Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems †
Abstract
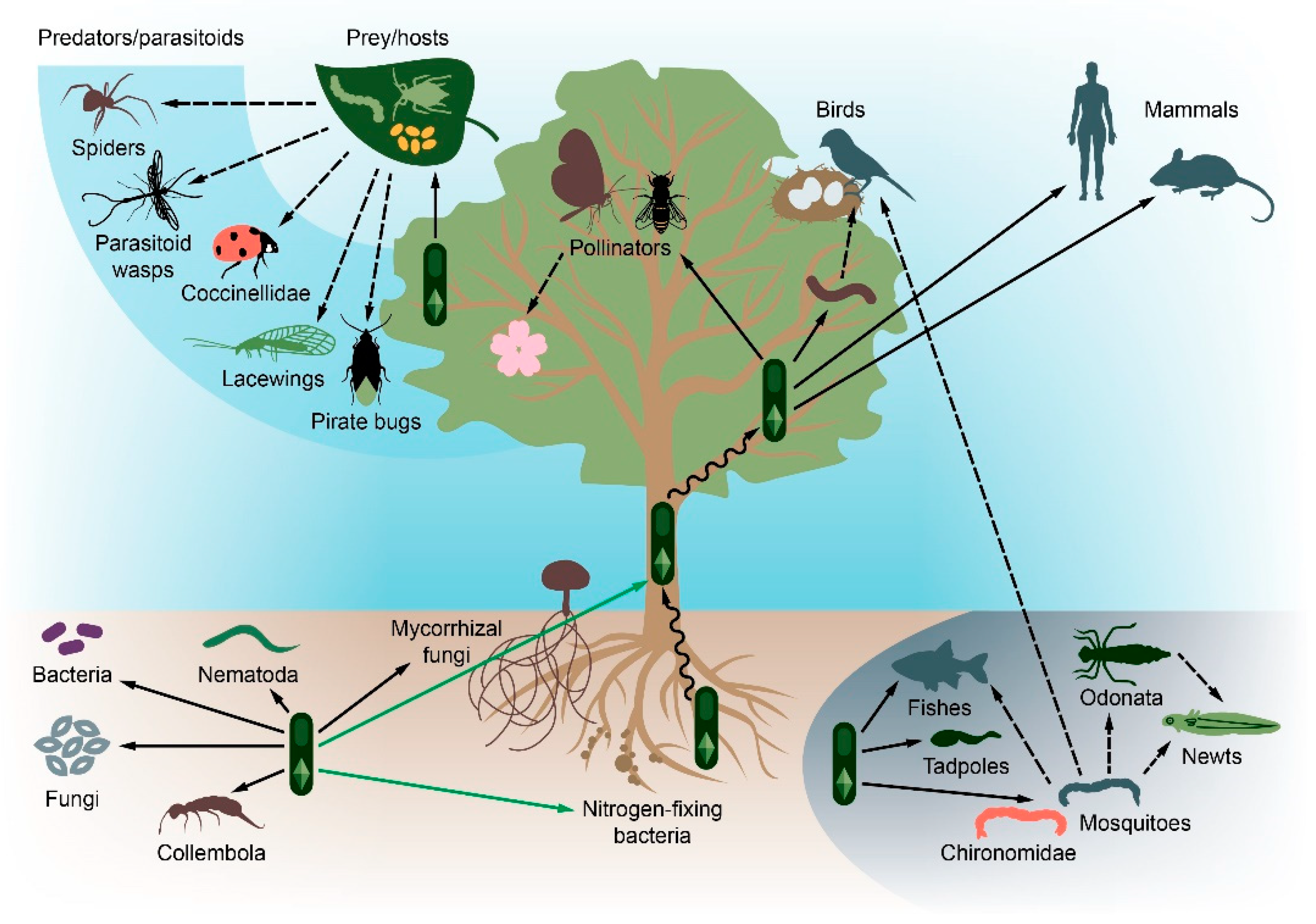
1. Introduction
2. Soil Microbial Communities
3. Plants
4. Invertebrates
4.1. Soil Invertebrates
4.2. Arthropods
5. Vertebrates
6. Land Ecosystems
7. Aquatic Ecosystems
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasko, D.A.; Altherr, M.R.; Han, C.S.; Ravel, J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 2005, 29, 303–329. [Google Scholar] [CrossRef]
- Melo, A.L.D.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Griko, N.; Junker, M.; Bulla, L.A. Bacillus thuringiensis. Bioeng. Bugs 2010, 1, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Milner, R.J. History of Bacillus thuringiensis. Agric. Ecosyst. Environ. 1994, 49, 9–13. [Google Scholar] [CrossRef]
- Angus, T.A. A bacterial toxin paralysing silkworm larvae. Nature 1954, 173, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Hannay, C.L.; Fitz-James, P. The protein crystals of Bacillus thuringiensis Berliner. Can. J. Microbiol. 1955, 1, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Torres-Manno, M.A.; Repizo, G.D.; Magni, C.; Dunlap, C.A.; Espariz, M. The assessment of leading traits in the taxonomy of the Bacillus cereus group. Int. J. Gen. Mol. Microbiol. 2020, 113, 2223–2242. [Google Scholar] [CrossRef]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a Taxonomic Nomenclature for the Bacillus cereus Group which Reconciles Genomic Definitions of Bacterial Species with Clinical and Industrial Phenotypes. MBio 2020, 11. [Google Scholar] [CrossRef]
- Vilas-Bôas, G.T.; Peruca, A.P.S.; Arantes, O.M.N. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can. J. Microbiol. 2007, 53, 673–687. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Helgason, E.; Okstad, O.A.; Caugant, D.A.; Johansen, H.A.; Fouet, A.; Mock, M.; Hegna, I.; Kolstø, A.B. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—One species on the basis of genetic evidence. Appl. Environ. Microbiol. 2000, 66, 2627–2630. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Estrada, L.J.; Hernández-Velázquez, V.M.; Arenas-Sosa, I.; Flores-Pérez, F.I.; Morales-Montor, J.; Penã-Chora, G. Anthelmintic Effect of Bacillus thuringiensis Strains against the Gill Fish Trematode Centrocestus formosanus. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Malovichko, Y.V.; Nizhnikov, A.A.; Antonets, K.S. Repertoire of the Bacillus thuringiensis Virulence Factors Unrelated to Major Classes of Protein Toxins and Its Role in Specificity of Host-Pathogen Interactions. Toxins 2019, 11, 347. [Google Scholar] [CrossRef]
- Martínez-Zavala, S.A.; Barboza-Pérez, U.E.; Hernández-Guzmán, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, Modular Structure, and Applied Potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef]
- Fang, S.; Wang, L.; Guo, W.; Zhang, X.; Peng, D.; Luo, C.; Yu, Z.; Sun, M. Bacillus thuringiensis bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl. Environ. Microbiol. 2009, 75, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, E.; Cadot, C.; Tran, S.L.; Guinebretière, M.H.; Lereclus, D.; Ramarao, N. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 2010, 192, 286–294. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Huang, Q.; Zheng, J.; Zhou, W.; Peng, D.; Ruan, L.; Sun, M. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl. Environ. Microbiol. 2013, 79, 460–468. [Google Scholar] [CrossRef]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Lereclus, D. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell. Microbiol. 2005, 7, 1357–1364. [Google Scholar] [CrossRef]
- Dalhmmar, G.; Steiner, H. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. Eur. J. Biochem. 1984, 139, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Fagerlund, A.; Buisson, C.; Gilois, N.; Kolstø, A.; Økstad, O.A.; Aymerich, S.; Nielsen-Leroux, C.; Lereclus, D.; Gohar, M. CalY is a major virulence factor and a biofilm matrix protein. Mol. Microbiol. 2019, 111, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Nisnevitch, M.; Cohen, S.; Ben-Dov, E.; Zaritsky, A.; Sofer, Y.; Cahan, R. Cyt2Ba of Bacillus thuringiensis israelensis: Activation by putative endogenous protease. Biochem. Biophys. Res. Commun. 2006, 344, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Nisnevitch, M.; Sigawi, S.; Cahan, R.; Nitzan, Y. Isolation, characterization and biological role of camelysin from Bacillus thuringiensis subsp. israelensis. Curr. Microbiol. 2010, 61, 176–183. [Google Scholar] [CrossRef]
- Yin, J.; Ding, X.; Xia, L.; Yu, Z.; Lv, Y.; Hu, S.; Huang, S.; Cao, Z.; Xiao, X. Transcription of gene in an acrystalliferous strain of Bacillus thuringiensis XBU001 positively regulated by the metalloprotease camelysin gene at the onset of stationary phase. FEMS Microbiol. Lett. 2011, 318, 92–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tonello, F.; Zornetta, I. Bacillus anthracis Factors for Phagosomal Escape. Toxins 2012, 4, 536–553. [Google Scholar] [CrossRef]
- Lyu, Y.; Ye, L.; Xu, J.; Yang, X.; Chen, W.; Yu, H. Recent research progress with phospholipase C from Bacillus cereus. Biotechnol. Lett. 2016, 38, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ruan, L.; Peng, D.; Li, L.; Sun, M.; Yu, Z. Thuringiensin: A Thermostable Secondary Metabolite from Bacillus thuringiensis with Insecticidal Activity against a Wide Range of Insects. Toxins 2014, 6, 2229–2238. [Google Scholar] [CrossRef]
- Du, C.; Cao, S.; Shi, X.; Nie, X.; Zheng, J.; Deng, Y.; Ruan, L.; Peng, D.; Sun, M. Genetic and biochemical characterization of a gene operon for trans-aconitic acid, a novel nematicide from Bacillus thuringiensis. J. Biol. Chem. 2017, 292, 3517–3530. [Google Scholar] [CrossRef]
- He, H.; Silo-Suh, L.A.; Handelsman, J.; Clardy, J. Zwittermicin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett. 1994, 35, 2499–2502. [Google Scholar] [CrossRef]
- Broderick, N.A.; Robinson, C.J.; McMahon, M.D.; Holt, J.; Handelsman, J.; Raffa, K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009, 7, 11. [Google Scholar] [CrossRef]
