The Deleterious Effects of Shiga Toxin Type 2 Are Neutralized In Vitro by FabF8:Stx2 Recombinant Monoclonal Antibody
Abstract
:1. Introduction
2. Results
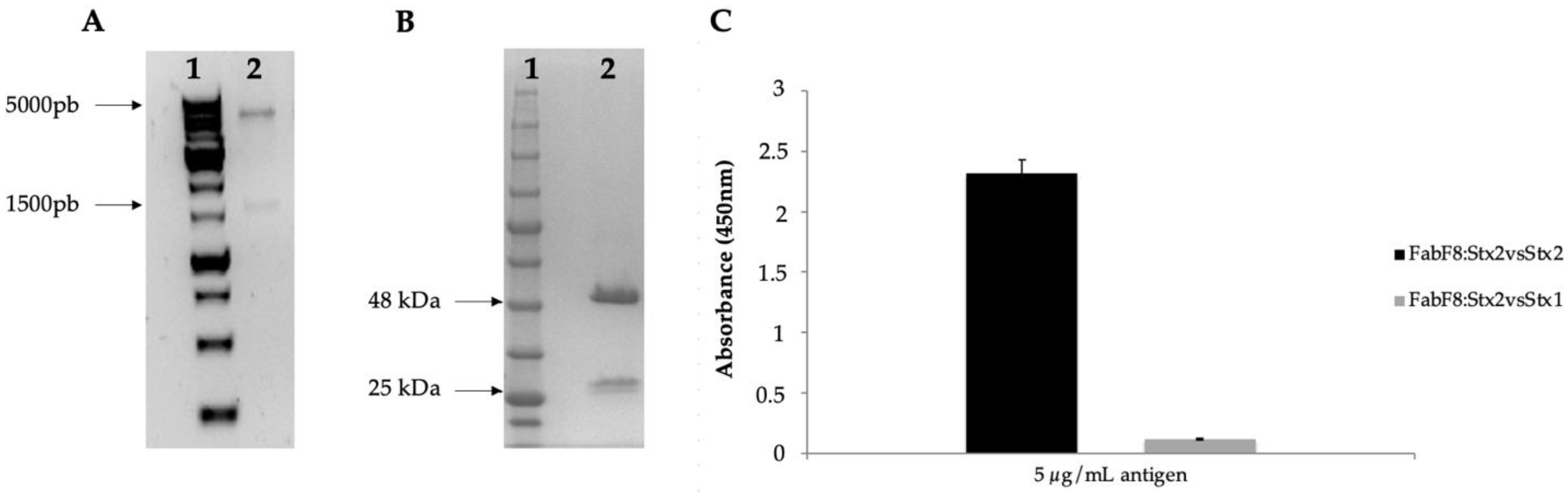
2.1. Selection of FabF8:Stx2 from a Human Antibody Fragment Phage Display Library
2.2. FabF8:Stx2 Neutralizes the Cytotoxic Effect of Supernatants from Different Stx2-Producing Strains
2.3. FabF8:Stx2 Protects Cell Viability of Human Glomerular Endothelial Cells (HGEC) from Stx2 Effects
2.4. FabF8:Stx2 Antibodies Prevent Detachment and Swelling Caused by Stx2 in HGEC
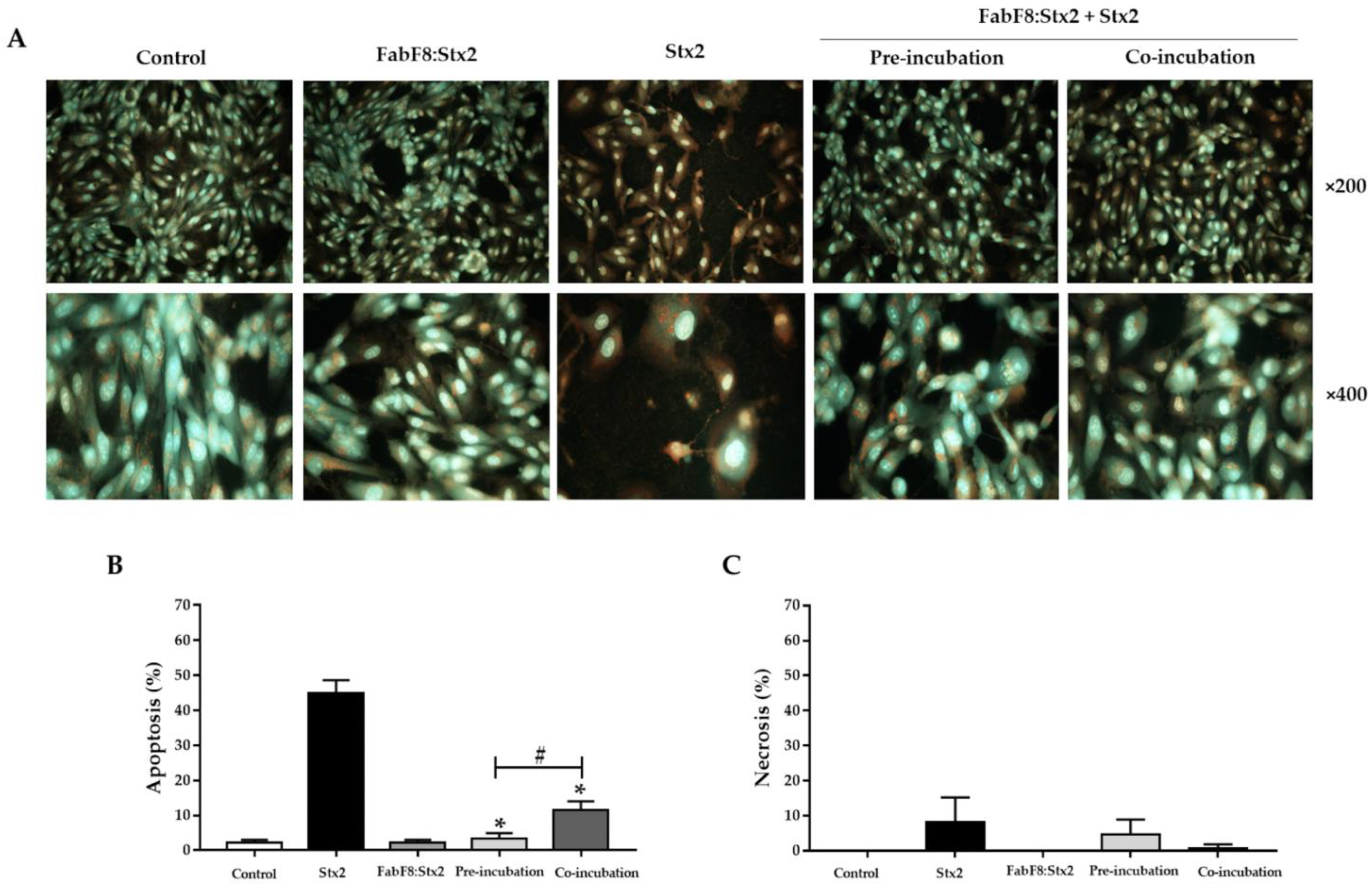
2.5. FabF8:Stx2 Antibodies Avoid Apoptosis Induced by Stx2 in HGEC
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains, Plasmids, and Antigen
5.2. Antibody Generation and Characterization
FabF8:Stx2 Characterization
5.3. Vero Cell Antibody Neutralization Assay
5.4. Primary Culture
5.5. Stx2 Neutralization Assay in HGEC
5.6. Neutral Red Viability Assay
5.7. Cell Morphology Analysis
5.8. Necrosis and Apoptosis Analysis
5.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 Outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic Profile of Shiga-Toxin–Producing Escherichia coli O104:H4 Outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terajima, J.; Iyoda, S.; Ohnishi, M.; Watanabe, H. Shiga Toxin (Verotoxin)-Producing Escherichia coli in Japan. Microbiol. Spectr. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Salud de la Nación Argentina. Boletín Integrado de Vigilancia. 2021. Available online: https://bancos.salud.gob.ar/recurso/boletin-integrado-de-vigilancia-n560-se-302021 (accessed on 13 October 2021).
