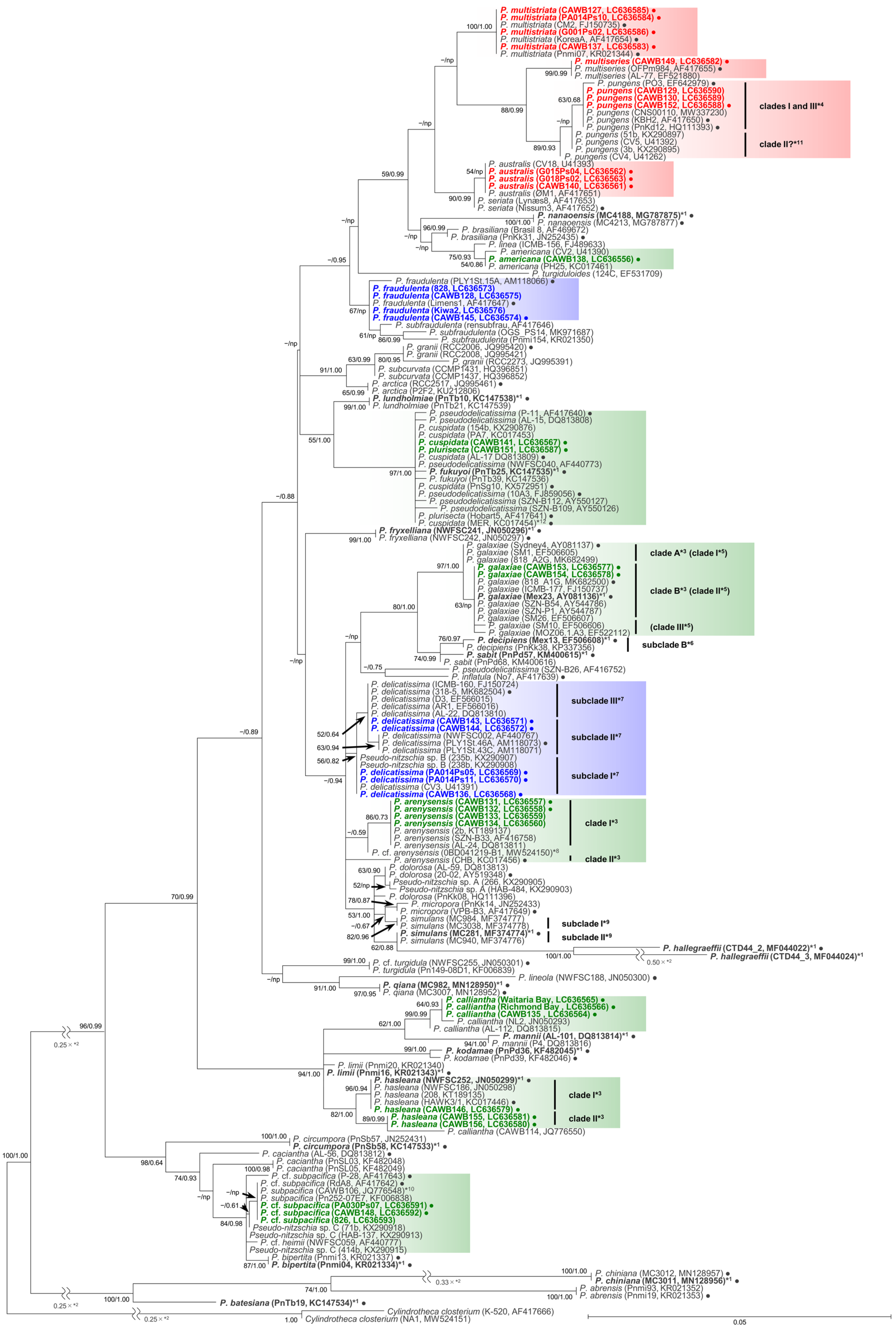
2.1. Molecular Phylogenetic Characteristics
2.1.1. Molecular Phylogeny
2.1.2. Sequence Analysis
2.2. Distribution
2.3. Toxin Production
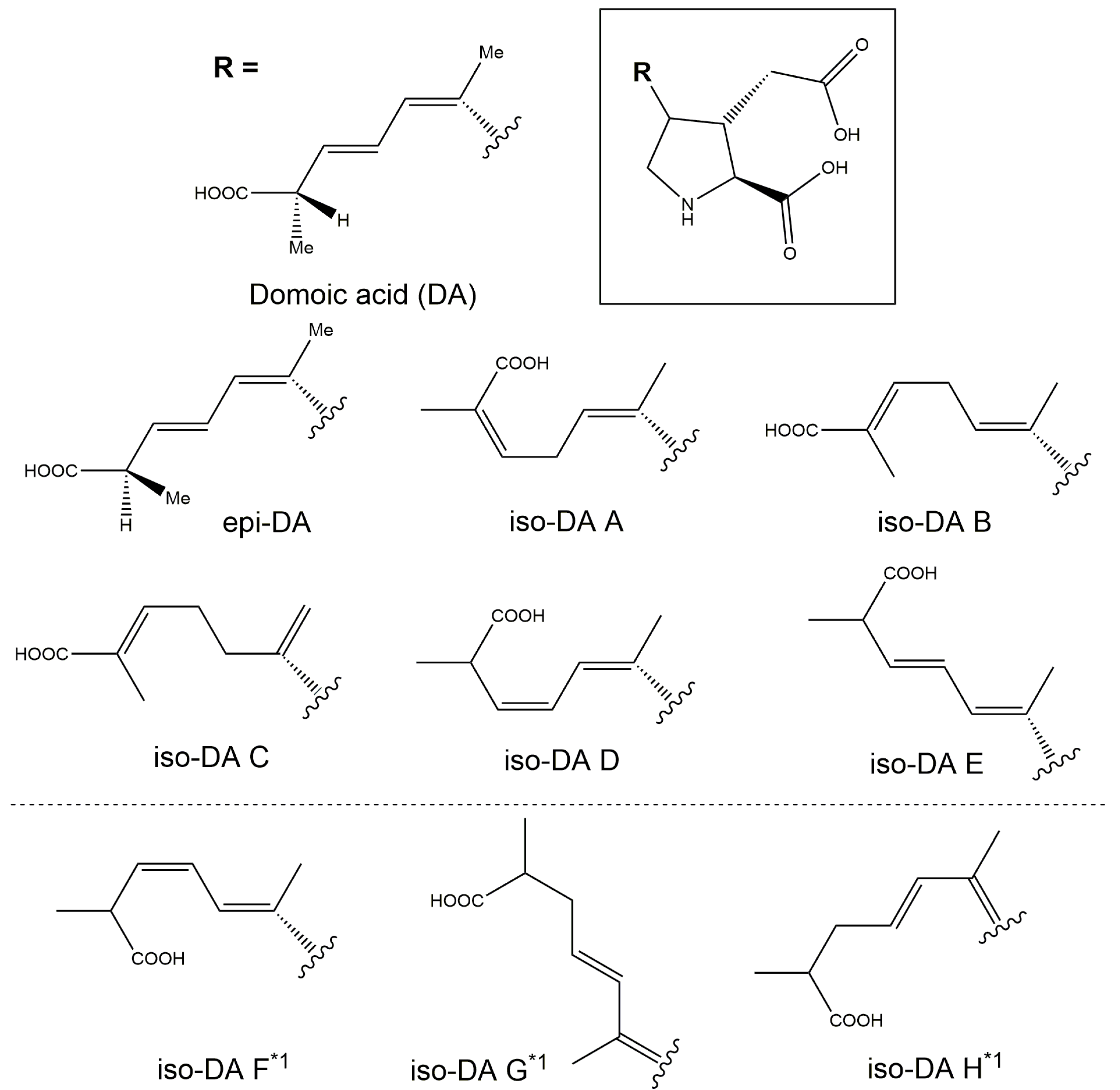
2.3.1. Production of Domoic Acid (DA) and Its Isomers
2.3.2. Relative Proportion of DA and Its Isomers
2.4. Potential ASP Risk of Each Pseudo-nitzschia Species in New Zealand Coastal Waters
2.4.1. Classification of Potential ASP Risk for Each Species
2.4.2. Distribution of Each Species
3. Discussion
3.1. Diversity of Pseudo-nitzschia Species in New Zealand
3.2. Toxin Production of Pseudo-nitzschia Species in New Zealand
3.3. Distribution of Pseudo-nitzschia Species with Potential ‘High, Low, or No ASP Risk’ in New Zealand
4. Materials and Methods
4.1. Sampling and Establishment of Clonal Isolates
4.2. Molecular Phylogenetic Characterisation
4.2.1. DNA Extraction, PCR, and Sequencing
4.2.2. Molecular Phylogenetic Analyses
4.2.3. Sequence Analysis
4.3. Toxin Analysis
4.3.1. Culturing, Harvesting, and Toxin Extraction
4.3.2. Instrument Analysis
4.4. Classification of Potential ASP Risk and Distribution Mapping of Each Pseudo-nitzschia Species in New Zealand Coastal Waters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Subba Rao, D.V.; Quilliam, M.A.; Pocklington, R. Domoic Acid—A Neurotoxic Amino Acid Produced by the Marine Diatom Nitzschia pungens in Culture. Can. J. Fish. Aquat Sci. 1988, 45, 2076–2079. [Google Scholar] [CrossRef]
