Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination
Abstract
:1. Introduction
2. Results
2.1. Description of the Population
2.2. Food Consumption Reported by the Participants
2.3. Occurrence and Concentration of Mycotoxins in Urine
2.4. Association Between Food Consumption and Mycotoxins in Urine
2.5. Association Between Occupation and Mycotoxins in Urine
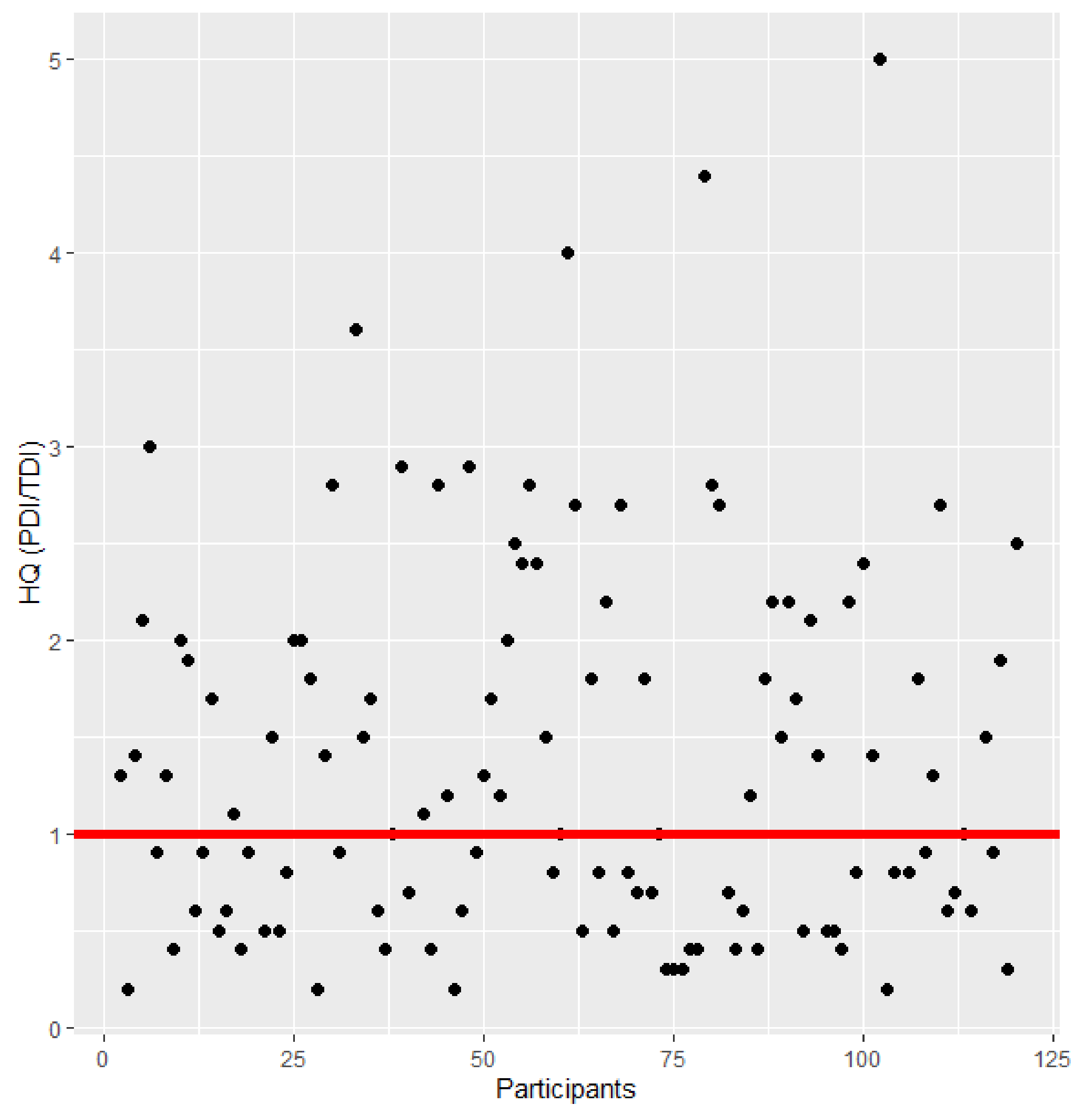
2.6. Dietary Exposure and Risk Assessment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Diet and Occupation Assessment
5.3. Reagents and Chemicals
5.4. Urine Sample Extraction
5.5. Chromatographic Conditions
5.6. Exposure Assessment
5.7. Risk Characterization
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the presence of mycotoxins in biological samples: An overview. Toxins 2017, 251. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 89, ISBN 9780128171714. [Google Scholar]
- Mycotoxins and human health. IARC Sci. Publ. 2012, 87–104.