- De la Fuente-Salcido, N.M.; Casados-Vázquez, L.E.; Barboza-Corona, J.E. Bacteriocins of Bacillus thuringiensis can expand the potential of this bacterium to other areas rather than limit its use only as microbial insecticide. Can. J. Microbiol. 2013, 59, 515–522. [Google Scholar] [CrossRef]
- Martínez, C.; Ibarra, J.E.; Caballero, P. Association analysis between serotype, cry gene content, and toxicity to Helicoverpa armigera larvae among Bacillus thuringiensis isolates native to Spain. J. Invertebr. Pathol. 2005, 90, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.E.; Malovichko, Y.V.; Lobov, A.A.; Belousova, M.E.; Nizhnikov, A.A.; Antonets, K.S. The Distribution of Several Genomic Virulence Determinants Does Not Corroborate the Established Serotyping Classification of Bacillus thuringiensis. Int. J. Mol. Sci. 2021, 22, 2244. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Gwynn, R. (Ed.) The Manual of Biocontrol Agents, 5th ed.; BCPC: Cambridge, UK, 2014; ISBN 9781901396874. [Google Scholar]
- Eski, A.; Demir, İ.; Sezen, K.; Demirbağ, Z. A new biopesticide from a local Bacillus thuringiensis var. tenebrionis (Xd3) against alder leaf beetle (Coleoptera: Chrysomelidae). World J. Microbiol. Biotechnol. 2017, 33. [Google Scholar] [CrossRef]
- Grace, J.K.; Ewart, D.M. Recombinant cells of Pseudomonas fluorescens: A highly palatable encapsulation for delivery of genetically engineered toxins to subterranean termites (Isoptera: Rhinotermitidae). Lett. Appl. Microbiol. 2008, 23, 183–186. [Google Scholar] [CrossRef]
- Navon, A. Bacillus thuringiensis application in agriculture. In Entomopathogenic Bacteria: From Laboratory to Field Application; Springer: Dordrecht, The Netherlands, 2000; pp. 355–369. [Google Scholar]
- World Health Organization; International Programme on Chemical Safety. Microbial Pest Control Agent: Bacillus thuringiensis; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Belousova, M.E.; Grishechkina, S.D.; Ermolova, V.P.; Antonets, K.S.; Mardanov, A.V.; Rakitin, A.L.; Beletsky, A.V.; Ravin, N.V.; Nizhnikov, A.A. Whole genome sequencing of Bacillus thuringiensis var. darmstadiensis 56 strain and the study of insecticidal activity of the biological preparation on its basis. Agric. Biol. 2020, 55, 87–96. [Google Scholar] [CrossRef]
- Lipa, J.J.; Smits, P.H. Microbial Control of Pests in Greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops. Developments in Plant Pathology; Albajes, R., Gullino, L.M., van Lenteren, J.C., Elad, Y., Eds.; Springer: Dordrecht, The Netherlands, 1999; Volume 14, pp. 295–309. [Google Scholar]
- Pedersen, J.C.; Hansen, B.M.; Damgaard, P.H.; Eilenberg, J. Dispersal of Bacillus thuringiensis var. kurstaki in an experimental cabbage field. Can. J. Microbiol. 1995, 41, 118–125. [Google Scholar] [CrossRef]
- Van Cuyk, S.; Deshpande, A.; Hollander, A.; Duval, N.; Ticknor, L.; Layshock, J.; Gallegos-Graves, L.V.; Omberg, K.M. Persistence of Bacillus thuringiensis subsp. kurstaki in Urban environments following spraying. Appl. Environ. Microbiol. 2011, 77, 7954–7961. [Google Scholar] [CrossRef][Green Version]
- Vettori, C.; Paffetti, D.; Saxena, D.; Stotzky, G.; Giannini, R. Persistence of toxins and cells of Bacillus thuringiensis subsp. kurstaki introduced in sprays to Sardinia soils. Soil Biol. Biochem. 2003, 35, 1635–1642. [Google Scholar] [CrossRef]
- Allende, A.; Bolton, J.D.; Chemaly, M.; Davies, R.; Amez, P.S.F.; Herman, L.; Giron, R.; Koutsoumanis, K.; Lindqvist, R.; Norrung, B.; et al. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016. [Google Scholar] [CrossRef]
- Vilas-Bã’as, L.A.; Vilas-Bã´as, G.F.L.T.; Saridakis, H.O.; Lemos, M.V.F.; Lereclus, D.; Arantes, O.M.N. Survival and conjugation of Bacillus thuringiensis in a soil microcosm. FEMS Microbiol. Ecol. 2000, 31, 255–259. [Google Scholar] [CrossRef]
- Jensen, G.B.; Larsen, P.; Jacobsen, B.L.; Madsen, B.; Wilcks, A.; Smidt, L.; Andrup, L. Isolation and characterization of Bacillus cereus-like bacteria from faecal samples from greenhouse workers who are using Bacillus thuringiensis-based insecticides. Int. Arch. Occup. Environ. Health 2002, 75, 191–196. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do Biopesticides Affect the Demographic Traits of a Parasitoid Wasp and Its Biocontrol Services through Sublethal Effects? PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Federici, B.A. In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—A response to EFSA. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Bhattarai, B. Variation of Soil Microbial Population in Different Soil Horizons. J. Microbiol. Exp. 2015, 2, 44. [Google Scholar] [CrossRef]
- Argôlo-Filho, R.; Loguercio, L. Bacillus thuringiensis Is an Environmental Pathogen and Host-Specificity Has Developed as an Adaptation to Human-Generated Ecological Niches. Insects 2013, 5, 62–91. [Google Scholar] [CrossRef]
- Martin, P.A.W.; Travers, R.S. Worldwide Abundance and Distribution of Bacillus thuringiensis Isolates. Appl. Environ. Microbiol. 1989, 55, 2437–2442. [Google Scholar] [CrossRef]
- Reinoso-Pozo, Y.; Del Rincón-Castro, M.C.; Ibarra, J.E. Characterization of a highly toxic strain of Bacillus thuringiensis serovar kurstaki very similar to the HD-73 strain. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Ammouneh, H.; Harba, M.; Idris, E.; Makee, H. Isolation and characterization of native Bacillus thuringiensis isolates from Syrian soil and testing of their insecticidal activities against some insect pests. Turkish J. Agric. For. 2011. [Google Scholar] [CrossRef]
- Landén, R.; Bryne, M.; Abdel-Hameed, A. Distribution of Bacillus thuringiensis strains in Southern Sweden. World J. Microbiol. Biotechnol. 1994, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, R.G.; Soares, C.M.; Capdeville, G.; Jones, G.; Martins, É.S.; Praça, L.; Cordeiro, B.A.; Braz, S.V.; dos Santos, R.C.; Berry, C. Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microb. Biotechnol. 2009, 2, 512–520. [Google Scholar] [CrossRef]