- Bates, S.S.; Bird, C.J.; de Freitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington, R.; et al. Pennate Diatom Nitzschia pungens as the Primary Source of Domoic Acid, a Toxin in Shellfish from Eastern Prince Edward Island, Canada. Can. J. Fish. Aquat Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Wright, J.L.C. The Amnesic Shellfish Poisoning Mystery. Anal. Chem. 1989, 61, 1053A–1060A. [Google Scholar] [CrossRef]
- Hasle, G.R. Pseudo-nitzschia pungens and P. multiseries (Bacillariophyceae): Nomenclatural History, Morphology, and Distribution. J. Phycol. 1995, 31, 428–435. [Google Scholar] [CrossRef]
- Hay, B.E.; Grant, C.M.; McCoubrey, D.-J. A Review of the Marine Biotoxin Monitoring Programme for Non-Commercially Harvested Shellfish; Part 1: Technical report, A report prepared for the Ministry of Health by AquaBio consultants Ltd.; NZ Ministry of Health: Wellington, New Zealand, 2000; ISBN 0-478-24349-9.
- CODEX STAN 292-2008 Standard for Live and Raw Bivalve Molluscs. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B292-2008%252FCXS_292e_2015.pdf (accessed on 20 July 2021).
- Ministry for Primary Industries Animal Products Notice: Regulated Control Scheme-Bivalve Molluscan Shellfish for Human Consumption. Available online: https://www.mpi.govt.nz/dmsdocument/30282/direct (accessed on 20 July 2021).