- Habschied, K.; Kanižai Šarić, G.; Krstanović, V.; Mastanjević, K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of Aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2008. Food Chem. Toxicol. 2019, 129. [Google Scholar]
- Warensjö Lemming, E.; Montano Montes, A.; Schmidt, J.; Cramer, B.; Humpf, H.U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents—possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Tuanny Franco, L.; Mousavi Khaneghah, A.; In Lee, S.H.; Fernandes Oliveira, C.A. Biomonitoring of mycotoxin exposure using urinary biomarker approaches: A review. Toxin Rev. 2019, 1–21. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; Muñoz, K.; Degen, G.H. Ochratoxin A and its metabolites in urines of German adults—An assessment of variables in biomarker analysis. Toxicol. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef]
- Mengelers, M.; Zeilmaker, M.; Vidal, A.; De Boevre, M.; De Saeger, S.; Hoogenveen, R. Biomonitoring of deoxynivalenol and deoxynivalenol-3-glucoside in human volunteers: Renal excretion profiles. Toxins 2019, 11, 466. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Assunção, R.; Nunes, C.; Torres, D.; Alvito, P. Are Data from Mycotoxins’ Urinary Biomarkers and Food Surveys Linked? A Review Underneath Risk Assessment. Food Rev. Int. 2020, 37, 373–398. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A review. Toxins 2020, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renwick AG, W.R. An Analysis of the Risk of Exceeding the Acceptable or Tolerable Daily Intake. Regul. Toxicol. Pharmacol. 1993, 18, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Benford, D.; Bolger, P.M.; Carthew, P.; Coulet, M.; DiNovi, M.; Leblanc, J.C.; Renwick, A.G.; Setzer, W.; Schlatter, J.; Smith, B.; et al. Application of the Margin of Exposure (MOE) approach to substances in food that are genotoxic and carcinogenic. Food Chem. Toxicol. 2010, 48, S2–S24. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Foerster, C.; Muñoz, K.; Delgado-Rivera, L.; Rivera, A.; Cortés, S.; Müller, A.; Arriagada, G.; Ferreccio, C.; Rios, G. Occurrence of relevant mycotoxins in food commodities consumed in Chile. Mycotoxin Res. 2020, 36. [Google Scholar] [CrossRef]
- Muñoz, K.; Vega, M.; Rios, G.; Muñoz, S.; Madariaga, R. Preliminary study of Ochratoxin A in human plasma in agricultural zones of Chile and its relation to food consumption. Food Chem. Toxicol. 2006. [Google Scholar] [CrossRef]
- Muñoz, K.; Campos, V.; Blaszkewicz, M.; Vega, M.; Alvarez, A.; Neira, J.; Degen, G.H. Exposure of neonates to ochratoxin A: First biomonitoring results in human milk (colostrum) from Chile. Mycotoxin Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Blaszkewicz, M.; Campos, V.; Vega, M.; Degen, G.H. Exposure of infants to ochratoxin A with breast milk. Arch. Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Foerster, C.; Groopman, J.; Egner, P.; Koshiol, J.; Ferreccio, C. Association of aflatoxin with gallbladder cancer in Chile. JAMA - J. Am. Med. Assoc. 2015, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, I.; Yamamoto, M.; Calvo, A.; Cavada, G.; Báez, S.; Endoh, K.; Watanabe, H.; Tajima, K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cancer 2002, 102, 407–411. [Google Scholar] [CrossRef]
- Foerster, C.; Koshiol, J.; Guerrero, A.R.; Kogan, M.J.; Ferreccio, C. The case for aflatoxins in the causal chain of gallbladder cancer. Med. Hypotheses 2016, 86. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Viegas, C.; Martins, C. Occupational Exposure to Mycotoxins — Di ff erent Sampling Strategies Telling a Common Story. Toxins 2020, 12, 513. [Google Scholar] [CrossRef]
