Morphology of the Cutaneous Poison and Mucous Glands in Amphibians with Particular Emphasis on Caecilians (Siphonops annulatus)
Abstract
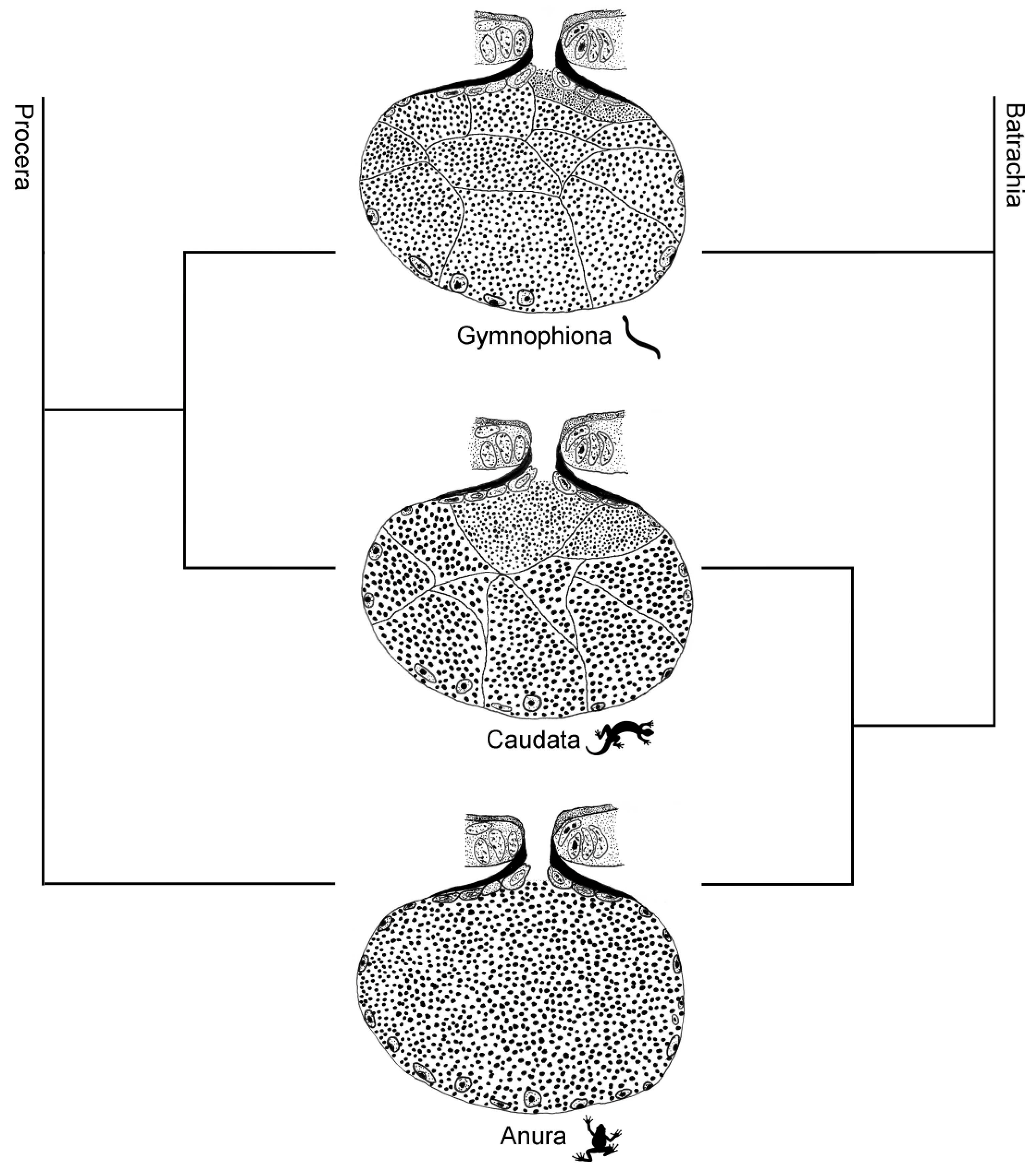
