Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality
Abstract
:1. Introduction
2. Results
2.1. Individual Variation of Venom Composition Is Associated with Habitats
2.2. Population-Level Differentiation between Spatially Disparate B. atrox Groups
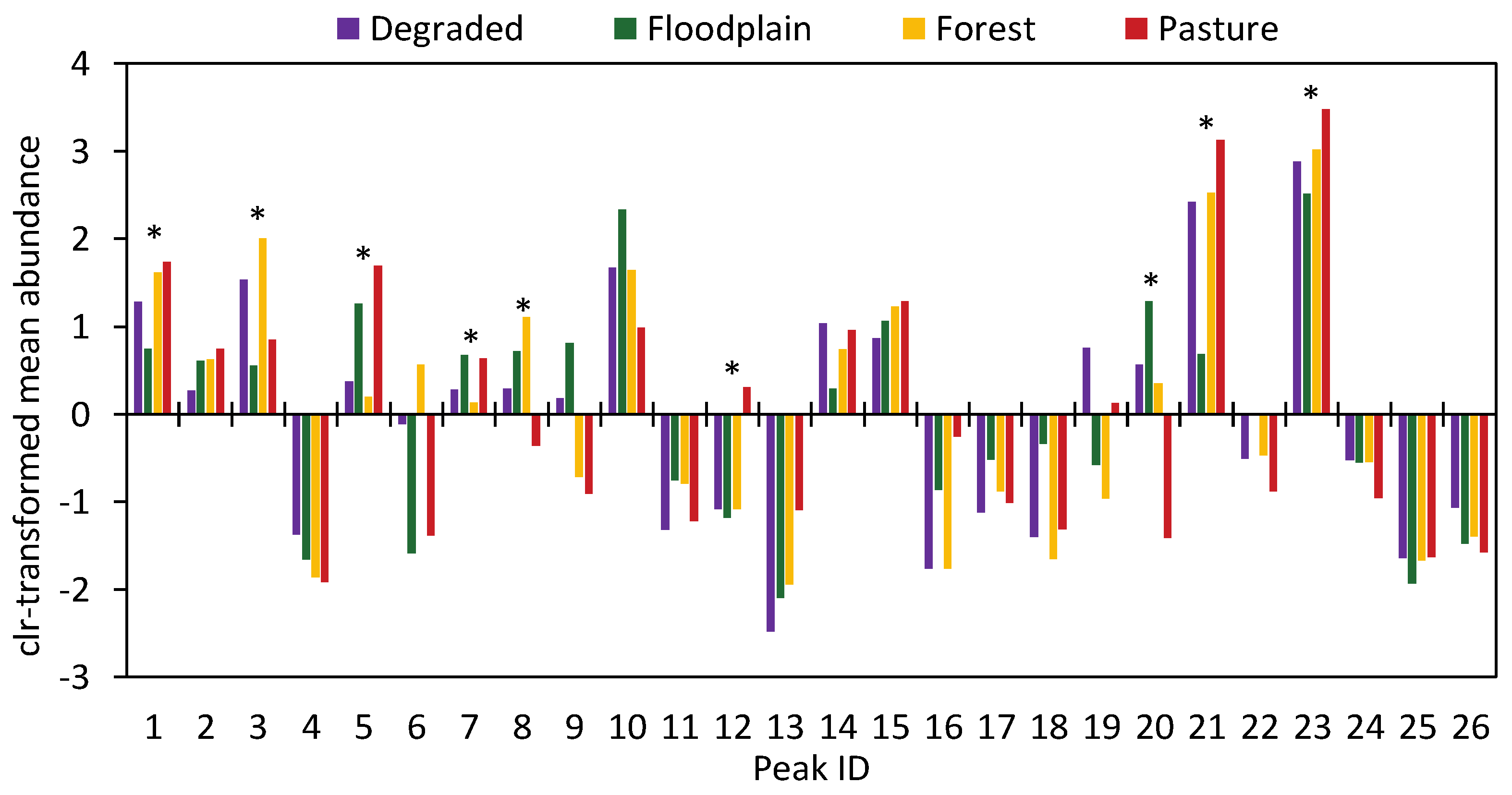
2.3. Venom Differentiation Is Not Limited to Rare (Low Abundance) Proteins
2.4. Specific-Level Differentiation among Venoms of Snakes Collected in the Same Environment
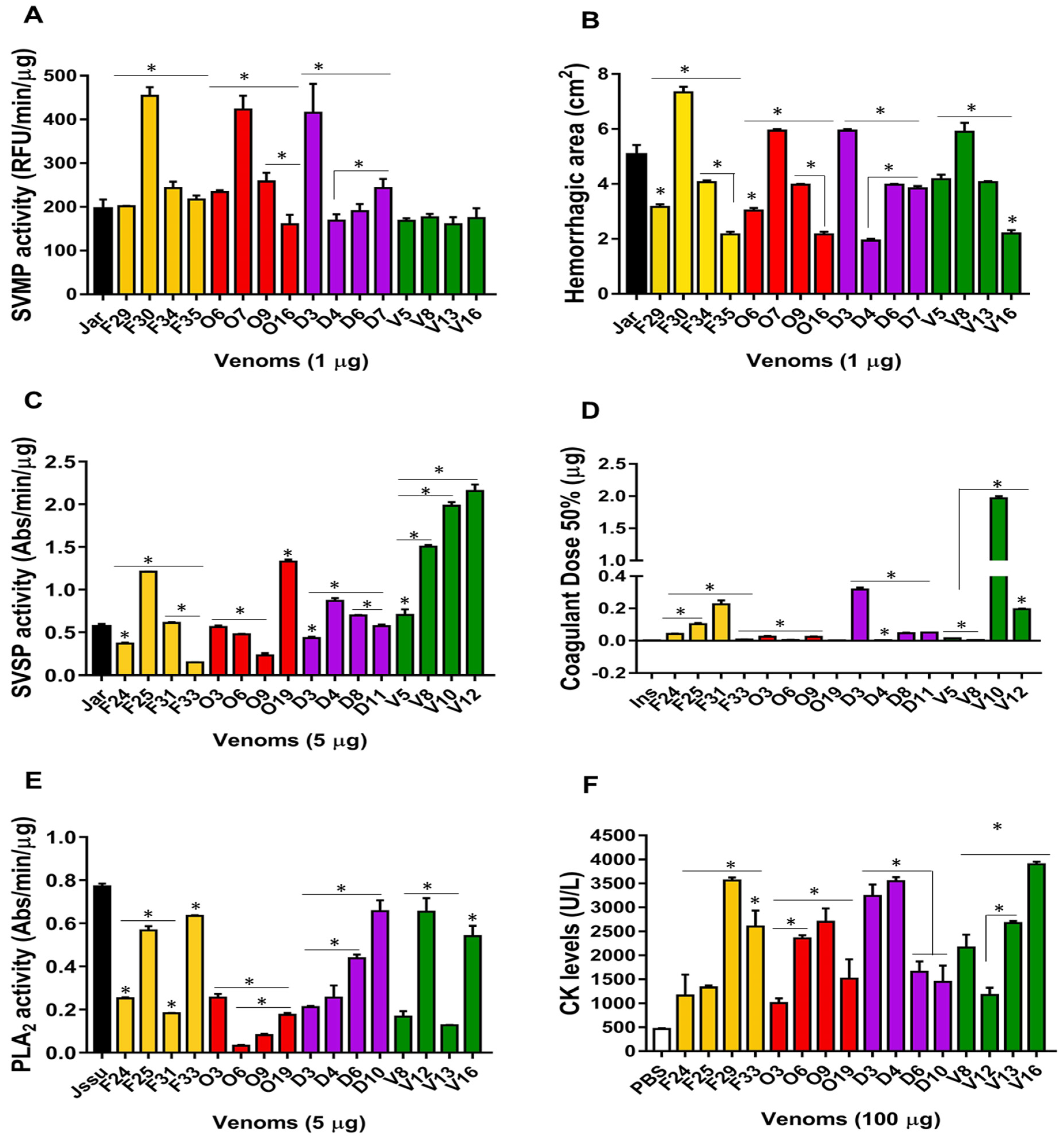
2.5. Differences in the Composition of Individual Venoms Resulting in Functional Variability
2.6. Individual Heterogeneity in Venom-Induced Lethality
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Snakes and Venoms
5.2. Chromatographic Analysis
5.3. Functional Assays
5.3.1. Enzymatic Assays on Synthetic Substrates
5.3.2. Procoagulant Activity
5.3.3. Venom Activities Using Animal Models
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama-Jr, M.Y.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.T.; Zingali, R.B.; Moura-da-Silva, A.M. Functional Variability of Snake Venom Metalloproteinases: Adaptive Advantages in Targeting Different Prey and Implications for Human Envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.L.; Mackessy, S.P. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon 2009, 53, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Campbell, J.A.; Lamar, W.W. The Venomous Reptiles of the Western Hemisphere; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- López-Lozano, J.L.; de Sousa, M.V.; Ricart, C.A.; Chávez-Olortegui, C.; Flores Sanchez, E.; Muniz, E.G.; Bührnheim, P.F.; Morhy, L. Ontogenetic variation of metalloproteinases and plasma coagulant activity in venoms of wild Bothrops atrox specimens from Amazonian rain forest. Toxicon 2002, 40, 997–1006. [Google Scholar] [CrossRef]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Guércio, R.A.; Shevchenko, A.; López-Lozano, J.L.; Paba, J.; Sousa, M.V.; Ricart, C.A. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteom. Sci. 2006, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Salazar, A.M.; Rodriguez-Acosta, A.; Girón, M.E.; Aguilar, I.; Guerrero, B. A comparative analysis of the clotting and fibrinolytic activities of the snake venom (Bothrops atrox) from different geographical areas in Venezuela. Thromb. Res. 2007, 120, 95–104. [Google Scholar] [CrossRef]