- Ferreccio, C.; Huidobro, A.; Cortés, S.; Bambs, C.; Toro, P.; Van De Wyngard, V.; Acevedo, J.; Paredes, F.; Venegas, P.; Verdejo, H.; et al. Cohort Profile: The Maule Cohort (MAUCO). Int. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; Roa, J.C.; Bambs, C.; Vives, A.; Corvalán, A.H.; Cortés, S.; Foerster, C.; Acevedo, J.; Huidobro, A.; Passi, A.; et al. Study protocol for the Maule Cohort (MAUCO) of chronic diseases, Chile 2014-2024. BMC Public Health 2015, 16. [Google Scholar] [CrossRef] [Green Version]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.D.S.S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Env. Int 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins 2014, 6, 523. [Google Scholar] [CrossRef]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Föllmann, W.; Ali, N.; Blaszkewicz, M.; Degen, G.H. Biomonitoring of Mycotoxins in Urine: Pilot Study in Mill Workers. J. Toxicol. Environ. Heal. Part A Curr. Issues 2016, 79. [Google Scholar] [CrossRef] [PubMed]
- Wallin, S.; Gambacorta, L.; Kotova, N.; Lemming, E.W.; Nälsén, C.; Solfrizzo, M.O.M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem Toxicol. 2015, 83, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Alvito, P.; Assunção, R.; Oliveira, C.A.F. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019, 128. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.H.; Thrasher, J.D.; Straus, D.C.; Madison, R.A.; Hooper, D. Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins 2013, 5, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Šarkanj, B.; Warth, B.; Uhlig, S.; Abia, W.A.; Sulyok, M.; Klapec, T.; Krska, R.; Banjari, I. Urinary analysis reveals high deoxynivalenol exposure in pregnant women from Croatia. Food Chem. Toxicol. 2013, 62, 231–237. [Google Scholar] [CrossRef]
- Stolpe, N.; Undurraga, P. Long term climatic trends in Chile and effects on soil moisture and temperature regimes. Chil. J. Agric. Res. 2016, 76, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Doohan, F.M.; Brennan, J.; Cookie, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. In Epidemiology of Mycotoxin Producing Fungi; Xu, X., Bailey, J.A., Cooke, C.B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Amigo, H.; Pizarro, M.; Bustos, P.; Castillo, E.; Cerda, R.; Jelvez, I.; Quijada, S.; Valencia, A. Anexos ENCA. Available online: https://www.minsal.cl/encadescarga/ (accessed on 20 April 2021).
- Pérez, C.F.; Hernández, Á.E. Análisis del comportamiento reciente del precio de la harina de panificación. Available online: https://www.odepa.gob.cl/wp-content/uploads/2019/02/articulo-harina_trigo2019.pdf (accessed on 20 April 2021).
- Information, N.N.C. for E. Global Climate Report—Annual 2017. Available online: https://www.ncdc.noaa.gov/sotc/global/201713 (accessed on 20 April 2021).
- Vaughan, M.; Backhouse, D.P.E. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 1, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Arrúa, A.A.; Mendes, J.M.; Arrúa, P.; Ferreira, F.P.; Caballero, G.; Cazal, C.; Kohli, M.M.; Peralta, I.; Ulke, G.; Ríos, D.F. Occurrence of deoxynivalenol and ochratoxin a in beers and wines commercialized in Paraguay. Toxins 2019, 11, 308. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, K.C.; Savi, G.D.; Olivo, G.; Scussel, V.M. Quality and occurrence of deoxynivalenol and fumonisins in craft beer. Food Control 2015, 50, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Van Dam, R.; Van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS One 2017, 12, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Baccigaluppi, J.L. Facultad De Ciencias Fisicas Y Matematicas. Available online: http://repositorio.uchile.cl/handle/2250/173980 (accessed on 20 April 2021).