- Clayden, J.; Read, B.; Hebditch, K.R. Chemistry of Domoic Acid, Isodomoic Acids, and Their Analogues. Tetrahedron 2005, 61, 5713–5724. [Google Scholar] [CrossRef]
- FAO/WHO. Toxicity Equivalence Factors for Marine Biotoxins Associated with Bivalve Molluscs; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-5-109345-0. [Google Scholar]
- Rhodes, L.; Jiang, W.; Knight, B.; Adamson, J.; Smith, K.; Langi, V.; Edgar, M. The Genus Pseudo-nitzschia (Bacillariophyceae) in New Zealand: Analysis of the Last Decade’s Monitoring Data. N. Z. J. Mar. Freshw. Res. 2013, 47, 490–503. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M.; Schweibold, L.; Jaffrezic, E.; Rhodes, L.; MacKenzie, L.; Hay, B.; Farrell, H. Overview of Australian and New Zealand Harmful Algal Species Occurrences and Their Societal Impacts in the Period 1985 to 2018, Including a Compilation of Historic Records. Harmful Algae 2021, 102, 101848. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; White, D.; Syhre, M.; Atkinson, M. Pseudo-nitzschia species isolated from New Zealand coastal waters: Domoic acid production in vitro and links with shellfish toxicity. In Harmful and Toxic Algal Blooms: Proceedings of the Seventh International Conference on Toxic Phytoplankton, Sendai, Japan, 12–16 July 1995; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovermental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 155–158. [Google Scholar]
- Chen, X.M.; Pang, J.X.; Huang, C.X.; Lundholm, N.; Teng, S.T.; Li, A.; Li, Y. Two New and Nontoxigenic Pseudo-nitzschia Species (Bacillariophyceae) from Chinese Southeast Coastal Waters. J. Phycol. 2021, 57, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Stonik, I.V.; Isaeva, M.P.; Aizdaicher, N.A.; Balakirev, E.S.; Ayala, F.J. Morphological and Genetic Identification of Pseudo-nitzschia H. Peragallo, 1900 (Bacillariophyta) from the Sea of Japan. Russ. J. Mar. Biol. 2018, 44, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Gai, F.F.; Hedemand, C.K.; Louw, D.C.; Grobler, K.; Krock, B.; Moestrup, Ø.; Lundholm, N. Morphological, Molecular and Toxigenic Characteristics of Namibian Pseudo-nitzschia Species–Including Pseudo-nitzschia bucculenta Sp. Nov. Harmful Algae 2018, 76, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, B.S.; Kim, J.-H.; Wang, P.; Han, M.-S. Intraspecific Diversity and Distribution of the Cosmopolitan Species Pseudo-nitzschia pungens (Bacillariophyceae): Morphology, Genetics, and Ecophysiology of the Three Clades. J. Phycol. 2015, 51, 159–172. [Google Scholar] [CrossRef]
- Ajani, P.A.; Lim, H.C.; Verma, A.; Lassudrie, M.; McBean, K.; Doblin, M.A.; Murray, S.A. First Report of the Potentially Toxic Marine Diatom Pseudo-nitzschia simulans (Bacillariophyceae) from the East Australian Current. Phycol. Res. 2020, 68, 254–259. [Google Scholar] [CrossRef]
- Ajani, P.A.; Verma, A.; Kim, J.H.; Woodcock, S.; Nishimura, T.; Farrell, H.; Zammit, A.; Brett, S.; Murray, S.A. Using QPCR and High-Resolution Sensor Data to Model a Multi-Species Pseudo-nitzschia (Bacillariophyceae) Bloom in Southeastern Australia. Harmful Algae 2021, 108, 102095. [Google Scholar] [CrossRef]
- McDonald, S.M.; Sarno, D.; Zingone, A. Identifying Pseudo-nitzschia Species in Natural Samples Using Genus-Specific PCR Primers and Clone Libraries. Harmful Algae 2007, 6, 849–860. [Google Scholar] [CrossRef]
- Giulietti, S.; Romagnoli, T.; Siracusa, M.; Bacchiocchi, S.; Totti, C.; Accoroni, S. Integrative Taxonomy of the Pseudo-nitzschia (Bacillariophyceae) Populations in the NW Adriatic Sea, with a Focus on a Novel Cryptic Species in the P. delicatissima Species Complex. Phycologia 2021, 60, 247–264. [Google Scholar] [CrossRef]
- Scholin, C.A.; Villac, M.C.; Buck, K.R.; Krupp, J.M.; Powers, D.A.; Fryxell, G.A.; Chavez, F.P. Ribosomal DNA Sequences Discriminate among Toxic and Non-Toxic Pseudo-nitzschia Species. Nat. Toxins 1994, 2, 152–165. [Google Scholar] [CrossRef]
- Bowers, H.A.; Marin, R.; Birch, J.M.; Scholin, C.A. Sandwich Hybridization Probes for the Detection of Pseudo-nitzschia (Bacillariophyceae) Species: An Update to Existing Probes and a Description of New Probes. Harmful Algae 2017, 70, 37–51. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid Extraction and Cleanup for Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/ (accessed on 20 July 2021).