- Ammons, D.R.; Reyna, A.; Granados, J.C.; Samlal, M.S.; Rampersad, J.N. An Investigation of Bacillus thuringiensis in Rectal-Collected Fecal Samples of Cows. Curr. Microbiol. 2009, 59, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Cha, I.H.; Woo, D.S.; Ohba, M. Microbial ecology of Bacillus thuringiensis: Fecal populations recovered from wildlife in Korea. Can. J. Microbiol. 2003, 49, 465–471. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Wu, S.; Sun, L.; Huang, E.; Huang, T.; Xu, L.; Wu, C.; Gelbič, I.; Guan, X. Microbial ecology and association of Bacillus thuringiensis in chicken feces originating from feed. Curr. Microbiol. 2012, 65, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.F.; Bishop, A.H. The ecology of Bacillus thuringiensis on the phylloplane: Colonization from soil, plasmid transfer, and interaction with larvae of Pieris brassicae. Microb. Ecol. 2008, 56, 133–139. [Google Scholar] [CrossRef]
- Glare, T.R.; O’Callaghan, M. Bacillus thuringiensis: Biology, Ecology, and Safety; John Wiley & Sons, Ltd.: New York, NY, USA, 2000; ISBN 9780471496304. [Google Scholar]
- Palm, C.J.; Donegan, K.; Harris, D.; Seidler, R.J. Quantification in soil of Bacillus thuringiensis var. kurstaki δ-endotoxin from transgenic plants. Mol. Ecol. 1994, 3, 145–151. [Google Scholar] [CrossRef]
- Sims, S.R.; Ream, J.E. Soil Inactivation of the Bacillus thuringiensis Subsp. kurstaki CryIIA Insecticidal Protein within Transgenic Cotton Tissue: Laboratory Microcosm and Field Studies. J. Agric. Food Chem. 1997, 45, 1502–1505. [Google Scholar] [CrossRef]
- Venkateswerlu, G.; Stotzky, G. Binding of the protoxin and toxin proteins of Bacillus thuringiensis subsp. kurstaki on clay minerals. Curr. Microbiol. 1992, 25, 225–233. [Google Scholar] [CrossRef]
- Saxena, D. Fate and Effects in Soil of Cry Proteins from Bacillus thuringiensis: Influence of Physicochemical and Biological Characteristics of Soil. Open Toxinol. J. 2013, 3, 151–171. [Google Scholar] [CrossRef]
- Smith, R.A.; Barry, J.W. Environmental persistence of Bacillus thuringiensis spores following aerial application. J. Invertebr. Pathol. 1998, 71, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Duchet, C.; Tetreau, G.; Marie, A.; Rey, D.; Besnard, G.; Perrin, Y.; Paris, M.; David, J.P.; Lagneau, C.; Després, L. Persistence and recycling of bioinsecticidal Bacillus thuringiensis subsp. israelensis spores in contrasting environments: Evidence from field monitoring and laboratory experiments. Microb. Ecol. 2014, 67, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Petras, S.F.; Casida, L.E. Survival of Bacillus thuringiensis Spores in Soil. Appl. Environ. Microbiol. 1985, 50, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- West, A.W.; Burges, H.D.; Dixon, T.J.; Wyborn, C.H. Effect of incubation in non-sterilised and autoclaved arable soil on survival of Bacillus thuringiensis and Bacillus cereus spore inocula. N. Z. J. Agric. Res. 1985, 28, 559–566. [Google Scholar] [CrossRef]
- Ferreira, L.H.P.L.; Molina, J.C.; Brasil, C.; Andrade, G. Evaluation of Bacillus thuringiensis bioinsecticidal protein effects on soil microorganisms. Plant Soil 2003, 256, 161–168. [Google Scholar] [CrossRef]
- Tetreau, G.; Alessi, M.; Veyrenc, S.; Périgon, S.; David, J.P.; Reynaud, S.; Després, L. Fate of Bacillus thuringiensis subsp. israelensis in the field: Evidence for spore recycling and differential persistence of toxins in leaf litter. Appl. Environ. Microbiol. 2012, 78, 8362–8367. [Google Scholar] [CrossRef]
- Rojas-Ruiz, N.E.; Sansinenea-Royano, E.; Cedillo-Ramirez, M.L.; Marsch-Moreno, R.; Sanchez-Alonso, P.; Vazquez-Cruz, C. Analysis of Bacillus thuringiensis population dynamics and its interaction with Pseudomonas fluorescens in soil. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef]
- Chehimi, S.; Pons, A.M.; Sablé, S.; Hajlaoui, M.R.; Limam, F. Mode of action of thuricin S, a new class IId bacteriocin from Bacillus thuringiensis. Can. J. Microbiol. 2010, 56, 162–167. [Google Scholar] [CrossRef]
- Su, X.; Li, L.; Pan, J.; Fan, X.; Ma, S.; Guo, Y.; Idris, A.L.; Zhang, L.; Pan, X.; Gelbič, I.; et al. Identification and partial purification of thuricin 4AJ1 produced by Bacillus thuringiensis. Arch. Microbiol. 2020, 202, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.; Chehimi, S.; Limem, F.; Hansen, B.M.; Hendriksen, N.B.; Daffonchio, D.; Boudabous, A. Detection and characterization of the novel bacteriocin entomocin 9, and safety evaluation of its producer, Bacillus thuringiensis ssp. entomocidus HD9. J. Appl. Microbiol. 2003, 95, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Ahern, M.; Verschueren, S.; Sinderen, D. Isolation and characterisation of a novel bacteriocin produced by Bacillus thuringiensis strain B439. FEMS Microbiol. Lett. 2003, 220, 127–131. [Google Scholar] [CrossRef]
- Honda, S.; Kunii, T.; Nohara, K.; Wakita, S.; Sugahara, Y.; Kawakita, M.; Oyama, F.; Sakaguchi, M. Characterization of a Bacillus thuringiensis chitinase that binds to cellulose and chitin. AMB Express. 2017, 7, 51. [Google Scholar] [CrossRef]
- Kamoun, F.; Fguira, I.B.; Hassen, N.B.B.; Mejdoub, H.; Lereclus, D.; Jaoua, S. Purification and Characterization of a New Bacillus thuringiensis Bacteriocin Active against Listeria monocytogenes, Bacillus cereus and Agrobacterium tumefaciens. Appl. Biochem. Biotechnol. 2011, 165, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Cherif, A.; Ouzari, H.; Daffonchio, D.; Cherif, H.; Ben Slama, K.; Hassen, A.; Jaoua, S.; Boudabous, A. Thuricin 7: A novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett. Appl. Microbiol. 2001, 32, 243–247. [Google Scholar] [CrossRef]
- Kamoun, F.; Mejdoub, H.; Aouissaoui, H.; Reinbolt, J.; Hammami, A.; Jaoua, S. Purification, amino acid sequence and characterization of Bacthuricin F4, a new bacteriocin produced by Bacillus thuringiensis. J. Appl. Microbiol. 2005, 98, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ramírez, A.; Escudero-Abarca, B.I.; Aguilar-Uscanga, G.; Hayward-Jones, P.M.; Eleazar Barboza-Corona, J. Antifungal activity of Bacillus thuringiensis chitinase and its potential for the biocontrol of phytopathogenic fungi in soybean seeds. J. Food Sci. 2004, 69. [Google Scholar] [CrossRef]
- Barboza-Corona, J.E.; Vázquez-Acosta, H.; Bideshi, D.K.; Salcedo-Hernández, R. Bacteriocin-like inhibitor substances produced by Mexican strains of Bacillus thuringiensis. Arch. Microbiol. 2007, 187, 117–126. [Google Scholar] [CrossRef]