:1. Introduction
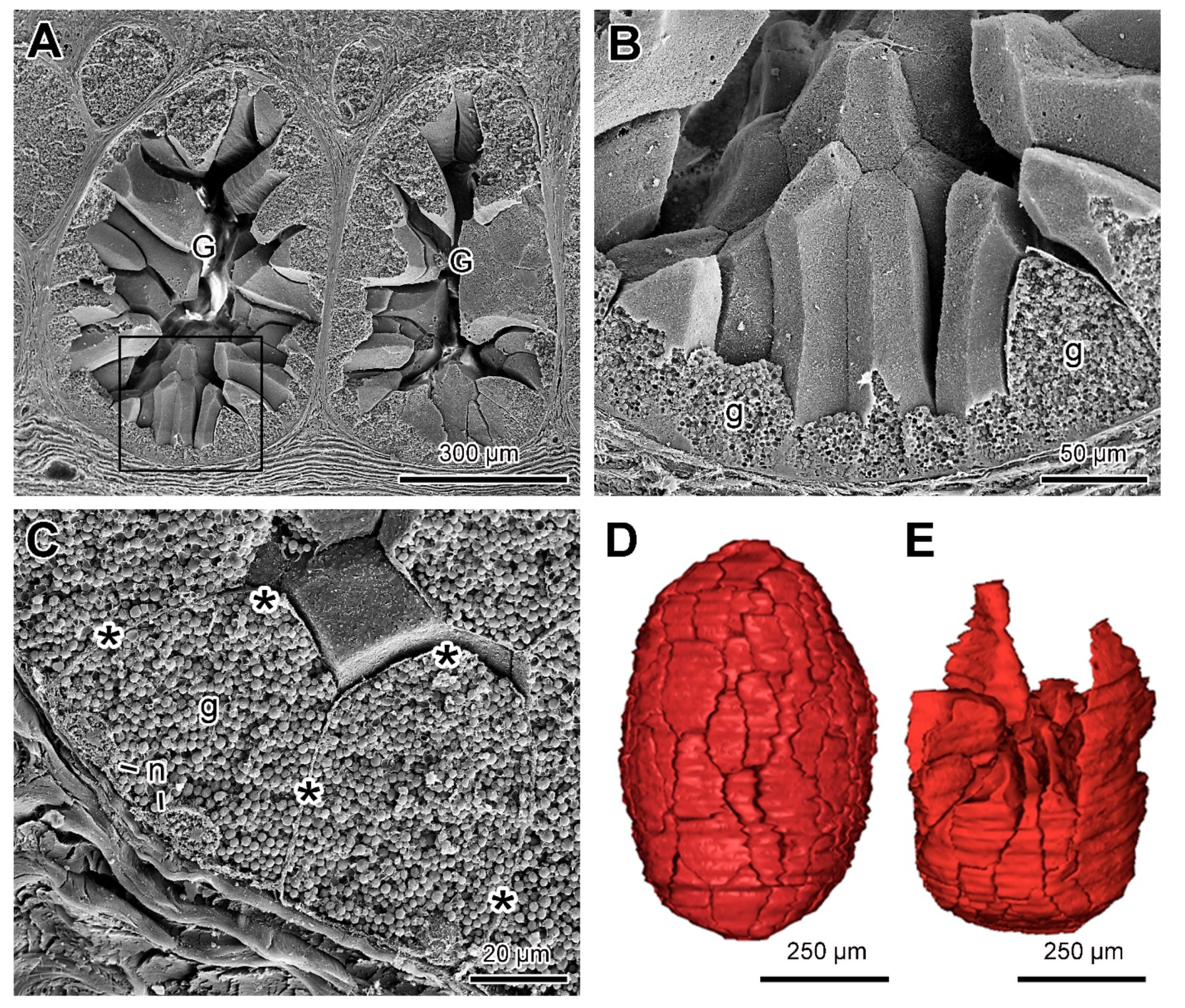