- Pianciola, L.; Rivas, M. Genotypic Features of Clinical and Bovine Escherichia coli O157 Strains Isolated in Countries with Different Associated-Disease Incidences. Microorganisms 2018, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Alconcher, L.F.; Rivas, M.; Lucarelli, L.I.; Galavotti, J.; Rizzo, M. Shiga toxin-producing Escherichia coli in household members of children with hemolytic uremic syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 427–432. [Google Scholar] [CrossRef]
- Repetto, H.A. Epidemic hemolytic-uremic syndrome in children. Kidney Int. 1997, 52, 1708–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repetto, H.A.; Rodríguez de Córdoba, E.; Arrizurieta, E.; Rivas, M.; y Ibarra, C. Microangiopatía trombótica y Síndrome Hemolítico Urémico. In Nefrología Clínica, 3rd ed.; Editorial Médica Panamericana: Buenos Aires, Argentina, 2014; Chapter 25; pp. 352–363. [Google Scholar]
- Spinale, J.M.; Ruebner, R.L.; Copelovitch, L.; Kaplan, B.S. Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr. Nephrol. 2013, 28, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.A.; Pellino, C.A.; Flagler, M.J.; Strasser, J.E.; Weiss, A.A. Shiga toxin subtypes display dramatic differences in potency. Infect Immun. 2011, 79, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavaliauskiene, S.; Lingelem, A.B.D.; Skotland, T.; Sandvig, K. Protection against Shiga Toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef]
- Sandvig, K.; Garred, O.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; Deurs, B.V. Retrograde transport of endocytosed Shiga toxin to the endoplasmatic reticulum. Nature 1992, 358, 510–512. [Google Scholar] [CrossRef]
- Hall, G.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga Toxin Therapeutics: Beyond Neutralization. Toxins 2017, 9, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: A global overview. J. Infect. 2019, 79, 75–94. [Google Scholar] [CrossRef]
- Cody, E.M.; Dixon, B.P. Hemolytic Uremic Syndrome. Pediatr. Clin. North Am. 2019, 66, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Melton-Celsa, A.R.; O’Brien, A.D. New Therapeutic Developments against Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Mühlen, S.; Dersch, P. Treatment Strategies for Infections with Shiga Toxin-Producing Escherichia coli. Front. Cell. Infect. Microbiol. 2020, 10, 169. [Google Scholar] [CrossRef]
- Dowling, T.C.; Chavaillaz, P.A.; Young, D.G.; Melton-Celsa, A.; O’Brien, A.; Thuning-Roberson, C.; Edelman, R.; Tacket, C.O. Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody c alpha Stx2 administered intravenously to healthy adult volunteers. Antimicrob. Agents Chemother. 2005, 49, 1808–1812. [Google Scholar] [CrossRef] [Green Version]
- Bitzan, M.; Poole, R.; Mehran, M.; Sicard, E.; Brockus, C.; Thuning-Roberson, C.; Rivière, M. Safety and Pharmacokinetics of Chimeric Anti-Shiga Toxin 1 and Anti-Shiga Toxin 2 Monoclonal Antibodies in Healthy Volunteers. Antimicrob. Agents Chemother. 2009, 53, 3081–3087. [Google Scholar] [CrossRef] [Green Version]
- López, E.L.; Contrini, M.M.; Glatstein, E.; Ayala, S.G.; Santoro, R.; Allende, D.; Ezcurra, G.; Teplitz, E.; Koyama, T.; Matsumoto, Y.; et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.M.; Bitzan, M.; Reymond, D. A Phase II study assessing monoclonal antibodies against Shiga toxin 1 and 2 in Shiga toxin-producing E. coli-infected children. Shigatec, Boston, USA, October 21, 2011. Pediatr. Nephrol. 2011, 26, 1595–1596. [Google Scholar]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Nilvebrant, J.; Sidhu, S.S. Construction of Synthetic Antibody Phage-Display Libraries. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1701, pp. 45–60. [Google Scholar]