- Ugras, S.; Sezen, K.; Kati, H.; Demirbag, Z. Purification and characterization of the Bacteriocin thuricin Bn1 produced by Bacillus thuringiensis subsp. kurstaki Bn1 isolated from a hazelnut pest. J. Microbiol. Biotechnol. 2013, 23, 167–176. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, Z.; Hoefel, D.; Tang, L.; Yang, Z.; Zhuang, G.; Yang, J.; Zhang, H. Assessing the impact of the biological control agent Bacillus thuringiensis on the indigenous microbial community within the pepper plant phyllosphere. FEMS Microbiol. Lett. 2008, 284, 102–108. [Google Scholar] [CrossRef]
- Houry, A.; Gohar, M.; Deschamps, J.; Tischenko, E.; Aymerich, S.; Gruss, A.; Briandet, R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 13088–13093. [Google Scholar] [CrossRef] [PubMed]
- Gage, D.J. Infection and Invasion of Roots by Symbiotic, Nitrogen-Fixing Rhizobia during Nodulation of Temperate Legumes. Microbiol. Mol. Biol. Rev. 2004, 68, 280–300. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J. Microbiol. Biotechnol. 2009, 25, 753–761. [Google Scholar] [CrossRef]
- Jung, W.J.; Mabood, F.; Souleimanov, A.; Park, R.D.; Smith, D.L. Chitinases produced by Paenibacillus illinoisensis and Bacillus thuringiensis subsp. pakistani degrade Nod factor from Bradyrhizobium japonicum. Microbiol. Res. 2008, 163, 345–349. [Google Scholar] [CrossRef]
- Djenane, Z.; Nateche, F.; Amziane, M.; Gomis-Cebolla, J.; El-Aichar, F.; Khorf, H.; Ferré, J. Assessment of the Antimicrobial Activity and the Entomocidal Potential of Bacillus thuringiensis Isolates from Algeria. Toxins 2017, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.M.; El-Fatih, M.M.; Helmy, K.G. Antifungal activity of Bacillus thuringiensis strains and their efficacy against the cotton leaf worm Spodoptera littoralis. Arch. Phytopathol. Plant Prot. 2013, 46, 2420–2427. [Google Scholar] [CrossRef]
- Batista, C.B.; Albino, U.B.; Martines, A.M.; Saridakis, D.P.; Matsumoto, L.S.; Avanzi, M.A.; Andrade, G. Efeito fungistático de Bacillus thuringiensis e de outras bactérias sobre alguns fungos fitopatogênicos. Pesqui. Agropecu. Bras. 2002, 37, 1189–1194. [Google Scholar] [CrossRef]
- Lelman, E.K.; Osir, E.O.; Jefwa, J.; Anyango, B.; Boga, H.I. Preliminary investigations reveal that Bacillus thuringiensis δ-endotoxin CryIA(c) incorporated in soil does not affect arbuscular mycorrhiza in Sorghum bicolor (L.) (Moench). J. Trop. Microbiol. Biotechnol. 2007, 3, 12–18. [Google Scholar] [CrossRef]
- Praça, L.B.; Mendes-Gomes, A.C.M.; Cabral, G.; Martins, E.S.; Ryoiti-Sujii, E.; Gomes-Monnerat, R. Endophytic Colonization by Brazilian Strains of Bacillus thuringiensis on Cabbage Seedlings Grown In Vitro. Bt Res. 2012. [Google Scholar] [CrossRef]
- Subrahmanyam, P.; Reddy, M.N.; Rao, A.S. Exudation of certain organic compounds from seeds of groundnut. Seed Sci. Technol. 1983, 11, 267–272. [Google Scholar]
- Hendriksen, N.B.; Carstensen, J. Long-term survival of Bacillus thuringiensis subsp. kurstaki in a field trial. Can. J. Microbiol. 2013, 59, 34–38. [Google Scholar] [CrossRef]
- Hendriksen, N.B.; Hansen, B.M. Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can. J. Microbiol. 2002, 48, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Suarez, R.; Verduzco-Rosas, L.A.; Del Rincon-Castro, M.C.; Delano-Frier, J.P.; Ibarra, J.E. Translocation of Bacillus thuringiensis in Phaseolus vulgaris tissues and vertical transmission in Arabidopsis thaliana. J. Appl. Microbiol. 2017, 122, 1092–1100. [Google Scholar] [CrossRef]
- Vidal-Quist, J.C.; Rogers, H.J.; Mahenthiralingam, E.; Berry, C. Bacillus thuringiensis colonises plant roots in a phylogeny-dependent manner. FEMS Microbiol. Ecol. 2013, 86, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Sadfi, N.; Chérif, M.; Fliss, I.; Boudabbous, A.; Antoun, H. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of fusarium dry rot of potato tubers. J. Plant Pathol. 2001, 83, 101–118. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012, 50, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Zhang, X.F.; Xu, J.L.; Zhang, L.H. Insecticidal Bacillus thuringiensis Silences Erwinia carotovora Virulence by a New Form of Microbial Antagonism, Signal Interference. Appl. Environ. Microbiol. 2004, 70, 954–960. [Google Scholar] [CrossRef]
- Azizoglu, U. Bacillus thuringiensis as a Biofertilizer and Biostimulator: A Mini-Review of the Little-Known Plant Growth-Promoting Properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef]
- Raddadi, N.; Cherif, A.; Boudabous, A.; Daffonchio, D. Screening of plant growth promoting traits of Bacillus thuringiensis. Ann. Microbiol. 2008, 58, 47–52. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, S3–S15. [Google Scholar] [CrossRef]
- Schulte, R.D.; Makus, C.; Hasert, B.; Michiels, N.K.; Schulenburg, H. Multiple reciprocal adaptations and rapid genetic change upon experimental coevolution of an animal host and its microbial parasite. Proc. Natl. Acad. Sci. USA 2010, 107, 7359–7364. [Google Scholar] [CrossRef]
- Ruan, L.; Crickmore, N.; Peng, D.; Sun, M. Are nematodes a missing link in the confounded ecology of the entomopathogen Bacillus thuringiensis? Trends Microbiol. 2015, 23, 341–346. [Google Scholar] [CrossRef]
- Swiecicka, I.; Mahillon, J. Diversity of commensal Bacillus cereus sensu lato isolated from the common sow bug (Porcellio scaber, Isopoda). FEMS Microbiol. Ecol. 2006, 56, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W.; Coleman, D.C. Role of nematodes in decomposition. In Nematodes in Soil Ecosystems; Freckman, D.W., Ed.; University of Texas Press: Austin, TX, USA, 1982; pp. 55–80. ISBN 978-0-292-74091-4. [Google Scholar]
- Wei, J.Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.C.; Aroian, R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Peng, D.; Ji, S.; Wang, P.; Yu, Z.; Sun, M. New strategy for isolating novel nematicidal crystal protein genes from Bacillus thuringiensis strain YBT-1518. Appl. Environ. Microbiol. 2008, 74, 6997–7001. [Google Scholar] [CrossRef] [PubMed]