- Núñez, V.; Cid, P.; Sanz, L.; De La Torre, P.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J. Proteom. 2009, 73, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Perez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Maria Gutierrez, J.; Chalkidis, H.M.; Mourao, R.H.V.; et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Moretto Del-Rei, T.H.; Sousa, L.F.; Rocha, M.M.T.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional variability of Bothrops atrox venoms from three distinct areas across the Brazilian Amazon and consequences for human envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y., Jr.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourao, R.H.V.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Contreras-Bernal, J.C.; Bisneto, P.F.; Sachett, J.; Mendonça da Silva, I.; Lacerda, M.; Guimarães da Costa, A.; Val, F.; Brasileiro, L.; Sartim, M.A.; et al. Bothrops atrox, the most important snake involved in human envenomings in the amazon: How venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Contreras-Bernal, J.C.; Cirilo Gimenes, S.N.; Freitas-de-Sousa, L.A.; Portes-Junior, J.A.; da Silva Peixoto, P.; Kei Iwai, L.; Mourão de Moura, V.; Ferreira Bisneto, P.; Lacerda, M.; et al. The relationship between clinics and the venom of the causative Amazon pit viper (Bothrops atrox). PLoS Negl. Trop. Dis. 2020, 14, e0008299. [Google Scholar] [CrossRef]
- Sousa, L.F.; Bernardoni, J.L.; Zdenek, C.N.; Dobson, J.; Coimbra, F.; Gillett, A.; Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Fry, B.G. Differential coagulotoxicity of metalloprotease isoforms from Bothrops neuwiedi snake venom and consequent variations in antivenom efficacy. Toxicol. Lett. 2020, 333, 211–221. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Nachtigall, P.G.; Portes-Junior, J.A.; Holding, M.L.; Nystrom, G.S.; Ellsworth, S.A.; Guimarães, N.C.; Tioyama, E.; Ortiz, F.; Silva, B.R.; et al. Size matters: An evaluation of the molecular basis of ontogenetic modifications in the composition of Bothrops jararacussu snake venom. Toxins 2020, 12, 791. [Google Scholar] [CrossRef]
- Schonour, R.B.; Huff, E.M.; Holding, M.L.; Claunch, N.M.; Ellsworth, S.A.; Hogan, M.P.; Wray, K.; McGivern, J.; Margres, M.J.; Colston, T.J.; et al. Gradual and Discrete Ontogenetic Shifts in Rattlesnake Venom Composition and Assessment of Hormonal and Ecological Correlates. Toxins 2020, 12, 659. [Google Scholar] [CrossRef]
- Harvey, M.G.; Aleixo, A.; Ribas, C.C.; Brumfield, R.T. Habitat Association Predicts Genetic Diversity and Population Divergence in Amazonian Birds. Am. Nat. 2017, 190, 631–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, H.L.; Sovic, M.; Amazonas, D.; Chalkidis, H.; Salazar-Valenzuela, D.; Moura-Da-Silva, A.M. Recent lineage diversification in a venomous snake through dispersal across the Amazon River. Biol. J. Linnean Soc. 2018, 123, 651–665. [Google Scholar] [CrossRef] [Green Version]