- Mejías, M.P.; Hiriart, Y.; Lauché, C.; Fernández-Brando, R.J.; Pardo, R.; Bruballa, A.; Ramos, M.V.; Goldbaum, F.A.; Palermo, M.S.; Zylberman, V. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS). Sci. Rep. 2016, 6, 24913. [Google Scholar] [CrossRef] [Green Version]
- Luz, D.; Chen, G.; Maranhão, A.; Rocha, L.B.; Sidhu, S.; Piazza, R.M.F. Development and Characterization of Recombinant Antibody Fragments That Recognize and Neutralize In Vitro Stx2 Toxin from Shiga Toxin-Producing Escherichia coli. PLoS ONE 2015, 10, e0120481. [Google Scholar] [CrossRef] [Green Version]
- Luz, D.; Amaral, M.M.; Sacerdoti, F.; Bernal, A.M.; Quintilio, W.; Moro, A.M.; Palermo, M.S.; Ibarra, C.; Piazza, R.M.F. Human Recombinant Fab Fragment Neutralizes Shiga Toxin Type 2 Cytotoxic Effects in vitro and in vivo. Toxins 2018, 10, 508. [Google Scholar] [CrossRef] [Green Version]
- Persson, H.; Ye, W.; Wernimont, A.; Adams, J.J.; Koide, A.; Koide, S.; Lam, R.; Sidhu, S.S. CDR-H3 diversity is not required for antigen recognition by synthetic antibodies. J. Mol. Biol. 2012, 425, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Amaral, M.M.; Sacerdoti, F.; Jancic, C.; Repetto, H.A.; Paton, A.W.; Paton, J.C.; Ibarra, C. Action of Shiga Toxin Type-2 and Subtilase Cytotoxin on Human Microvascular Endothelial Cells. PLoS ONE 2013, 8, e70431. [Google Scholar] [CrossRef] [Green Version]
- Bitzan, M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int. 2009, 75, S62–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, J.M.; Mukherjee, J.; Leysath, C.E.; Debatis, M.; Ofori, K.; Baldwin, K.; Boucher, C.; Peters, R.; Beamer, G.; Sheoran, A.; et al. A Single VHH-Based Toxin-Neutralizing Agent and an Effector Antibody Protect Mice against Challenge with Shiga Toxins 1 and 2. Infect. Immun. 2013, 81, 4592–4603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Mao, X.; Li, J.; Li, H.; Feng, Y.; Chen, H.; Luo, P.; Gu, J.; Yu, S.; Zeng, H.; et al. Engineering an anti-Stx2 antibody to control severe infections of EHEC O157:H7. Immunol. Lett. 2008, 121, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, D.E.; Sheoran, A.S.; Rich, C.M.; Richard, L.; Chapman-Bonofiglio, S.; Tzipori, S. Evaluation of Fab and F(ab′) 2 Fragments and Isotype Variants of a Recombinant Human Monoclonal Antibody against Shiga Toxin 2. Infect. Immun. 2010, 78, 1376–1382. [Google Scholar] [CrossRef] [Green Version]
- Obrig, T.G. Escherichia coli Shiga Toxin Mechanisms of Action in Renal Disease. Toxins 2010, 2, 2769–2794. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Santos, A.R.R.; Rocha, L.B.; Caetano, B.A.; Mitsunari, T.; Santos, L.I.; Polatto, J.M.; Horton, D.S.P.Q.; Guth, B.E.C.; Dos Santos, L.F.; et al. Development and Validation of Shiga Toxin-Producing Escherichia coli Immunodiagnostic Assay. Microorganisms 2019, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Perna, N.T.; Plunkett, G., 3rd; Burland, V.; Mau, B.; Glasner, J.D.; Rose, D.J.; Mayhew, G.F.; Evans, P.S.; Gregor, J.; Kirkpatrick, H.A.; et al. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 2001, 409, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, S.S.; Fellouse, F.A. Synthetic therapeutic antibodies. Nat. Chem. Biol. 2006, 2, 682–688. [Google Scholar] [CrossRef]