- Ravari, S.B.; Moghaddam, E.M. Efficacy of Bacillus thuringiensis Cry14 toxin against root knot nematode, meloidogyne javanica. Plant Prot. Sci. 2015, 51, 46–51. [Google Scholar] [CrossRef]
- Zi-Quan, Y.; Qian-Lan, W.; Bin, L.; Xue, Z.; Zi-Niu, Y.; Ming, S. Bacillus thuringiensis crystal protein toxicity against plant-parasitic nematodes. Chin. J. Agric. Biotechnol. 2008, 5, 13–17. [Google Scholar] [CrossRef]
- van Zyl, C.; Malan, A.P. The Role of Entomopathogenic Nematodes as Biological Control Agents of Insect Pests, with Emphasis on the History of Their Mass Culturing and In Vivo Production. Afr. Entomol. 2014, 22, 235–249. [Google Scholar] [CrossRef]
- Kaya, H.K.; Burlando, T.M.; Choo, H.Y.; Thurston, G.S. Integration of entomopathogenic nematodes with Bacillus thuringiensis or pesticidal soap for control of insect pests. Biol. Control 1995, 5, 432–441. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Kaya, H.K. Additive and Synergistic Interaction between Entomopathogenic Nematodes and Bacillus thuringiensis for Scarab Grub Control. Biol. Control 1997, 8, 131–137. [Google Scholar] [CrossRef]
- Bhadauria, T.; Saxena, K.G. Role of earthworms in soil fertility maintenance through the production of biogenic structures. Appl. Environ. Soil Sci. 2010, 2010. [Google Scholar] [CrossRef]
- Benz, G.; Altwegg, A. Safety of Bacillus thuringiensis for earthworms. J. Invertebr. Pathol. 1975, 26, 125–126. [Google Scholar] [CrossRef]
- Heimpel, A.M. A crystalliferous bacterium associated with a “blister disease” in the earthworm, Eisenia foetida (Savigny). J. Invertebr. Pathol. 1966, 8, 295–298. [Google Scholar] [CrossRef]
- Beck, L.; Römbke, J.; Ruf, A.; Prinzing, A.; Woas, S. Effects of diflubenzuron and Bacillus thuringiensis var. kurstaki toxin on soil invertebrates of a mixed deciduous forest in the Upper Rhine Valley, Germany. Eur. J. Soil Biol. 2004, 40, 55–62. [Google Scholar] [CrossRef]
- Prabhakar, A.; Bishop, A.H. Invertebrate pathogenicity and toxin-producing potential of strains of Bacillus thuringiensis endemic to Antarctica. J. Invertebr. Pathol. 2011, 107, 132–138. [Google Scholar] [CrossRef]
- Margulis, L.; Jorgensen, J.Z.; Dolan, S.; Kolchinsky, R.; Rainey, F.A.; Lo, S.C. The Arthromitus stage of Bacillus cereus: Intestinal symbionts of animals. Proc. Natl. Acad. Sci. USA 1998, 95, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Pigott, C.R.; Ellar, D.J. Role of Receptors in Bacillus thuringiensis Crystal Toxin Activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef]
- Paula, D.P.; Andow, D.A. Uptake and bioaccumulation of Cry toxins by an aphidophagous predator. Environ. Pollut. 2016, 209, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Dutton, A.; Bigler, F. Bacillus thuringiensis toxin (Cry1Ab) has no direct effect on larvae of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). J. Insect Physiol. 2004, 50, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Simón, A.; De Maagd, R.A.; Avilla, C.; Bakker, P.L.; Molthoff, J.; González-Zamora, J.E.; Ferré, J. Lack of detrimental effects of Bacillus thuringiensis cry toxins on the insect predator Chrysoperla carnea: A toxicological, histopathological, and biochemical analysis. Appl. Environ. Microbiol. 2006, 72, 1595–1603. [Google Scholar] [CrossRef]
- Hilbeck, A.; Moar, W.J.; Pusztai-Carey, M.; Filippini, A.; Bigler, F. Toxicity of Bacillus thuringiensis CrylAb Toxin to the Predator Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 1998, 27, 1255–1263. [Google Scholar] [CrossRef]
- Hilbeck, A.; Moar, W.J.; Pusztai-Carey, M.; Filippini, A.; Bigler, F. Prey-mediated effects of Cry1Ab toxin and protoxin and Cry2A protoxin on the predator Chrysoperla carnea. Entomol. Exp. Appl. 1999, 91, 305–316. [Google Scholar] [CrossRef]
- Dibelli, W.; De Bortoli, S.A.; Volpe, H.X.L.; Vacari, A.M.; De Magalhães, G.O.; Duarte, R.T.; Polanczyk, R.A. Effect of Bacillus thuringiensis on the biological parameters and phytophagy of podisus nigrispinus (Hemiptera: Pentatomidae). Entomol. Gen. 2013, 34, 313–321. [Google Scholar] [CrossRef]
- Magalhães, G.O.; Vacari, A.M.; Laurentis, V.L.; De Bortoli, S.A.; Polanczyk, R.A. Interactions of Bacillus thuringiensis bioinsecticides and the predatory stink bug Podisus nigrispinus to control Plutella xylostella. J. Appl. Entomol. 2015, 139, 123–133. [Google Scholar] [CrossRef]
- Carvalho, V.F.P.; Vacari, A.M.; Pomari, A.F.; De Bortoli, C.P.; Ramalho, D.G.; De Bortoli, S.A. Interaction between the Predator Podisus nigrispinus (Hemiptera: Pentatomidae) and the Entomopathogenic Bacteria Bacillus thuringiensis. Environ. Entomol. 2013, 41, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Caetano, F.H.; Wanderley-Teixeira, V.; Torres, J.B.; Teixeira, Á.A.C.; Alves, L.C. Ultra-structure and histochemistry of digestive cells of Podisus nigrispinus (Hemiptera: Pentatomidae) fed with prey reared on bt-cotton. Micron 2012, 43, 245–250. [Google Scholar] [CrossRef]
- Hafez, M.; Abol-Ela, R.; Zaki, F.N.; Salama, H.S.; Ragaei, M. The potential of the predator Orius albidepennis on Agrotis ypsilon as affected by Bacillus thuringiensis. Anz. Schadl. Pflanzenschutz Umweltschutz 1997, 70, 127–130. [Google Scholar] [CrossRef]
- Hoefler, C.D.; Chen, A.; Jakob, E.M. The potential of a jumping spider, Phidippus clarus, as a biocontrol agent. J. Econ. Entomol. 2006, 99, 432–436. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, K.; Wei, B.; Wang, Z.; Tian, Y.; Tian, Y.; Song, Q. Bioaccumulation of Cry1Ab Protein from an Herbivore Reduces Anti-Oxidant Enzyme Activities in Two Spider Species. PLoS ONE 2014, 9, e84724. [Google Scholar] [CrossRef][Green Version]
- Marchetti, E.; Alberghini, S.; Battisti, A.; Squartini, A.; Baronio, P.; Dindo, M.L. Effects of conventional and transgenic Bacillus thuringiensis galleriae toxin on Exorista larvarum (Diptera: Tachinidae), a parasitoid of forest defoliating Lepidoptera. Biocontrol Sci. Technol. 2009, 19, 463–473. [Google Scholar] [CrossRef]
- Da Rolim, G.S.; Plata-Rueda, A.; Martínez, L.C.; Ribeiro, G.T.; Serrão, J.E.; Zanuncio, J.C. Side effects of Bacillus thuringiensis on the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae). Ecotoxicol. Environ. Saf. 2020, 189. [Google Scholar] [CrossRef]