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and Domoic Acid: New Research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Lim, H.C.; Tan, S.N.; Teng, S.T.; Lundholm, N.; Orive, E.; David, H.; Quijano-Scheggia, S.; Leong, S.C.Y.; Wolf, M.; Bates, S.S.; et al. Phylogeny and Species Delineation in the Marine Diatom Pseudo-nitzschia (Bacillariophyta) Using Cox1, LSU, and ITS2 RRNA Genes: A Perspective in Character Evolution. J. Phycol. 2018, 54, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Kooistra, W.H.C.F.; Levialdi Ghiron, J.H.; Mann, D.G.; Pröschold, T.; Montresor, M. Reproductive Isolation among Sympatric Cryptic Species in Marine Diatoms. Protist 2007, 158, 193–207. [Google Scholar] [CrossRef]
- Dong, H.C.; Lundholm, N.; Teng, S.T.; Li, A.; Wang, C.; Hu, Y.; Li, Y. Occurrence of Pseudo-nitzschia Species and Associated Domoic Acid Production along the Guangdong Coast, South China Sea. Harmful Algae 2020, 98, 101899. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, G.; Chepurnov, V.A.; Leliaert, F.; Mann, D.G.; Bates, S.S.; Lundholm, N.; Rhodes, L.; Sabbe, K.; Vyverman, W. Pseudo-nitzschia pungens (Bacillariophyceae): A Cosmopolitan Diatom Species? Harmful Algae 2008, 7, 241–257. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.-H.; Park, B.S.; Wang, P.; Patidar, S.K.; Han, M.-S. Development of a QPCR Assay for Tracking the Ecological Niches of Genetic Sub-Populations within Pseudo-nitzschia pungens (Bacillariophyceae). Harmful Algae 2017, 63, 68–78. [Google Scholar] [CrossRef]
- Rhodes, L.; Scholin, C.; Garthwaite, I.; Haywood, A.; Thomas, A. Domoic acid producing Pseudo-nitzschia species educed by whole cell DNA probe-based and immunochemical assay. In Harmful algae = Algas nocivas: Proceedings of the VIII International Conference on Harmful Algae. Vigo, Spain, 25–29 June 1997; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, NY, USA, 1998; pp. 274–277. ISBN 84-453-2166-8. [Google Scholar]
- Rhodes, L.; Scholin, C.; Garthwaite, I. Pseudo-nitzschia in New Zealand and the Role of DNA Probes and Immunoassays in Refining Marine Biotoxin Monitoring Programmes. Nat. Toxins 1998, 6, 105–111. [Google Scholar] [CrossRef]
- Rhodes, L.L. Identification of Potentially Toxic Pseudo-nitzschia (Bacillariophyceae) in New Zealand Coastal Waters, Using Lectins. N. Z. J. Mar. Freshw. Res. 1998, 32, 537–544. [Google Scholar] [CrossRef]
- Dermastia, T.T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological Time Series and Integrative Taxonomy Unveil Seasonality and Diversity of the Toxic Diatom Pseudo-nitzschia H. Peragallo in the Northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ajani, P.; Murray, S.A.; Kim, J.-H.; Lim, H.C.; Teng, S.T.; Lim, P.T.; Han, M.-S.; Park, B.S. Sexual Reproduction and Genetic Polymorphism within the Cosmopolitan Marine Diatom Pseudo-nitzschia pungens. Sci. Rep. 2020, 10, 10653. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Hasle, G.R.; Hoef-Emden, K. A Study of the Pseudo-nitzschia pseudodelicatissima/cuspidata Complex (Bacillariophyceae): What Is P. pseudodelicatissima? J. Phycol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Orive, E.; Pérez-Aicua, L.; David, H.; García-Etxebarria, K.; Laza-Martínez, A.; Seoane, S.; Miguel, I. The Genus Pseudo-nitzschia (Bacillariophyceae) in a Temperate Estuary with Description of Two New Species: Pseudo-nitzschia plurisecta Sp. Nov. and Pseudo-nitzschia abrensis Sp. Nov. J. Phycol. 2013, 49, 1192–1206. [Google Scholar] [CrossRef]