- Shiga, E.; Guth, B.; Piazza, R.; Luz, D. Comparative analysis of rapid agglutination latex test using single-chain antibody fragments (scFv) versus the gold standard Vero cell assay for Shiga toxin (Stx) detection. J. Microbiol. Methods 2020, 175, 105965. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.B.; Luz, D.; Moraes, C.T.P.; Caravelli, A.; Fernandes, I.; Guth, B.E.C.; Horton, D.S.P.Q.; Piazza, R.M.F. Interaction between Shiga Toxin and Monoclonal Antibodies: Binding Characteristics and in Vitro Neutralizing Abilities. Toxins 2012, 4, 729–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Strain | Serotype | Source | Stx | Subtype | Neutralization Rate (%) |
|---|---|---|---|---|---|
| EPM 50 | O87:H16 | Animal | Stx2 | 2b | 90 |
| EPM 96 | O93:H19 | Food | Stx2 | 2a, 2d | 86 |
| EPM 82 | O112:H21 | Animal | Stx2 | 2c | 90 |
| EPM 1 | O157:H7 | Human | Stx2 | 2a, 2c | 0 |
| EPM 2 | O157:H7 | Human | Stx2 | 2a, 2c | 97 |
| EPM 94 | O157:H7 | Animal | Stx2 | 2c | 95 |
| Raph/4 | O165:H- | Human | Stx2 | 2a, 2c | 0 |
| EPM O3 | O172:NM | Animal | Stx2 | 2a | 44 |
| EPM O22 | ONT:H16 | Animal | Stx2 | 2b | 46 |
| EPM 59 | ONT:H16 | Animal | Stx2 | 2d | 97 |
| EPM 81 | ONT:H38 | Animal | Stx2 | 2a | 27 |
| BA 1189 | ONT:H49 | Human | Stx2 | 2a, 2d | 24 |
| BA 1132 | ONT:H49 | Human | Stx2 | 2a, 2c, 2d | 7 |
| EPM 79 | O22:H16 | Animal | Stx1/2 | 1a, 2c, 2d | 53 |
| BA 3003 | O48:H7 | Human | Stx1/2 | 1a, 2a | 0 |
| EPM O36 | O75:H8 | Animal | Stx1/2 | 1c, 2b | 0 |
| EPM 4 | O93:H19 | Human | Stx1/2 | 1a, 2d | 78 |
| EPM 53 | O98:H17 | Animal | Stx1/2 | 1a, 2a, 2c | 100 |
| EPM 55 | O98:H17 | Animal | Stx1/2 | 1a, 2a, 2c | 85 |
| EPM 9 | O103:H2 | Human | Stx1/2 | 1a, 2c | 46 |
| EPM 66 | O105:H18 | Animal | Stx1/2 | 1a, 2a, 2b | 7 |
| EPM O55 | O146:H21 | Animal | Stx1/2 | 1a, 2a, 2b | 24 |
| 3104-88 | O157:H7 | Human | Stx1/2 | 1a, 2a | 27 |
| C7-88 | O157:H7 | Human | Stx1/2 | 1a, 2NT | 15 |
| EDL 933 | O157:H7 | Food | Stx1/2 | 1a, 2a | 80 |
| EPM 45 | O181:H4 | Animal | Stx1/2 | 1a, 2a | 80 |
| 18 (ICB) | ND | ND | Stx1/2 | 1NT, 2NT | 87 |
| Purified Stx2 | - | - | Stx2 | 2a | 84 |
| FabF8:Stx2 (g/mL) | Stx2 Cytotoxicity Prevention (%) | |
|---|---|---|
| Pre-Incubation | Co-Incubation | |
| 0 | 0 | 0 |
| 0.1 | 30.5 ± 1.5 | 27.1 ± 4.2 |
| 1 | 58.5 ± 5.4 | 53.5 ± 2.6 |
| 10 | 83.0 ± 5.1 | 67.5 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz, D.; Gómez, F.D.; Ferreira, R.L.; Melo, B.S.; Guth, B.E.C.; Quintilio, W.; Moro, A.M.; Presta, A.; Sacerdoti, F.; Ibarra, C.; et al. The Deleterious Effects of Shiga Toxin Type 2 Are Neutralized In Vitro by FabF8:Stx2 Recombinant Monoclonal Antibody. Toxins 2021, 13, 825. https://doi.org/10.3390/toxins13110825
Luz D, Gómez FD, Ferreira RL, Melo BS, Guth BEC, Quintilio W, Moro AM, Presta A, Sacerdoti F, Ibarra C, et al. The Deleterious Effects of Shiga Toxin Type 2 Are Neutralized In Vitro by FabF8:Stx2 Recombinant Monoclonal Antibody. Toxins. 2021; 13(11):825. https://doi.org/10.3390/toxins13110825
Chicago/Turabian StyleLuz, Daniela, Fernando D. Gómez, Raíssa L. Ferreira, Bruna S. Melo, Beatriz E. C. Guth, Wagner Quintilio, Ana Maria Moro, Agostina Presta, Flavia Sacerdoti, Cristina Ibarra, and et al. 2021. "The Deleterious Effects of Shiga Toxin Type 2 Are Neutralized In Vitro by FabF8:Stx2 Recombinant Monoclonal Antibody" Toxins 13, no. 11: 825. https://doi.org/10.3390/toxins13110825
APA StyleLuz, D., Gómez, F. D., Ferreira, R. L., Melo, B. S., Guth, B. E. C., Quintilio, W., Moro, A. M., Presta, A., Sacerdoti, F., Ibarra, C., Chen, G., Sidhu, S. S., Amaral, M. M., & Piazza, R. M. F. (2021). The Deleterious Effects of Shiga Toxin Type 2 Are Neutralized In Vitro by FabF8:Stx2 Recombinant Monoclonal Antibody. Toxins, 13(11), 825. https://doi.org/10.3390/toxins13110825