- Ksentini, I.; Monje, J.C.; Jardak, T.; Zeghal, N. Naturally occurring egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) in a pomegranate orchard in Tunisia. Entomol. Sci. 2010, 13, 99–106. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sahragard, A.; Talaei-Hassanloui, R. Effect of Bacillus thuringiensis var. kurstaki on survival and mortality of immature and mature stages of Diadegma insulare parasitizing Plutella xylostella. Phytoparasitica 2012, 40, 393–401. [Google Scholar] [CrossRef]
- Schoenmaker, A.; Cusson, M.; Van Frankenhuyzen, K. Interactions between Bacillus thuringiensis and parasitoids of late-instar larvae of the spruce budworm (Lepidoptera: Tortricidae). Can. J. Zool. 2001, 79, 1697–1703. [Google Scholar] [CrossRef]
- Nascimento, P.T.; Fadini, M.A.M.; Valicente, F.H.; Ribeiro, P.E.A. Does Bacillus thuringiensis have adverse effects on the host egg location by parasitoid wasps? Rev. Bras. Entomol. 2018, 62, 260–266. [Google Scholar] [CrossRef]
- Malone, L.A.; Burgess, E.P.J.; Stefanovic, D. Effects of a Bacillus thuringiensis toxin, two Bacillus thuringiensis biopesticide formulations, and a soybean trypsin inhibitor on honey bee (Apis mellifera L.) survival and food consumption. Apidologie 1999, 30, 465–473. [Google Scholar] [CrossRef]
- Libardoni, G.; De Gouvea, A.; Costa-Maia, F.M.; Lozano, E.R.; De Freitas, P.F.; Colombo, F.C.; Raulino, F.; Maciel, R.M.A.; Potrich, M. Effect of different Bacillus thuringiensis strains on the longevity of Africanized honey bee. Semin. Agrar. 2018, 39, 329–338. [Google Scholar] [CrossRef]
- Dai, P.-L.; Zhou, W.; Zhang, J.; Jiang, W.-Y.; Wang, Q.; Cui, H.-J.; Sun, J.-H.; Wu, Y.-Y.; Zhou, T. The effects of Bt Cry1Ah toxin on worker honeybees (Apis mellifera ligustica and Apis cerana cerana). Apidologie 2012, 43. [Google Scholar] [CrossRef]
- Mommaerts, V.; Jans, K.; Smagghe, G. Impact of Bacillus thuringiensis strains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci. 2010, 66, 520–525. [Google Scholar] [CrossRef]
- MacGregor, C.J.; Pocock, M.J.O.; Fox, R.; Evans, D.M. Pollination by nocturnal Lepidoptera, and the effects of light pollution: A review. Ecol. Entomol. 2015, 40, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.P. The mammalian safety of Bacillus thuringiensis-based insecticides. J. Invertebr. Pathol. 2001, 77, 13–21. [Google Scholar] [CrossRef]
- Grisolia, C.K.; Oliveira-Filho, E.C.; Ramos, F.R.; Lopes, M.C.; Muniz, D.H.F.; Monnerat, R.G. Acute toxicity and cytotoxicity of Bacillus thuringiensis and Bacillus sphaericus strains on fish and mouse bone marrow. Ecotoxicology 2009, 18, 22–26. [Google Scholar] [CrossRef]
- Meher, S.M.; Bodhankar, S.L.; Dhuley, J.N.; Khodape, D.J.; Naik, S.R. Toxicity studies of microbial insecticide Bacillus thuringiensis var. kenyae in rats, rabbits, and fish. Int. J. Toxicol. 2002, 21, 99–105. [Google Scholar] [CrossRef]
- Fisher, R.; Rosner, L. Toxicology of the Microbial Insecticide, Thuricide. J. Agric. Food Chem. 1959, 7, 686–688. [Google Scholar] [CrossRef]
- Freire, I.S.; Miranda-Vilela, A.L.; Fascineli, M.L.; Oliveira-Filho, E.C.; Martins, E.S.; Monnerat, R.G.; Grisolia, C.K. Genotoxic evaluation in Oreochromis niloticus (Fish: Characidae) of recombinant spore-crystal complexes Cry1Ia, Cry10Aa and Cry1Ba6 from Bacillus thuringiensis. Ecotoxicology 2014, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Horwitz, R.J.; Sweeney, B.W. Effects of Bacillus thuringiensis israelensis on Black Flies and Nontarget Macroinvertebrates and Fish in a Large River. Trans. Am. Fish. Soc. 2002, 131, 910–930. [Google Scholar] [CrossRef]
- Najib Ahmad, M.; Ramlah Ahmad Ali, S.; Mazmira Mohd Masri, M.; Ghazali, R.; Basri Wahid, M. Ecotoxicity of Bacillus thuringiensis, Terakil-1 ® and Teracon-1 ® against freshwater fish, Tilapia nilotica. J. Oil Palm Res. 2011, 23, 1036–1039. [Google Scholar]
- Snarski, V.M. Interactions between Bacillus thuringiensis subsp. israelensis and fathead minnows, Pimephales promelas rafinesque, under laboratory conditions. Appl. Environ. Microbiol. 1990, 56, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, E.C.; Ramos, F.R.; Miranda, B.C.G.; Muniz, D.H.F.; Monnerat, R.G. Evaluating the Elimination of Brazilian Entomopathogenic Bacillus by Non-Target Aquatic Species: An Experimental Study. Bull. Environ. Contam. Toxicol. 2014, 93, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mariano, W.S.; Azevedo, S.B.; Gomes, F.L.; Lima, L.B.D.; Moron, S.E.; Tavares-Dias, M. Physiological parameters of Piaractus Mesopotamicus (Osteichthyes: Characidae) exposed to a biopesticide based on Bacillus thuringiensis. An. Acad. Bras. Cienc. 2019, 91. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Miksch, L.; Köhler, H.R.; Triebskorn, R. Does Bti (Bacillus thuringiensis var. israelensis) affect Rana temporaria tadpoles? Ecotoxicol. Environ. Saf. 2019, 181, 121–129. [Google Scholar] [CrossRef]
- Allgeier, S.; Frombold, B.; Mingo, V.; Brühl, C.A. European common frog Rana temporaria (Anura: Ranidae) larvae show subcellular responses under field-relevant Bacillus thuringiensis var. israelensis (Bti) exposure levels. Environ. Res. 2018, 162, 271–279. [Google Scholar] [CrossRef]
- Glare, T.R.; O’Callaghan, M. Environmental and Health Impacts of Bacillus thuringiensis israelensis; Report for the Ministry of Health; Beyond Pesticides: Washington, DC, USA, 1998. [Google Scholar]
- Lajmanovich, R.C.; Junges, C.M.; Cabagna-Zenklusen, M.C.; Attademo, A.M.; Peltzer, P.M.; Maglianese, M.; Márquez, V.E.; Beccaria, A.J. Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Environ. Res. 2015, 136, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Fiscarelli, E.; Senesi, S. Bacillus thuringiensis pulmonary infection: Critical role for bacterial membrane-damaging toxins and host neutrophils. Microbes Infect. 2007, 9, 591–598. [Google Scholar] [CrossRef]
- Hernandez, E.; Ramisse, F.; Gros, P.; Cavallo, J.-D. Super-infection by Bacillus thuringiensis H34 or 3a3b can lead to death in mice infected with the influenza A virus. FEMS Immunol. Med. Microbiol. 2000, 29, 177–181. [Google Scholar] [CrossRef]