- Margres, M.J.; Wray, K.P.; Seavy, M.; McGivern, J.J.; Herrera, N.D.; Rokyta, D.R. Expression Differentiation Is Constrained to Low-Expression Proteins over Ecological Timescales. Genetics 2016, 202, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Freitas-de-Sousa, L.A.; Amazonas, D.R.; Sousa, L.F.; Sant’Anna, S.S.; Nishiyama, M.Y., Jr.; Serrano, S.M.T.; Junqueira-de-Azevedo, I.L.M.; Chalkidis, H.M.; Moura-da-Silva, A.M.; Mourao, R.H.V. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of Batroxrhagin, the predominant class PIII metalloproteinase from the venom of this species. Biochimie 2015, 118, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguiura, N.; Kapronezai, J.; Ribeiro, T.; Rocha, M.M.; Medeiros, C.R.; Marcelino, J.R.; Prezoto, B.C. An alternative micromethod to access the procoagulant activity of Bothrops jararaca venom and the efficacy of antivenom. Toxicon 2014, 90, 148–154. [Google Scholar] [CrossRef]
- Prezoto, B.C.; Tanaka-Azevedo, A.M.; Marcelino, J.R.; Tashima, A.K.; Nishiduka, E.S.; Kapronezai, J.; Mota, J.O.; Rocha, M.M.T.; Serino-Silva, C.; Oguiura, N. A functional and thromboelastometric-based micromethod for assessing crotoxin anticoagulant activity and antiserum relative potency against Crotalus durissus terrificus venom. Toxicon 2018, 148, 26–32. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Colombini, M.; Lopes-Ferreira, M.; Serrano, S.M.T.; Moura-da-Silva, A.M. Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase. Toxins 2017, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Greene, H.W. Snakes. In The Evolution of Mistery in Nature; University of Califórnia Press: Berkeley, CA, USA, 1997. [Google Scholar]
- Saviola, A.J.; Gandara, A.J.; Bryson, R.W.; Mackessy, S.P. Venom phenotypes of the Rock Rattlesnake (Crotalus lepidus) and the Ridge-nosed Rattlesnake (Crotalus willardi) from México and the United States. Toxicon 2017, 138, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Smiley-Walters, S.A.; Farrell, T.M.; Gibbs, H.L. Evaluating local adaptation of a complex phenotype: Reciprocal tests of pigmy rattlesnake venoms on treefrog prey. Oecologia 2017, 184, 739–748. [Google Scholar] [CrossRef]
- Zelanis, A.; de Souza Ventura, J.; Chudzinski-Tavassi, A.M.; de Fátima Domingues Furtado, M. Variability in expression of Bothrops insularis snake venom proteases: An ontogenetic approach. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 601–609. [Google Scholar] [CrossRef]