- Rivera-Vilarelle, M.; Valdez-Velázquez, L.L.; Quijano-Scheggia, S.I. Description of Pseudo-nitzschia cuspidata Var. manzanillensis Var. Nov. (Bacillariophyceae): Morphology and Molecular Characterization of a Variety from the Central Mexican Pacific. Diatom Res. 2018, 33, 55–68. [Google Scholar] [CrossRef]
- Huang, C.X.; Dong, H.C.; Lundholm, N.; Teng, S.T.; Zheng, G.C.; Tan, Z.J.; Lim, P.T.; Li, Y. Species Composition and Toxicity of the Genus Pseudo-nitzschia in Taiwan Strait, Including P. chiniana Sp. Nov. and P. qiana Sp. Nov. Harmful Algae 2019, 84, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, N.; Moestrup, Ø.; Kotaki, Y.; Hoef-Emden, K.; Scholin, C.; Miller, P. Inter- and Intraspecific Variation of the Pseudo-nitzschia delicatissima Complex (Bacillariophyceae) Illustrated by RRNA Probes, Morphological Data and Phylogenetic Analyses. J. Phycol. 2006, 42, 464–481. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.I.; Garcés, E.; Lundholm, N.; Moestrup, Ø.; Andree, K.; Camp, J. Morphology, Physiology, Molecular Phylogeny and Sexual Compatibility of the Cryptic Pseudo-nitzschia delicatissima Complex (Bacillariophyta), Including the Description of P. arenysensis Sp. Nov. Phycologia 2009, 48, 492–509. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø. The Marine Diatom Pseudo-nitzschia galaxiae Sp. Nov. (Bacillariophyceae): Morphology and Phylogenetic Relationships. Phycologia 2002, 41, 594–605. [Google Scholar] [CrossRef]
- Lundholm, N.; Bates, S.S.; Baugh, K.A.; Bill, B.D.; Connell, L.B.; Léger, C.; Trainer, V.L. Cryptic and Pseudo-Cryptic Diversity in Diatoms—with Descriptions of Pseudo-nitzschia hasleana Sp. Nov. and P. fryxelliana Sp. Nov. J. Phycol. 2012, 48, 436–454. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.I.; Olivos-Ortiz, A.; Garcia-Mendoza, E.; Sánchez-Bravo, Y.; Sosa-Avalos, R.; Marias, N.S.; Lim, H.C. Phylogenetic Relationships of Pseudo-nitzschia subpacifica (Bacillariophyceae) from the Mexican Pacific, and Its Production of Domoic Acid in Culture. PLoS ONE 2020, 15, e0231902. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A.; Sim, P.G.; McCulloch, A.W.; McInnes, A.G. High-Performance Liquid Chromatography of Domoic Acid, a Marine Neurotoxin, with Application to Shellfish and Plankton. Int. J. Environ. Anal. Chem. 1989, 36, 139–154. [Google Scholar] [CrossRef]
- Quilliam, M.A. Chemical methods for domoic acid, the amnesic shellfish poisoning (ASP) toxin. In Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2003; pp. 247–266. ISBN 92-3-103948-2. [Google Scholar]
- European Food Safety Authority Marine Biotoxins in Shellfish–Domoic Acid. EFSA J. 2009, 7, 1181. [CrossRef]
- Kotaki, Y.; Lundholm, N.; Katayama, T.; Furio, E.F.; Romero, M.L.; Relox, J.R.; Yasumoto, T.; Naoki, H.; Hirose, M.Y.; Thanh, T.D.; et al. ASP toxins of pennate diatoms and bacterial effects on the variation in toxin composition. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; Moestrup, Ø., Doucette, G., Enevoldsen, H., Godhe, A., Hallegraeff, G., Luckas, B., Lundholm, N., Lewis, J., Rengefors, K., Sellner, K., et al., Eds.; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO: Copenhagen, Denmark, 2008; pp. 300–302. [Google Scholar]