- Hernandez, E.; Ramisse, F.; Ducoureau, J.P.; Cruel, T.; Cavallo, J.D. Bacillus thuringiensis subsp. konkukian (Serotype H34) superinfection: Case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 1998, 36, 2138–2139. [Google Scholar] [CrossRef]
- Dash, S.K.; Achary, P.M.; Sharma, C.B. Metaphase arrest in the bone marrow cells of Rattus norvegicus by the Beta-exotoxin of Bacillus thuringiensis. Acta Biol. Acad. Sci. Hung. 1978, 29, 189–191. [Google Scholar]
- Tsai, S.F.; Liu, B.L.; Liao, J.W.; Wang, J.S.; Hwang, J.S.; Wang, S.C.; Tzeng, Y.M.; Ho, S.P. Pulmonary toxicity of thuringiensin administered intratracheally in Sprague-Dawley rats. Toxicology 2003, 186, 205–216. [Google Scholar] [CrossRef]
- Tsai, S.F.; Yang, C.; Wang, S.C.; Wang, J.S.; Hwang, J.S.; Ho, S.P. Effect of thuringiensin on adenylate cyclase in rat cerebral cortex. Toxicol. Appl. Pharmacol. 2004, 194, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.F.; Yang, C.; Liu, B.L.; Hwang, J.S.; Ho, S.P. Role of oxidative stress in thuringiensin-induced pulmonary toxicity. Toxicol. Appl. Pharmacol. 2006, 216, 347–353. [Google Scholar] [CrossRef]
- Pendleton, I.R.; Bernheimer, A.W.; Grushoff, P. Purification and partial characterization of hemolysins from Bacillus thuringiensis. J. Invertebr. Pathol. 1973, 21, 131–135. [Google Scholar] [CrossRef]
- Budarina, Z.I.; Sinev, M.A.; Mayorov, S.G.; Tomashevski, A.Y.; Shmelev, I.V.; Kuzmin, N.P. Hemolysin II is more characteristic of Bacillus thuringiensis than Bacillus cereus. Arch. Microbiol. 1994, 161, 252–257. [Google Scholar] [CrossRef]
- Ngamwongsatit, P.; Buasri, W.; Pianariyanon, P.; Pulsrikarn, C.; Ohba, M.; Assavanig, A.; Panbangred, W. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 2008, 121, 352–356. [Google Scholar] [CrossRef]
- Gaviria Rivera, A.M.; Granum, P.E.; Priest, F.G. Common occurrence of enterotoxin genes and enterotoxicity in Bacillus thuringiensis. FEMS Microbiol. Lett. 2000, 190, 151–155. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Hu, X.M.; Liu, H.Z.; Hansen, B.M.; Yan, J.P.; Yuan, Z.M. Kinetics of plasmid transfer among Bacillus cereus group strains within lepidopteran larvae. Arch. Microbiol. 2007, 187, 425–431. [Google Scholar] [CrossRef]
- Böhm, M.-E.; Huptas, C.; Krey, V.M.; Scherer, S. Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15, 246. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; Timmery, S.; Hoton, F.; Mahillon, J. Plasmid exchanges among members of the Bacillus cereus group in foodstuffs. Int. J. Food Microbiol. 2007, 113, 164–172. [Google Scholar] [CrossRef]
- Pannucci, J.; Okinaka, R.T.; Williams, E.; Sabin, R.; Ticknor, L.O.; Kuske, C.R. DNA sequence conservation between the Bacillus anthracis pXO2 plasmid and genomic sequence from closely related bacteria. BMC Genom. 2002, 3, 34. [Google Scholar] [CrossRef][Green Version]
- Baldwin, V.M. You Can’t B. cereus—A Review of Bacillus cereus Strains That Cause Anthrax-Like Disease. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Green, M.; Heumann, M.; Sokolow, R.; Foster, L.R.; Bryant, R.; Skeels, M. Public health implications of the microbial pesticide Bacillus thuringiensis: An epidemiology study, Oregon, 1985–1986. Am. J. Public Health 1990, 80, 848–852. [Google Scholar] [CrossRef]
- Jackson, S.G.; Goodbrand, R.B.; Ahmed, R.; Kasatiya, S. Bacillus cereus and Bacillus thuringiensis isolated in a gastroenteritis outbreak investigation. Lett. Appl. Microbiol. 1995, 21, 103–105. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.; Bernard, K.; Beniac, D.; Isaac-Renton, J.L.; Naseby, D.C. Identification of Bacillus cereus group species associated with food poisoning outbreaks in British Columbia, Canada. Appl. Environ. Microbiol. 2008, 74, 7451–7453. [Google Scholar] [CrossRef]
- Thompson, D.G. Ecological impacts of major forest-use pesticides. In Ecological Impacts of Toxic Chemicals (Open Access); Sánchez-Bayo, F., Brink, P.J., van den Mann, R.M., Eds.; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2012; pp. 88–110. [Google Scholar]
- Poulin, B.; Lefebvre, G.; Paz, L. Red flag for green spray: Adverse trophic effects of Bti on breeding birds. J. Appl. Ecol. 2010, 47, 884–889. [Google Scholar] [CrossRef]
- Miller, J.C. Monitoring the Effects of Bacillus thuringiensis kurstaki on Nontarget Lepidoptera in Woodlands and Forests of Western Oregon. Nontarget Eff. Biol. Control 2000, 277–286. [Google Scholar] [CrossRef]
- Norton, M.L.; Bendell, J.F.; Bendell-Young, L.I.; LeBlanc, C.W. Secondary effects of the pesticide Bacillus thuringiensis kurstaki on chicks of spruce grouse (Dendragapus canadensis). Arch. Environ. Contam. Toxicol. 2001, 41, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Rodenhouse, N.L.; Holmes, R.T. Results of experimental and natural food reductions for breeding black-throated blue warblers. Ecology 1992, 73, 357–372. [Google Scholar] [CrossRef]
- Awkerman, J.A.; Marshall, M.R.; Williams, A.B.; Gale, G.A.; Cooper, R.J.; Raimondo, S. Assessment of indirect pesticide effects on worm-eating warbler populations in a managed forest ecosystem. Environ. Toxicol. Chem. 2011, 30, 1843–1851. [Google Scholar] [CrossRef]
- Sopuck, L.; Ovaska, K.; Whittington, B. Responses of Songbirds To Aerial Spraying of the Microbial Insecticide Bacillus Thuringiensis Var. Kurstaki (Foray 48B ®) on Vancouver Island, British Columbia, Canada. Environ. Toxicol. Chem. 2002, 21, 1664. [Google Scholar] [CrossRef]
- Raimondo, S.; Pauley, T.K.; Butler, L. Potential Impacts of Bacillus thuringiensis var. kurstaki on Five Salamander Species in West Virginia. Northeast. Nat. 2003, 10, 25. [Google Scholar] [CrossRef]
- Sample, B.E.; Whitmore, R.C. Food Habits of the Endangered Virginia Big-Eared Bat in West Virginia. J. Mammal. 1993, 74, 428–435. [Google Scholar] [CrossRef]
- Blackburn, L.M.; Leonard, D.S.; Tobin, P.C. The Use of Bacillus thuringiensis kurstaki for Managing Gypsy Moth Populations under the Slow the Spread Program, 1996-2010, Relative to the Distributional Range of Threatened and Endangered Species; Res. Pap. NRS-18; US Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2011.