- McGrath, D.; Castro, F.; Futemma, C.; Amaral, B.; Calabria, J. Fisheries and resource management on the Lower Amazon floodplain. Human Ecol. 1993, 21, 167–195. [Google Scholar] [CrossRef]
- Thomas, R.G.; Pough, F.H. The effect of rattlesnake venom on digestion of prey. Toxicon 1979, 17, 221–228. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Schezaro-Ramos, R.; Floriano, R.S.; Rodrigues-Simioni, L.; Bernal-Bautista, M.H.; Alice da Cruz-Höfling, M. Biological activities of Leptodeira annulata (banded cat-eyed snake) venom on vertebrate neuromuscular preparations. Toxicon 2016, 119, 345–351. [Google Scholar] [CrossRef]
- Zimmerman, K.D.; Gates, G.R.; Heatwole, H. Effects of venom of the olive sea snake, Aipysurus laevis, on the behaviour and ventilation of three species of prey fish. Toxicon 1990, 28, 1469–1478. [Google Scholar] [CrossRef]
- Richards, D.P.; Barlow, A.; Wüster, W. Venom lethality and diet: Differential responses of natural prey and model organisms to the venom of the saw-scaled vipers (Echis). Toxicon 2012, 59, 110–116. [Google Scholar] [CrossRef]
- Martins, M.; Gordo, M. Bothrops atrox (common lancehead). Diet Herpetol. Rev. 1993, 24, 2. [Google Scholar]
- Martins, M.; Marques, O.A.V.; Sazima, I.; Schuett, G.; Höggren, M.; Green, H.W. Ecological and phylogenetic correlates of feeding habits in Neotropicalpitvipers of the genus Bothrops. In Biology of the Pit Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing: Tyler, TX, USA, 2002; pp. 307–328. [Google Scholar]
- Harrison, R.A.; Moura-da-Silva, A.M.; Laing, G.D.; Wu, Y.; Richards, A.; Broadhead, A.; Bianco, A.E.; Theakston, R.D.G. Antibody from mice immunized with DNA encoding the carboxyl-disintegrin and cysteine-rich domain (JD9) of the haemorrhagic metalloprotease, Jararhagin, inhibits the main lethal component of viper venom. Clin. Exp. Immunol. 2000, 121, 358–363. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the South Atlantic jellyfish Olindias sambaquiensis (Cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourao, R.H.V.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margres, M.J.; McGivern, J.J.; Seavy, M.; Wray, K.P.; Facente, J.; Rokyta, D.R. Contrasting modes and tempos of venom expression evolution in two snake species. Genetics 2015, 199, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Kindt, R.; O’Hara, B. Vegan: Community Ecology Package. R Package Version 1.17-0. Available online: http://cran.r-project.org/web/packages/vegan/ (accessed on 12 September 2021).