- Valencia, J. Trending Coffee. Available online: http://repositorio.uchile.cl/bitstream/handle/2250/143182/ValenciaMadridJorge.pdf?sequence=1&isAllowed= (accessed on 20 April 2021).
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.N.; Njobeh, P.B.; Dutton, M.F.; Moundipa, P.F. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Control 2013, 31, 438–453. [Google Scholar] [CrossRef]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Emin Erkan, M.; Başkaya, R.; Ciftcioglu, G. Determination of Aflatoxin B1 levels in powdered red pepper. Food Control 2007, 18, 1015–1018. [Google Scholar] [CrossRef]
- Ravi Kiran, D.; Narayana, K.J.P.; Vijayalakshmi, M. Aflatoxin B1 production in chillies (Capsicum annuum L.) kept in cold stores. African J. Biotechnol. 2005, 4, 791–795. [Google Scholar] [CrossRef]
- Costa, J.; Rodríguez, R.; Garcia-Cela, E.; Medina, A.; Magan, N.; Lima, N.; Battilani, P.; Santos, C. Overview of fungi and mycotoxin contamination in capsicum pepper and in its derivatives. Toxins 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.; Assunção, R.; Cunha, S.C.; Fernandes, J.O.; Jager, A.; Petta, T.; Oliveira, C.A.; Alvito, P. Assessment of multiple mycotoxins in breakfast cereals available in the Portuguese market. Food Chem. 2018, 239, 132–140. [Google Scholar] [CrossRef]
- Kujbida, P.; Maia, P.P.; de Araújo, A.N.; Mendes, L.D.; de Oliveira, M.L.; Silva-Rocha, W.P.; de Brito, G.Q.; Chaves, G.M.; Martins, I. Risk assessment of the occurrence of aflatoxin and fungi in peanuts and cashew nuts. Brazilian J. Pharm. Sci. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health 2019, 222. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Gong, Y.Y.; Zhao, Y.; Wu, Y. Human dietary and internal exposure to zearalenone based on a 24-hour duplicate diet and following morning urine study. Environ. Int. 2020, 142, 105852. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de Salud. Plan Nacional de Cáncer 2018–2028. Available online: https://www.minsal.cl/wp-content/uploads/2019/01/2019.01.23_PLAN-NACIONAL-DE-CANCER_web.pdf (accessed on 20 April 2021).
- Dalezios, J.I.; Hsieh, D.P.H.; Wogan, G.N. Excretion and metabolism of orally administered aflatoxin b1 by rhesus monkeys. Food Cosmet. Toxicol. 1973, 11, 605–616. [Google Scholar] [CrossRef]
- Berdegué, J.; Jara, E.; Modrego, F.; Sanclemente, X. Comunas Rurales de Chile; Documento de Trabajo N° 60. Programa Dinámicas Territoriales Rurales; Rimisp: Santiago, Chile, 2010. [Google Scholar]
- Chu, F.S.; Fremy, J.; Chen, J.S. Correlation of Dietary Aflatoxin B1 Levels with Excretion of Aflatoxin M1 in Human Urine. Cancer Res. 1987, 47, 1848–1852. [Google Scholar]
- Degen GH Are we ready to estimate daily ochratoxin A intake based on urinary concentrations? Env. Int 2016, Dec, 254–255.