- Hansen, L.R.; Soylu, S.í.; Kotaki, Y.; Moestrup, Ø.; Lundholm, N. Toxin Production and Temperature-Induced Morphological Variation of the Diatom Pseudo-nitzschia seriata from the Arctic. Harmful Algae 2011, 10, 689–696. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Holland, P.T.; Adamson, J.E.; McNabb, P.; Selwood, A.I. Production of a new isomer of domoic acid by New Zealand isolates of the diatom Pseudo-nitzschia australis. In Molluscan Shellfish Safety: Proceedings of the 4th International Conference on Molluscan Shellfish Safety, Santiago de Compostela, Spain, 4–8 June 2002; Villalba, A., Reguera, B., Romalde, J.L., Beiras, R., Eds.; Consellería de Pesca e Asuntos Marítimos da Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 2003; pp. 43–48. ISBN 84-453-3638-X. [Google Scholar]
- Holland, P.T.; Selwood, A.I.; Mountfort, D.O.; Wilkins, A.L.; McNabb, P.; Rhodes, L.L.; Doucette, G.J.; Mikulski, C.M.; King, K.L. Isodomoic Acid C, an Unusual Amnesic Shellfish Poisoning Toxin from Pseudo-nitzschia australis. Chem. Res. Toxicol. 2005, 18, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.J.; Leithoff, A.; Altenburger, A.; Krock, B.; Beszteri, B.; Eggers, S.L.; Lundholm, N. First Evidence of the Toxin Domoic Acid in Antarctic Diatom Species. Toxins 2021, 13, 93. [Google Scholar] [CrossRef]
- Caruana, A.M.N.; Ayache, N.; Raimbault, V.; Rétho, M.; Hervé, F.; Bilien, G.; Amzil, Z.; Chomérat, N. Direct Evidence for Toxin Production by Pseudo-nitzschia plurisecta (Bacillariophyceae) and Extension of Its Distribution Area. Eur. J. Phycol. 2019, 54, 585–594. [Google Scholar] [CrossRef]
- Rhodes, L.; Holland, P.; Adamson, J.; Selwood, A.; McNabb, P. Mass culture of New Zealand isolates of Pseudo-nitzschia australis for production of a new isomer of domoic acid. In Harmful Algae 2002. Proceedings of the Xth International Conference on Harmful Algae. St. Pete Beach, Florida, USA, 21–25 October 2002; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO: St. Petersburg, FL, USA, 2004; pp. 125–127. [Google Scholar]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) Species, Domoic Acid and Amnesic Shellfish Poisoning: Revisiting Previous Paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef] [Green Version]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia Physiological Ecology, Phylogeny, Toxicity, Monitoring and Impacts on Ecosystem Health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, A.L.; White, D.A.; Sim, P.G.; Holland, A.J. Domoic acid and the New Zealand Greenshell mussel (Perna canaliculus). In Toxic Phytoplankton Blooms in the Sea: Proceedings of the Fifth International Conference on Toxic Marine Phytoplankton, Newport, RI, USA, 28 October–1 November 1991; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, NY, USA, 1993; pp. 607–612. ISBN 978-0-444-89719-0. [Google Scholar]
- Rhodes, L.; Jiang, W.; Knight, B.; Adamson, J.; Smith, K.; Langi, V.; Edgar, M.; Munday, R. The Genus Pseudo-nitzschia (Bacillariophyceae) in New Zealand: A Review of the Last Decade’s Research Achievements and Monitoring Data. Prepared for Seafood Safety Programme Funded by Ministry of Science and Innovation. Cawthron Rep. 2012, 2035B. [Google Scholar]