- Templ, M.; Hron, K.; Filzmoser, P. robCompositions: An R-package for Robust Statistical Analysis of Compositional Data. In Compositiona Data Analysis: Theory and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 341–355. [Google Scholar]
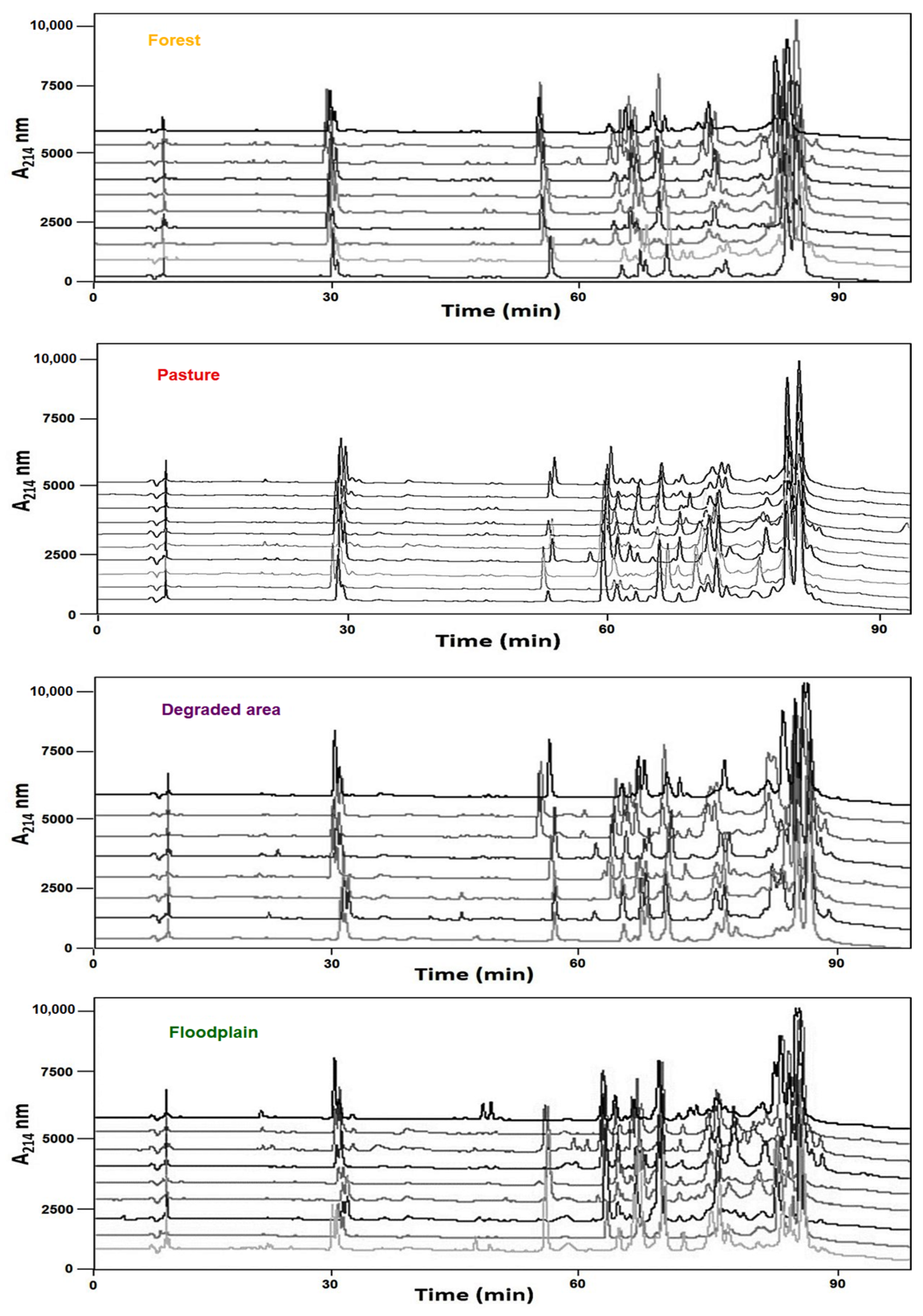
 ), pasture (
), pasture (  ), floodplain (
), floodplain (  ), and degraded (
), and degraded (  ) habitats.
) habitats.
 ), pasture (
), pasture (  ), floodplain (
), floodplain (  ), and degraded (
), and degraded (  ) habitats.
) habitats.


| Forest | Pasture | Floodplain | Degraded Area | |
|---|---|---|---|---|
| Forest | <0.0001 | <0.0001 | 0.34 | |
| Pasture | 19.4 | <0.0001 | <0.0001 | |
| Floodplain | 16.0 | 23.2 | 0.01 | |
| Degraded area | 6.4 | 17.4 | 12.2 |
| Area | Snake | Peaks (%Area) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | ||
| Forest | F 24 | 6.15 | 1.72 | 7.11 | 0.00 | 0.00 | 0.00 | 0.20 | 4.81 | 1.68 | 5.38 | 0.36 | 0.00 | 0.78 | 1.44 | 6.30 | 0.00 | 0.92 | 0.00 | 0.24 | 0.00 | 23.64 | 0.00 | 32.79 | 0.00 | 0.00 | 0.00 |
| F 25 | 4.54 | 2.15 | 7.84 | 0.00 | 0.00 | 0.00 | 0.59 | 5.04 | 3.83 | 9.77 | 0.64 | 0.00 | 0.00 | 4.40 | 3.47 | 0.00 | 0.59 | 0.00 | 1.97 | 10.29 | 12.28 | 0.00 | 29.10 | 0.00 | 0.00 | 0.70 | |
| F 26 | 4.68 | 2.19 | 8.12 | 1.02 | 0.00 | 0.00 | 9.06 | 1.24 | 6.49 | 3.98 | 1.20 | 0.00 | 0.00 | 4.53 | 7.61 | 0.31 | 0.75 | 0.00 | 0.30 | 9.38 | 19.40 | 0.00 | 5.57 | 7.04 | 0.45 | 1.17 | |
| F 28 | 5.20 | 2.89 | 6.02 | 0.00 | 0.00 | 0.00 | 0.60 | 3.15 | 2.93 | 8.83 | 0.30 | 0.00 | 1.79 | 6.51 | 0.00 | 0.00 | 0.39 | 0.28 | 0.69 | 16.06 | 13.16 | 0.00 | 27.72 | 0.00 | 0.22 | 0.00 | |
| F 29 | 5.65 | 2.11 | 12.00 | 0.00 | 0.00 | 0.00 | 0.53 | 5.62 | 9.84 | 8.93 | 1.04 | 0.00 | 0.00 | 2.38 | 6.73 | 0.68 | 0.43 | 0.22 | 2.25 | 0.13 | 12.96 | 10.32 | 8.23 | 3.88 | 0.92 | 0.00 | |
| F 30 | 5.96 | 2.70 | 9.88 | 0.00 | 0.00 | 0.00 | 0.28 | 4.70 | 3.55 | 6.26 | 0.33 | 0.00 | 1.76 | 1.19 | 0.79 | 0.00 | 0.67 | 0.16 | 2.05 | 14.50 | 12.61 | 0.00 | 28.62 | 0.00 | 0.00 | 0.00 | |
| F 31 | 6.63 | 1.81 | 6.69 | 0.00 | 0.00 | 0.00 | 0.54 | 3.78 | 0.94 | 9.28 | 0.63 | 0.00 | 1.29 | 4.85 | 0.00 | 0.00 | 0.72 | 1.56 | 0.72 | 0.00 | 23.17 | 0.00 | 35.78 | 0.00 | 0.00 | 0.00 | |
| F 33 | 6.14 | 1.65 | 9.98 | 0.00 | 0.97 | 0.91 | 5.08 | 0.24 | 7.66 | 0.00 | 0.80 | 0.00 | 0.00 | 0.00 | 3.48 | 1.18 | 0.00 | 0.71 | 0.00 | 1.75 | 1.12 | 14.45 | 36.50 | 0.00 | 0.00 | 0.79 | |