- Lacey, L.A. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 2007, 23, 133–163. [Google Scholar] [CrossRef]
- Ichimatsu, T.; Mizuki, E.; Nishimura, K.; Akao, T.; Saitoh, H.; Higuchi, K.; Ohba, M. Occurrence of Bacillus thuringiensis in fresh waters of Japan. Curr. Microbiol. 2000, 40, 217–220. [Google Scholar] [CrossRef]
- Boisvert, M.; Boisvert, J. Effects of Bacillus thuringiensis var. israelensis on Target and Nontarget Organisms: A Review of Laboratory and Field Experiments. Biocontrol Sci. Technol. 2000, 10, 517–561. [Google Scholar] [CrossRef]
- Manasherob, R.; Ben-Dov, E.; Zaritsky, A.; Barak, Z. Germination, growth, and sporulation of Bacillus thuringiensis subsp. israelensis in excreted food vacuoles of the protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 1998, 64, 1750–1758. [Google Scholar] [CrossRef]
- Olmo, C.; Marco, A.; Armengol, X.; Ortells, R. Effects of Bacillus thuringiensis var. israelensis on nonstandard microcrustacean species isolated from field zooplankton communities. Ecotoxicology 2016, 25, 1730–1738. [Google Scholar] [CrossRef]
- Liber, K.; Schmude, K.L.; Rau, D.M. Toxicity of Bacillus thuringiensis var. israelensis to chironomids in pond mesocosms. Ecotoxicology 1998, 7, 343–354. [Google Scholar] [CrossRef]
- Jakob, C.; Poulin, B. Indirect effects of mosquito control using Bti on dragonflies and damselflies (Odonata) in the Camargue. Insect Conserv. Divers. 2016, 9, 161–169. [Google Scholar] [CrossRef]
- Brühl, C.A.; Després, L.; Frör, O.; Patil, C.D.; Poulin, B.; Tetreau, G.; Allgeier, S. Environmental and socioeconomic effects of mosquito control in Europe using the biocide Bacillus thuringiensis subsp. israelensis (Bti). Sci. Total Environ. 2020, 137800. [Google Scholar] [CrossRef] [PubMed]
- Allgeier, S.; Friedrich, A.; Brühl, C.A. Mosquito control based on Bacillus thuringiensis israelensis (Bti) interrupts artificial wetland food chains. Sci. Total Environ. 2019, 686, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.O.; Schäfer, M.L.; Petersson, E.; Persson Vinnersten, T.Z.; Landin, J.; Brodin, Y. Production of wetland Chironomidae (Diptera) and the effects of using Bacillus thuringiensis israelensis for mosquito control. Bull. Entomol. Res. 2010, 100, 117–125. [Google Scholar] [CrossRef]
- Lagadic, L.; Schäfer, R.B.; Roucaute, M.; Szöcs, E.; Chouin, S.; de Maupeou, J.; Duchet, C.; Franquet, E.; Le Hunsec, B.; Bertrand, C.; et al. No association between the use of Bti for mosquito control and the dynamics of non-target aquatic invertebrates in French coastal and continental wetlands. Sci. Total Environ. 2016, 553, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Duchet, C.; Franquet, E.; Lagadic, L.; Lagneau, C. Effects of Bacillus thuringiensis israelensis and spinosad on adult emergence of the non-biting midges Polypedilum nubifer (Skuse) and Tanytarsus curticornis Kieffer (Diptera: Chironomidae) in coastal wetlands. Ecotoxicol. Environ. Saf. 2015, 115, 272–278. [Google Scholar] [CrossRef]
- Wolfram, G.; Wenzl, P.; Jerrentrup, H. A multi-year study following BACI design reveals no short-term impact of Bti on chironomids (Diptera) in a floodplain in Eastern Austria. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef]
- Land, M.; Bundschuh, M.; Hopkins, R.J.; Poulin, B.; McKie, B.G. What are the effects of control of mosquitoes and other nematoceran Diptera using the microbial agent Bacillus thuringiensis israelensis (Bti) on aquatic and terrestrial ecosystems? A systematic review protocol. Environ. Evid. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Capell, S.S. Palatability of leaf material contaminated with Bacillus thuringiensis var. kurstaki, to Hydatophylax argus, a detritivorous aquatic insect. Bull. Environ. Contam. Toxicol. 1996, 56, 80–84. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Capell, S.S.; Thomas, D.R. Aquatic insect responses to Bacillus thuringiensis var. kurstaki in a forest stream. Can. J. For. Res. 1994, 24, 2041–2049. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Holmes, S.B.; Capell, S.S.; Eichenberg, D.C. Lethal and sublethal effects of Bacillus thuringiensis var. kurstaki on aquatic insects in laboratory bioassays and outdoor stream channels. Bull. Environ. Contam. Toxicol. 1992, 49, 252–258. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousova, M.E.; Malovichko, Y.V.; Shikov, A.E.; Nizhnikov, A.A.; Antonets, K.S. Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems. Toxins 2021, 13, 355. https://doi.org/10.3390/toxins13050355
Belousova ME, Malovichko YV, Shikov AE, Nizhnikov AA, Antonets KS. Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems. Toxins. 2021; 13(5):355. https://doi.org/10.3390/toxins13050355
Chicago/Turabian StyleBelousova, Maria E., Yury V. Malovichko, Anton E. Shikov, Anton A. Nizhnikov, and Kirill S. Antonets. 2021. "Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems" Toxins 13, no. 5: 355. https://doi.org/10.3390/toxins13050355
APA StyleBelousova, M. E., Malovichko, Y. V., Shikov, A. E., Nizhnikov, A. A., & Antonets, K. S. (2021). Dissecting the Environmental Consequences of Bacillus thuringiensis Application for Natural Ecosystems. Toxins, 13(5), 355. https://doi.org/10.3390/toxins13050355