- Ajani, P.; Murray, S.; Hallegraeff, G.; Lundholm, N.; Gillings, M.; Brett, S.; Armand, L. The Diatom Genus Pseudo-nitzschia (Bacillariophyceae) in New South Wales, Australia: Morphotaxonomy, Molecular Phylogeny, Toxicity, and Distribution. J. Phycol. 2013, 49, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Wells, M.L.; Cochlan, W.P.; Trick, C.G.; Bill, B.D.; Baugh, K.A.; Beall, B.F.; Herndon, J.; Lundholm, N. An Ecological Study of a Massive Bloom of Toxigenic Pseudo-nitzschia cuspidata off the Washington State Coast. Limnol. Oceanogr. 2009, 54, 1461–1474. [Google Scholar] [CrossRef]
- Rhodes, L.; Selwood, A.; McNabb, P.; Briggs, L.; Adamson, J.; van Ginkel, R.; Laczka, O. Trace Metal Effects on the Production of Biotoxins by Microalgae. Afr. J. Mar. Sci. 2006, 28, 393–397. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Adamson, J.; Scholin, C. Pseudo-nitzschia multistriata (Bacillariophyceae) in New Zealand. N. Z. J. Mar. Freshw. Res. 2000, 34, 463–467. [Google Scholar] [CrossRef]
- Penna, A.; Bertozzini, E.; Battocchi, C.; Galluzzi, L.; Giacobbe, M.G.; Vila, M.; Garces, E.; Lugliè, A.; Magnani, M. Monitoring of HAB Species in the Mediterranean Sea through Molecular Methods. J. Plankton Res. 2007, 29, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, E.; Caron, D.A.; Schnetzer, A. Development and Environmental Application of a Genus-Specific Quantitative PCR Approach for Pseudo-nitzschia Species. Mar. Biol. 2010, 157, 1161–1169. [Google Scholar] [CrossRef]
- Andree, K.B.; Fernández-Tejedor, M.; Elandaloussi, L.M.; Quijano-Scheggia, S.; Sampedro, N.; Garcés, E.; Camp, J.; Diogène, J. Quantitative PCR Coupled with Melt Curve Analysis for Detection of Selected Pseudo-nitzschia Spp. (Bacillariophyceae) from the Northwestern Mediterranean Sea. Appl. Environ. Microbiol. 2011, 77, 1651–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings of the Conference on Culture of Marine Invertebrate Animals, Greenport, NY, USA, October 1972; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Richlen, M.L.; Barber, P.H. A Technique for the Rapid Extraction of Microalgal DNA from Single Live and Preserved Cells. Mol. Ecol. Notes 2005, 5, 688–691. [Google Scholar] [CrossRef]
- Bowers, H.A.; Tomas, C.; Tengs, T.; Kempton, J.W.; Lewitus, A.J.; Oldach, D.W. Raphidophyceae [Chadefaud Ex Silva] Systematics and Rapid Identification: Sequence Analyses and Real-Time PCR Assays. J. Phycol. 2006, 42, 1333–1348. [Google Scholar] [CrossRef] [Green Version]
- Nunn, G.B.; Theisen, B.F.; Christensen, B.; Arctander, P. Simplicity-Correlated Size Growth of the Nuclear 28S Ribosomal RNA D3 Expansion Segment in the Crustacean Order Isopoda. J. Mol. Evol. 1996, 42, 211–223. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Nylander, J.A.A. MrModeltest v2. Program. Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the Culture of Oceanic Ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- McNabb, P.; Selwood, A.I.; Holland, P.T. Collaborators: Multiresidue Method for Determination of Algal Toxins in Shellfish: Single-Laboratory Validation and Interlaboratory Study. J. AOAC Int. 2005, 88, 761–772. [Google Scholar] [CrossRef] [Green Version]


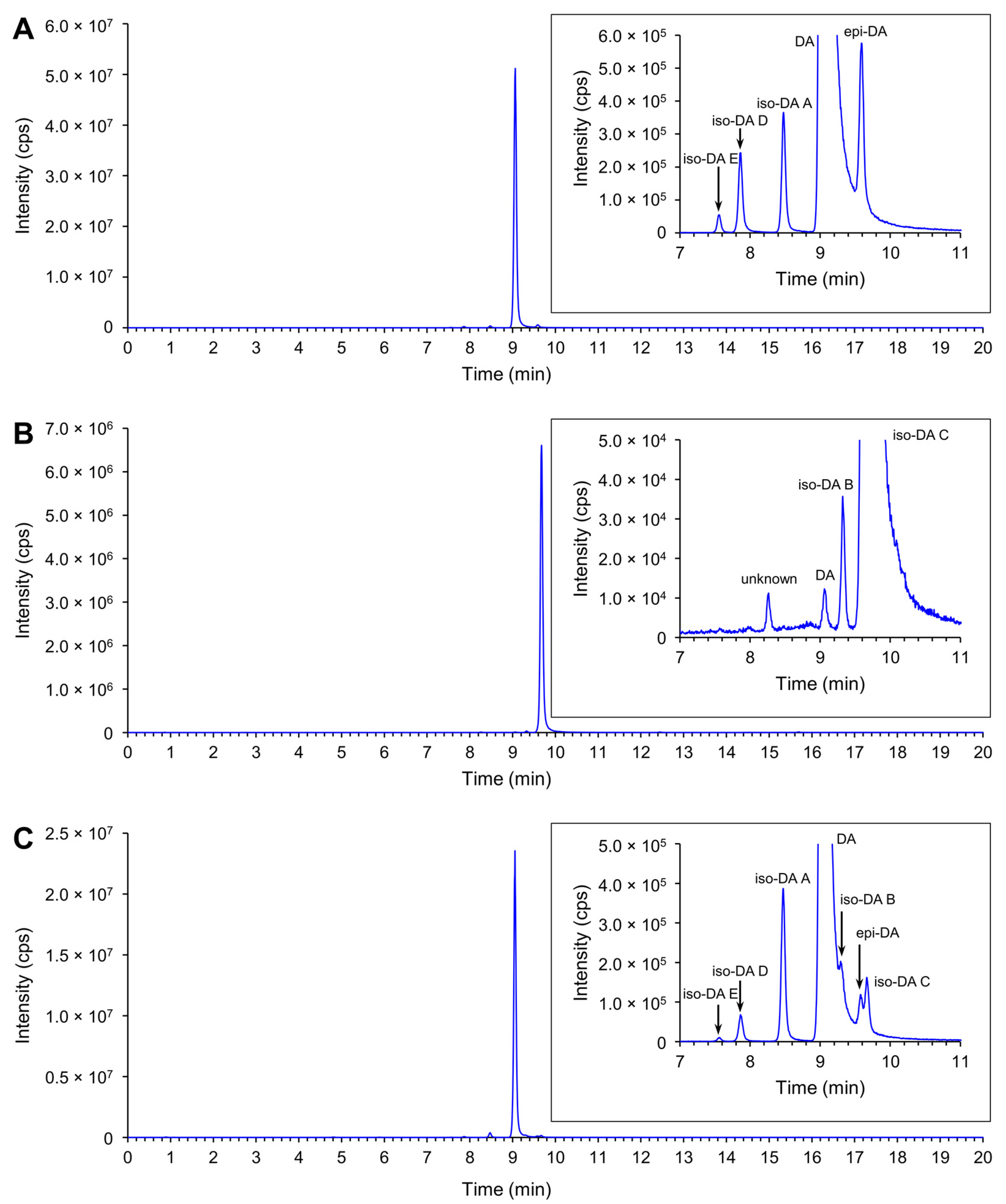
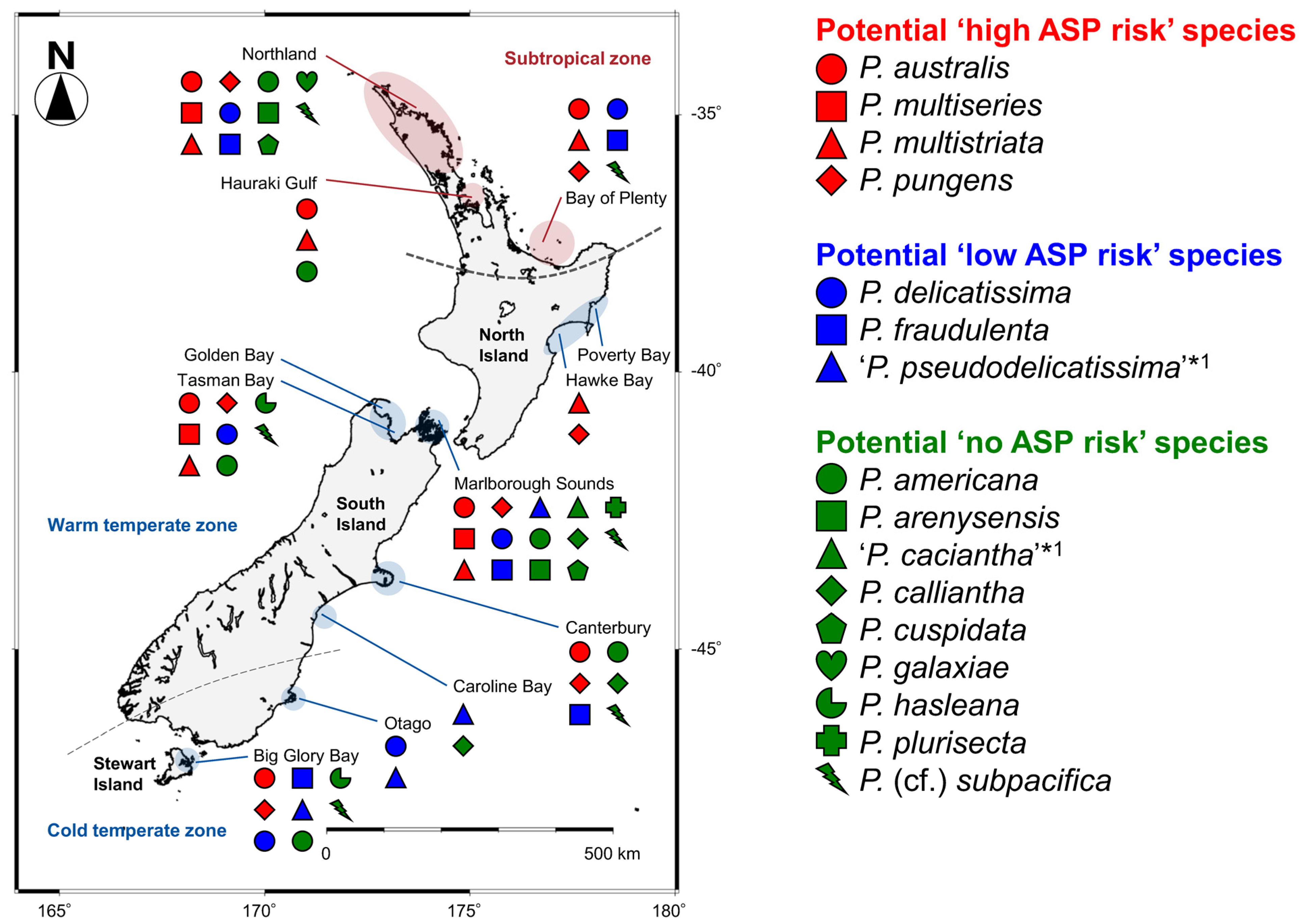
| Potential ASP Risk in New Zealand a | Species | First Record in New Zealand | Range of DA and DA Isomers Cell Quota (pg cell−1) b, c | Locality of Clonal Isolates in New Zealand d | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | epi-DA | iso-DA A | iso-DA B e | iso-DA C | iso-DA D | iso-DA E | Subtropical | Warm Temperate | Cold Temperate | |||||||||||||