| F 34 | 3.65 | 2.18 | 4.78 | 0.00 | 0.00 | 0.00 | 0.68 | 5.03 | 6.06 | 12.76 | 1.45 | 2.07 | 2.91 | 0.26 | 0.00 | 0.00 | 0.00 | 1.19 | 0.00 | 2.03 | 17.28 | 0.00 | 28.86 | 0.00 | 1.80 | 0.41 | |
| F 35 | 10.21 | 1.96 | 12.83 | 0.00 | 0.00 | 0.00 | 0.40 | 6.78 | 3.14 | 11.52 | 0.43 | 2.42 | 4.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.36 | 0.33 | 0.00 | 23.20 | 0.00 | 7.16 | 9.56 | 0.00 | 0.00 | |
| Degraded | D 3 | 7.64 | 2.21 | 8.76 | 0.00 | 0.00 | 0.00 | 0.32 | 5.15 | 4.74 | 5.70 | 0.31 | 0.00 | 0.00 | 5.41 | 0.53 | 0.00 | 0.53 | 0.26 | 2.72 | 21.16 | 13.46 | 0.00 | 7.37 | 7.63 | 0.25 | 0.00 |
| D 4 | 3.41 | 3.05 | 7.00 | 0.00 | 5.29 | 0.23 | 6.75 | 0.00 | 0.00 | 11.66 | 0.00 | 2.11 | 3.09 | 0.00 | 0.00 | 0.00 | 0.86 | 3.66 | 4.26 | 0.00 | 19.24 | 0.00 | 23.98 | 0.00 | 0.00 | 0.32 | |
| D 6 | 4.75 | 1.74 | 8.46 | 0.00 | 4.23 | 4.57 | 4.36 | 0.00 | 0.00 | 5.44 | 0.95 | 4.77 | 3.13 | 0.16 | 0.00 | 0.00 | 1.00 | 0.20 | 11.15 | 0.12 | 17.05 | 0.00 | 15.42 | 4.01 | 2.20 | 1.89 | |
| D 7 | 3.17 | 1.34 | 6.38 | 1.54 | 5.45 | 3.28 | 0.15 | 1.35 | 2.74 | 5.95 | 0.83 | 0.00 | 3.80 | 2.92 | 0.00 | 0.00 | 0.00 | 0.86 | 7.06 | 9.32 | 13.16 | 0.00 | 27.29 | 0.00 | 1.18 | 0.60 | |
| D 8 | 6.45 | 1.61 | 5.38 | 0.00 | 0.63 | 1.97 | 0.10 | 2.05 | 1.70 | 8.46 | 0.05 | 0.00 | 1.97 | 5.75 | 0.63 | 0.00 | 0.60 | 0.00 | 0.33 | 11.89 | 14.75 | 0.00 | 30.02 | 0.00 | 0.00 | 0.00 | |
| D 9 | 4.63 | 1.55 | 4.02 | 0.00 | 6.16 | 3.26 | 0.00 | 4.83 | 2.43 | 5.80 | 1.25 | 0.00 | 0.00 | 4.20 | 4.39 | 0.00 | 0.00 | 1.91 | 3.06 | 2.39 | 15.32 | 0.00 | 32.33 | 0.00 | 0.00 | 0.35 | |
| D 10 | 4.37 | 1.39 | 5.08 | 0.92 | 0.00 | 0.00 | 0.18 | 3.51 | 4.67 | 6.30 | 0.34 | 4.76 | 0.00 | 0.00 | 3.81 | 0.19 | 0.31 | 0.41 | 5.16 | 5.34 | 18.50 | 0.00 | 26.55 | 0.00 | 0.00 | 0.89 | |
| D 11 | 4.32 | 1.19 | 4.37 | 0.00 | 0.00 | 0.00 | 0.60 | 3.84 | 4.18 | 7.49 | 0.52 | 0.00 | 3.03 | 4.87 | 1.89 | 0.00 | 0.31 | 0.11 | 0.66 | 0.66 | 22.48 | 0.00 | 33.95 | 0.00 | 0.00 | 0.49 | |
| Floodplain | V 5 | 9.11 | 1.50 | 0.00 | 0.00 | 5.18 | 6.62 | 0.48 | 4.93 | 0.32 | 17.68 | 1.26 | 1.69 | 0.76 | 1.10 | 0.38 | 0.83 | 0.52 | 0.60 | 1.64 | 4.91 | 12.09 | 4.04 | 7.13 | 7.37 | 0.00 | 0.00 |