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; World Health Organization: Geneva, Switzerland, 1996; Volume 1, ISBN 951-802-158-9. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2018, 14. [CrossRef]
| Characteristics | All | Women | Men | p-Value Sex Difference |
|---|---|---|---|---|
| n = 172 | n = 81 | n = 91 | ||
| Age, mean ± SD (years old) | 57.4 ± 9.3 | 58.5 ± 8.8 | 56.4 ± 9.7 | 0.129 a |
| 35–44 | 10.5 | 6.2 | 14.3 | |
| 45–54 | 26.7 | 27.2 | 26.4 | |
| 55–64 | 39.0 | 40.7 | 37.4 | |
| 65–74 | 23.8 | 25.9 | 22.0 | |
| Body mass index (BMI) | 29.3 ± 4.5 | 29.5 ± 5.3 | 29.1 ± 3.8 | 0.536 a |
| Self-reported ethnicity (%) | 0.222 b | |||
| Chilean/Hispanic | 98.8 | 97.5 | 100 | |
| Mapuche | 1.2 | 2.5 | 0 | |
| Schooling (years) | 0.177 b | |||
| ≤8 | 55.8 | 61.7 | 50.5 | |
| 9–12 | 34.3 | 27.2 | 40.7 | |
| ≥13 | 9.9 | 11.1 | 8.8 | |
| Current smoking (%) | 43.9 | 51.1 | 37.7 | 0.269 b |
| Current alcohol drinking (%) | 66.7 | 60.5 | 72.2 | 0.143 b |
| Current physical activity (%) | 22.5 | 17.3 | 27.1 | 0.183 b |
| Agriculture worker (%) | 48.2 | 34.2 | 60.0 | 0.001 b |
| Food handling worker (%) | 11.4 | 7.8 | 14.4 | 0.226 b |
| Consumption | Food Item | All (Mean ± SD) | Women (Mean ± SD) | Man (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| Habitual consumption (g or mL/day) | Capsicum | 0.8 ± 2.0 | 0.7 ± 1.9 | 1.0 ± 2.0 | 0.230 |
| Cereal (whole) | 6.0 ± 13.1 | 8.2 ± 16.6 | 4.1 ± 8.5 | 0.540 | |
| Beer | 62.6 ± 268.2 | 45.6 ± 350 | 77.8 ± 165.2 | 0.002 | |
| Maize | 149.2 ± 166.5 | 125 ± 84.7 | 165.7 ± 204.3 | 0.898 | |
| Nuts | 9.8 ± 9.5 | 10 ± 9.9 | 9.5 ± 9.1 | 0.988 | |
| Dairy | 110.6 ± 95.8 | 117.4 ± 96.8 | 104.5 ± 95.1 | 0.338 | |
| Legumes | 53.4 ± 31.9 | 50.1 ± 28.8 | 56.4 ± 34.4 | 0.274 | |
| Wine | 134.9 ± 421.1 | 89.1 ± 309.7 | 175.6 ± 498.1 | 0.103 | |
| 24 h consumption (g or mL/day) | Capsicum | 0.7 ± 1.9 | 0.8 ± 2.1 | 0.7 ± 1.6 | 0.863 |
| Coffee | 1.9 ± 3.7 | 1.6 ± 2.9 | 2.2 ± 4.3 | 0.408 | |
| Meat | 60.4 ± 56.8 | 57.5 ± 55.9 | 63 ± 57.8 | 0.448 | |
| Cereal | 3.5 ± 10 | 3.9 ± 10.5 | 3.1 ± 9.5 | 0.607 | |
| Maize | 4.8 ± 19.7 | 5.2 ± 20.4 | 4.4 ± 19.2 | 0.623 | |
| Ginger | 0.2 ± 1.1 | 0.4 ± 1.4 | 0.1 ± 0.5 | 0.019 | |
| Legumes | 45.3 ± 99.9 | 44.6 ± 91.5 | 45.9 ± 107.3 | 0.767 | |
| Peanut | 3.0 ± 9.0 | 3.0 ± 9.0 | 3.0 ± 9.0 | 0.998 | |
| Walnut | 1.3 ± 5.9 | 1.3 ± 4.9 | 1.2 ± 6.7 | 0.595 | |
| Tea | 7.6 ± 6.5 | 8.1 ± 7.4 | 7.0 ± 5.5 | 0.551 |
| Mycotoxin | LOD (ng/mL) | LOQ (ng/mL) | Over LOQ (%) | Prevalence (%) | Mean (SD) (ng/mL) | Mean (SD) ng/mg Creat a | Median (IQ Range) (ng/mL) |