| Northland f | Hauraki Gulf | Bay of Plenty f | Poverty Bay/Hawke Bay | Golden Bay f/Tasman Bay f | Marlborough Sounds f | Canterbury | Caroline Bay | Otago | Big GloryBay f | |||||||||||||
| High | P. australis | 1993 | 0.004–2.20(0–35.00) g | 0–0.010 | 0–0.060 | trace | 0–1.700 (9.050 h) | 0–0.003 | 0–trace | ◎ | ◎ | ◎ i ● | ● | ◎ ● | ◎ i | ◎ ● | ||||||
| P. multiseries | 1997 | 0.47–7.20 | 0–0.012 | 0.014–0.206 | 0–trace | 0–0.239 | 0.003–0.020 | trace–0.005 | ◎ i | ◎ ● | ● | |||||||||||
| P. multistriata | 1997 | 0–1.60 | 0 | 0–trace | 0 | 0–0.200 | 0 | 0 | ◎ ● | ◎ | ◎ | ◎ i | ◎ ● | ◎ ● | ||||||||
| P. pungens | 1993 | 0–0.47 | 0 | 0 | 0 | 0 | 0 | 0 | ● | ◎ i | ◎ | ◎ ● | ◎ ● | ◎ | ◎ ● | |||||||
| Low | P. delicatissima j | 1996 | 0–0.12 | 0 | 0 | 0 | 0 | 0 | 0 | ◎ i ● | ◎ | ◎ i | ◎ ● | ◎ i | ◎ ● | |||||||
| P. fraudulenta | 1996 | 0–0.03 | 0 | 0 | 0 | 0 | 0 | 0 | ◎ ● | ◎ i | ◎ i | ◎ ● | ◎ | ◎ ● | ||||||||
| ‘P. pseudodelicatissima’ k | 1996 | 0.12 | NT | NT | NT | 0 | NT | NT | ◎ | ◎ | ◎ i | ◎ | ||||||||||
| No | P. americana | 1994 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● | ◎ | ● | ◎ ● | ◎ | ◎ | |||||||
| P. arenysensis | 2019 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● | ● | ||||||||||||
| ‘P. caciantha’ k, l | 2005 | 0 | NT | NT | NT | 0 | NT | NT | ◎ | |||||||||||||
| P. callianthal | 2005 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ◎ ● | ◎ | ◎ | |||||||||||
| P. cuspidatam | 2020 l | 0–trace | 0 | 0 | 0 | 0 | 0 | 0 | ● | |||||||||||||
| P. galaxiae | 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● | |||||||||||||
| P. hasleana | 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● | ● | ||||||||||||
| P. plurisectam | 2005 l | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● | ◎ | ||||||||||||
| P. (cf.) subpacifica n | 1996 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ◎ ● | ● | ● | ◎ ● | ◎ i | ◎ i | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, T.; Murray, J.S.; Boundy, M.J.; Balci, M.; Bowers, H.A.; Smith, K.F.; Harwood, D.T.; Rhodes, L.L. Update of the Planktonic Diatom Genus Pseudo-nitzschia in Aotearoa New Zealand Coastal Waters: Genetic Diversity and Toxin Production. Toxins 2021, 13, 637. https://doi.org/10.3390/toxins13090637
Nishimura T, Murray JS, Boundy MJ, Balci M, Bowers HA, Smith KF, Harwood DT, Rhodes LL. Update of the Planktonic Diatom Genus Pseudo-nitzschia in Aotearoa New Zealand Coastal Waters: Genetic Diversity and Toxin Production. Toxins. 2021; 13(9):637. https://doi.org/10.3390/toxins13090637
Chicago/Turabian StyleNishimura, Tomohiro, J. Sam Murray, Michael J. Boundy, Muharrem Balci, Holly A. Bowers, Kirsty F. Smith, D. Tim Harwood, and Lesley L. Rhodes. 2021. "Update of the Planktonic Diatom Genus Pseudo-nitzschia in Aotearoa New Zealand Coastal Waters: Genetic Diversity and Toxin Production" Toxins 13, no. 9: 637. https://doi.org/10.3390/toxins13090637
APA StyleNishimura, T., Murray, J. S., Boundy, M. J., Balci, M., Bowers, H. A., Smith, K. F., Harwood, D. T., & Rhodes, L. L. (2021). Update of the Planktonic Diatom Genus Pseudo-nitzschia in Aotearoa New Zealand Coastal Waters: Genetic Diversity and Toxin Production. Toxins, 13(9), 637. https://doi.org/10.3390/toxins13090637