| V 7 | 4.76 | 4.03 | 0.00 | 0.00 | 9.91 | 1.50 | 0.53 | 3.01 | 3.32 | 13.12 | 1.30 | 0.00 | 1.01 | 4.47 | 0.00 | 0.00 | 2.26 | 0.96 | 2.34 | 0.00 | 26.46 | 4.98 | 6.25 | 6.75 | 0.08 | 0.00 | |
| V 8 | 2.22 | 3.91 | 4.29 | 1.07 | 4.86 | 5.23 | 0.22 | 4.24 | 6.47 | 8.46 | 1.06 | 0.00 | 5.44 | 6.96 | 0.00 | 1.48 | 1.37 | 2.60 | 0.21 | 2.28 | 13.92 | 0.00 | 15.77 | 0.00 | 1.56 | 1.09 | |
| V 9 | 1.50 | 2.82 | 0.00 | 0.00 | 17.31 | 3.19 | 0.18 | 1.10 | 0.44 | 9.17 | 0.40 | 0.00 | 2.79 | 3.01 | 0.00 | 15.41 | 0.00 | 0.00 | 5.59 | 3.74 | 0.00 | 2.97 | 23.94 | 0.00 | 1.01 | 1.72 | |
| V 10 | 3.12 | 2.60 | 9.77 | 0.17 | 0.00 | 0.00 | 0.25 | 4.08 | 12.31 | 13.50 | 1.81 | 0.00 | 0.00 | 6.10 | 7.34 | 2.72 | 0.49 | 0.83 | 0.20 | 4.34 | 1.62 | 0.52 | 20.18 | 0.00 | 0.17 | 0.71 | |
| V 11 | 1.71 | 1.65 | 7.82 | 0.00 | 10.61 | 2.80 | 0.59 | 1.39 | 7.26 | 18.15 | 1.12 | 0.19 | 0.71 | 1.46 | 5.46 | 0.32 | 0.57 | 0.00 | 1.46 | 11.89 | 0.75 | 0.00 | 21.92 | 0.00 | 0.00 | 0.10 | |
| V 12 | 1.23 | 2.03 | 17.20 | 0.00 | 0.70 | 0.12 | 0.33 | 10.21 | 1.32 | 11.49 | 0.00 | 0.28 | 2.23 | 3.46 | 0.00 | 1.38 | 0.06 | 0.00 | 7.69 | 10.56 | 8.53 | 0.00 | 17.43 | 0.00 | 0.00 | 0.00 | |
| V 13 | 3.02 | 3.99 | 0.00 | 0.00 | 14.00 | 0.00 | 0.88 | 0.73 | 5.78 | 20.93 | 0.00 | 0.00 | 0.00 | 16.52 | 2.29 | 2.20 | 1.00 | 0.00 | 2.42 | 8.56 | 0.94 | 0.00 | 11.99 | 0.00 | 0.00 | 0.43 | |
| V 16 | 4.05 | 1.63 | 0.00 | 0.00 | 6.12 | 0.00 | 0.00 | 1.63 | 1.86 | 12.40 | 0.81 | 0.00 | 0.00 | 0.00 | 4.81 | 0.00 | 0.92 | 0.56 | 0.80 | 25.43 | 0.00 | 4.01 | 33.16 | 0.00 | 0.00 | 0.00 | |
| Pasture | O 1 | 5.50 | 4.14 | 4.57 | 0.20 | 6.92 | 0.88 | 0.00 | 0.88 | 0.59 | 4.49 | 1.54 | 1.50 | 2.21 | 2.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.82 | 0.00 | 25.56 | 0.00 | 35.50 | 0.00 | 0.00 | 0.00 |
| O 2 | 5.19 | 4.28 | 4.19 | 0.00 | 6.25 | 0.92 | 0.00 | 0.68 | 0.48 | 4.11 | 1.13 | 1.30 | 2.39 | 2.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.52 | 0.00 | 26.23 | 0.00 | 37.75 | 0.00 | 0.00 | 0.00 | |
| O 3 | 8.11 | 1.81 | 0.00 | 0.00 | 2.04 | 2.48 | 0.20 | 0.00 | 4.87 | 7.69 | 0.49 | 2.13 | 2.68 | 3.42 | 0.00 | 0.00 | 0.00 | 0.00 | 1.77 | 0.00 | 26.34 | 0.00 | 35.31 | 0.00 | 0.00 | 0.00 | |
| O 5 | 7.88 | 1.76 | 0.00 | 0.00 | 2.06 | 2.92 | 0.00 | 0.00 | 3.54 | 6.20 | 0.58 | 0.00 | 3.08 | 3.52 | 0.00 | 0.00 | 0.50 | 0.00 | 3.31 | 0.00 | 26.73 | 0.00 | 36.31 | 0.00 | 0.00 | 0.00 | |