|---|---|---|---|---|---|---|---|
| AFB1 | 0.08 | 0.1 | 7 | 8 | 0.3 (0.3) | 0.3 (0.2) | 0.3 (0.1–0.3) |
| AFM1 | 0.8 | 1.1 | 1 | 1 | 1.8 (1.0) | 4.3 (2.8) | 1.8 (1.5–2.2) |
| OTA | 0.4 | 2.1 | 0 | 0.6 | - | - | - |
| DON | 6.6 | 20.1 | 63 | 73 | 60.7 (78.7) | 64.6 (205.8) | 37.6 (23.2–61.1) |
| ZEN | 0.5 | 1.7 | 0 | 0.6 | - | - | - |
| α-ZEL | 1.2 | 3.7 | 6 | 8 | 41.8 (115.5) | 19.1 (25.2) | 11.8 (6.2–16.5) |
| β-ZEL | 0.7 | 2.3 | 7.5 | 7.5 | 17.4 (16.1) | 21.9 (57.8) | 8.6 (6.9–31.3) |
| Mycotoxin | Chile (This study) | Belgium [30] | Italy [31] | Germany [32] | Germany [33] | Sweden [34] | Brazil [35] | Haiti [32] | USA [36] | South Africa [37] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycotoxin Prevalence (%) | Aflatoxins | 9 | - | 6 | 0 | - | - | 12 | 11 | 13 | 0 |
| OTA | 0.6 | 35 | 100 | 15 | 77 | 51 | 27 | 47 | 87 | 96 | |
| DON | 55 | 37 | 96 | 8 | 100 | 63 | 88 | 24 | - | 87 | |
| ZEN | 0.6 | - | 100 | - | 100 | 37 | 7 | - | - | 100 | |
| α-ZEL | 8 | 0.4 | 100 | 0 | 46 | 21 | 0 | 4 | - | 92 | |
| β-ZEL | 6 | - | 98 | - | 23 | 18 | 0 | - | - | 75 | |
| Mycotoxin Mean Level (ng/L) | Aflatoxins | 500 | - | 68 | - | - | - | 20 * | 60 | 4670 | - |
| OTA | - | 27.8 | 140 | 97 | 66 | 460 | 20 * | 110 | 6200 | 24 | |
| DON | 60,700 | 3900 | 11,890 | 2000 | 6850 | 3370 | 12,000 * | 3200 | - | 4940 | |
| ZEN | 1100 | - | 60 | - | 31 | 30 | 20 * | - | - | 204 | |
| α-ZEL | 41,800 | 5000 | 80 | - | 16 | 30 | - | 1460 | - | 247 | |
| β-ZEL | 17400 | - | 90 | 1420 | 8 | 20 | - | - | - | 244 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foerster, C.; Ríos-Gajardo, G.; Gómez, P.; Muñoz, K.; Cortés, S.; Maldonado, C.; Ferreccio, C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins 2021, 13, 439. https://doi.org/10.3390/toxins13070439
Foerster C, Ríos-Gajardo G, Gómez P, Muñoz K, Cortés S, Maldonado C, Ferreccio C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins. 2021; 13(7):439. https://doi.org/10.3390/toxins13070439
Chicago/Turabian StyleFoerster, Claudia, Gisela Ríos-Gajardo, Patricia Gómez, Katherine Muñoz, Sandra Cortés, Carlos Maldonado, and Catterina Ferreccio. 2021. "Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination" Toxins 13, no. 7: 439. https://doi.org/10.3390/toxins13070439
APA StyleFoerster, C., Ríos-Gajardo, G., Gómez, P., Muñoz, K., Cortés, S., Maldonado, C., & Ferreccio, C. (2021). Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins, 13(7), 439. https://doi.org/10.3390/toxins13070439