| O 6 | 5.89 | 2.46 | 1.99 | 0.00 | 9.85 | 2.06 | 0.00 | 0.53 | 0.00 | 0.45 | 3.86 | 2.22 | 2.09 | 5.84 | 0.00 | 0.00 | 0.81 | 2.31 | 1.42 | 0.24 | 23.13 | 0.00 | 31.52 | 0.00 | 0.42 | 0.69 | |
| O 7 | 5.47 | 1.98 | 4.40 | 0.00 | 5.84 | 1.85 | 0.00 | 0.23 | 0.00 | 5.12 | 1.05 | 2.86 | 1.38 | 4.13 | 0.00 | 0.00 | 0.79 | 1.80 | 0.56 | 0.00 | 26.66 | 0.00 | 33.27 | 0.00 | 0.00 | 0.15 | |
| O 9 | 4.89 | 2.44 | 2.84 | 1.14 | 17.82 | 3.51 | 0.00 | 2.30 | 0.91 | 0.00 | 3.46 | 0.00 | 6.70 | 6.16 | 0.00 | 1.34 | 0.27 | 0.21 | 5.08 | 0.29 | 11.00 | 2.16 | 24.22 | 0.00 | 1.12 | 0.00 | |
| O 15 | 5.32 | 1.27 | 2.11 | 0.00 | 7.27 | 0.00 | 0.00 | 0.00 | 0.00 | 4.20 | 1.18 | 4.71 | 16.76 | 2.29 | 1.29 | 0.00 | 0.40 | 0.00 | 2.19 | 0.20 | 25.50 | 0.00 | 21.71 | 0.00 | 0.18 | 0.00 | |
| O 16 | 4.79 | 2.18 | 5.99 | 0.17 | 1.17 | 0.96 | 0.08 | 2.53 | 0.00 | 8.06 | 0.00 | 7.32 | 1.98 | 5.93 | 0.00 | 0.00 | 0.41 | 0.00 | 5.71 | 0.00 | 20.37 | 0.00 | 30.20 | 0.00 | 0.14 | 0.39 | |
| O 19 | 4.84 | 0.57 | 1.23 | 0.00 | 12.43 | 0.00 | 0.40 | 1.15 | 0.00 | 9.93 | 0.00 | 1.53 | 6.13 | 1.60 | 0.29 | 0.00 | 0.00 | 0.00 | 2.30 | 1.25 | 19.40 | 0.00 | 30.30 | 0.00 | 0.32 | 0.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.F.; Holding, M.L.; Del-Rei, T.H.M.; Rocha, M.M.T.; Mourão, R.H.V.; Chalkidis, H.M.; Prezoto, B.; Gibbs, H.L.; Moura-da-Silva, A.M. Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality. Toxins 2021, 13, 814. https://doi.org/10.3390/toxins13110814
Sousa LF, Holding ML, Del-Rei THM, Rocha MMT, Mourão RHV, Chalkidis HM, Prezoto B, Gibbs HL, Moura-da-Silva AM. Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality. Toxins. 2021; 13(11):814. https://doi.org/10.3390/toxins13110814
Chicago/Turabian StyleSousa, Leijane F., Matthew L. Holding, Tiago H. M. Del-Rei, Marisa M. T. Rocha, Rosa H. V. Mourão, Hipócrates M. Chalkidis, Benedito Prezoto, H. Lisle Gibbs, and Ana M. Moura-da-Silva. 2021. "Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality" Toxins 13, no. 11: 814. https://doi.org/10.3390/toxins13110814
APA StyleSousa, L. F., Holding, M. L., Del-Rei, T. H. M., Rocha, M. M. T., Mourão, R. H. V., Chalkidis, H. M., Prezoto, B., Gibbs, H. L., & Moura-da-Silva, A. M. (2021). Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality. Toxins, 13(11), 814. https://doi.org/10.3390/toxins13110814