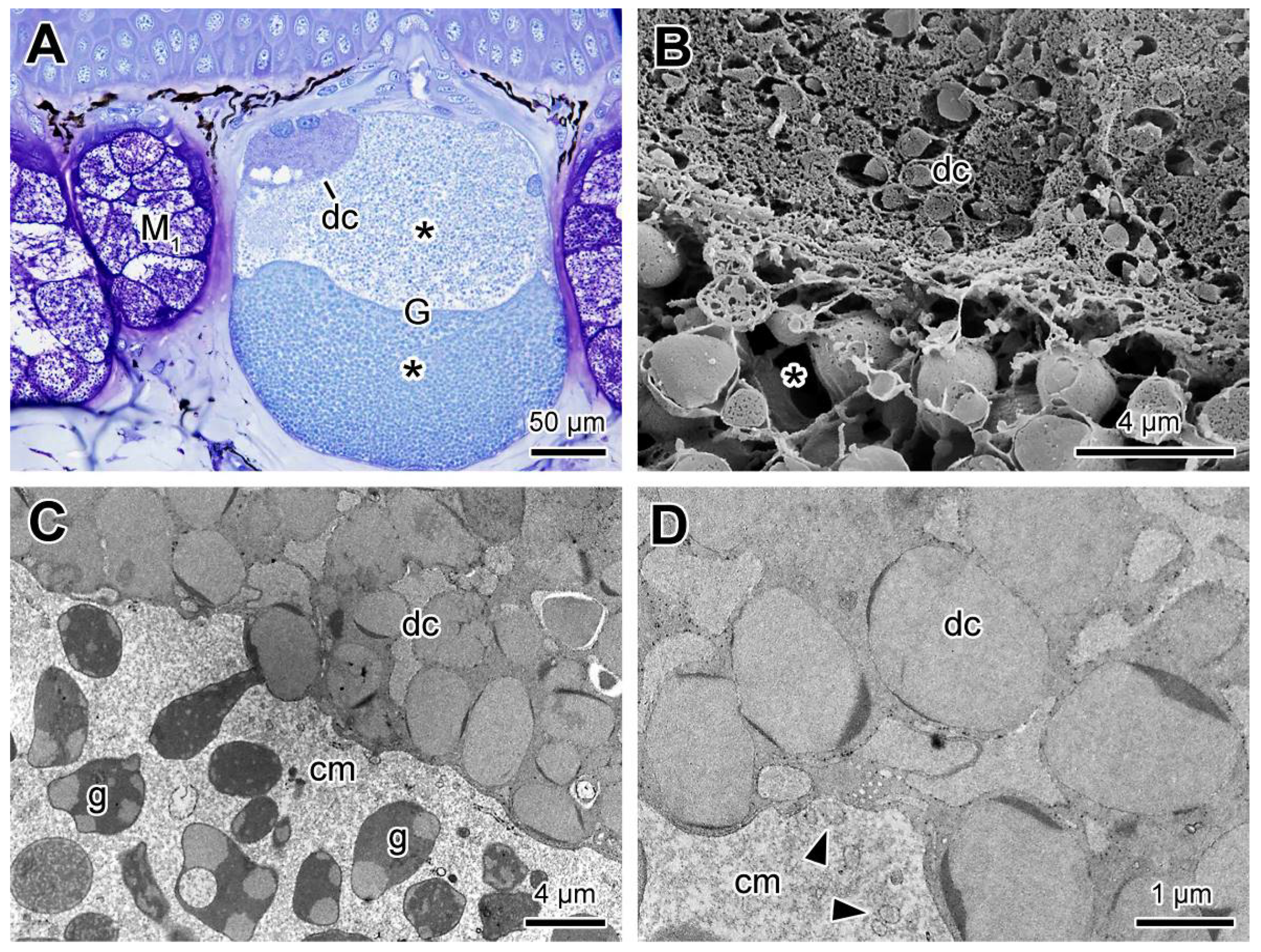
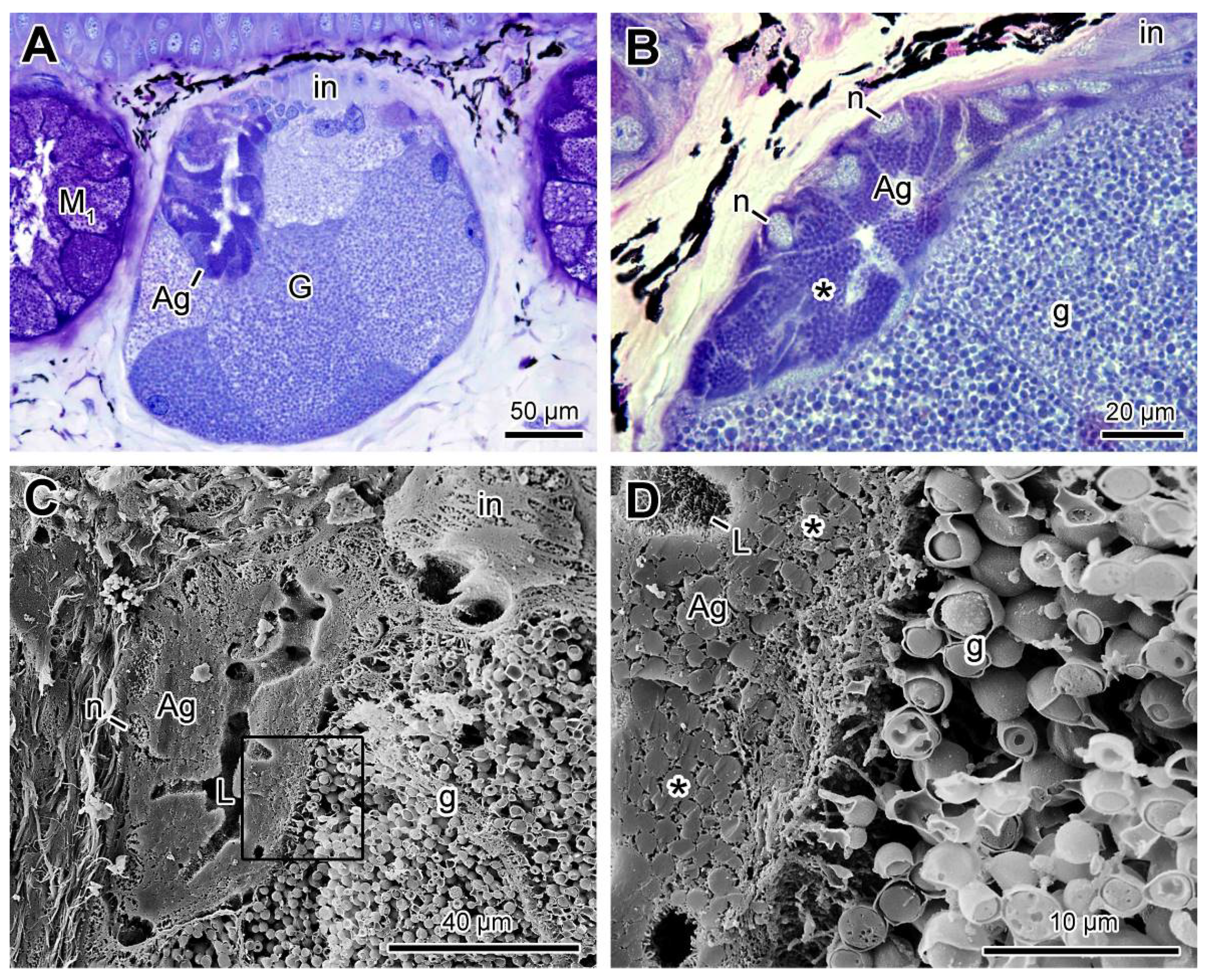
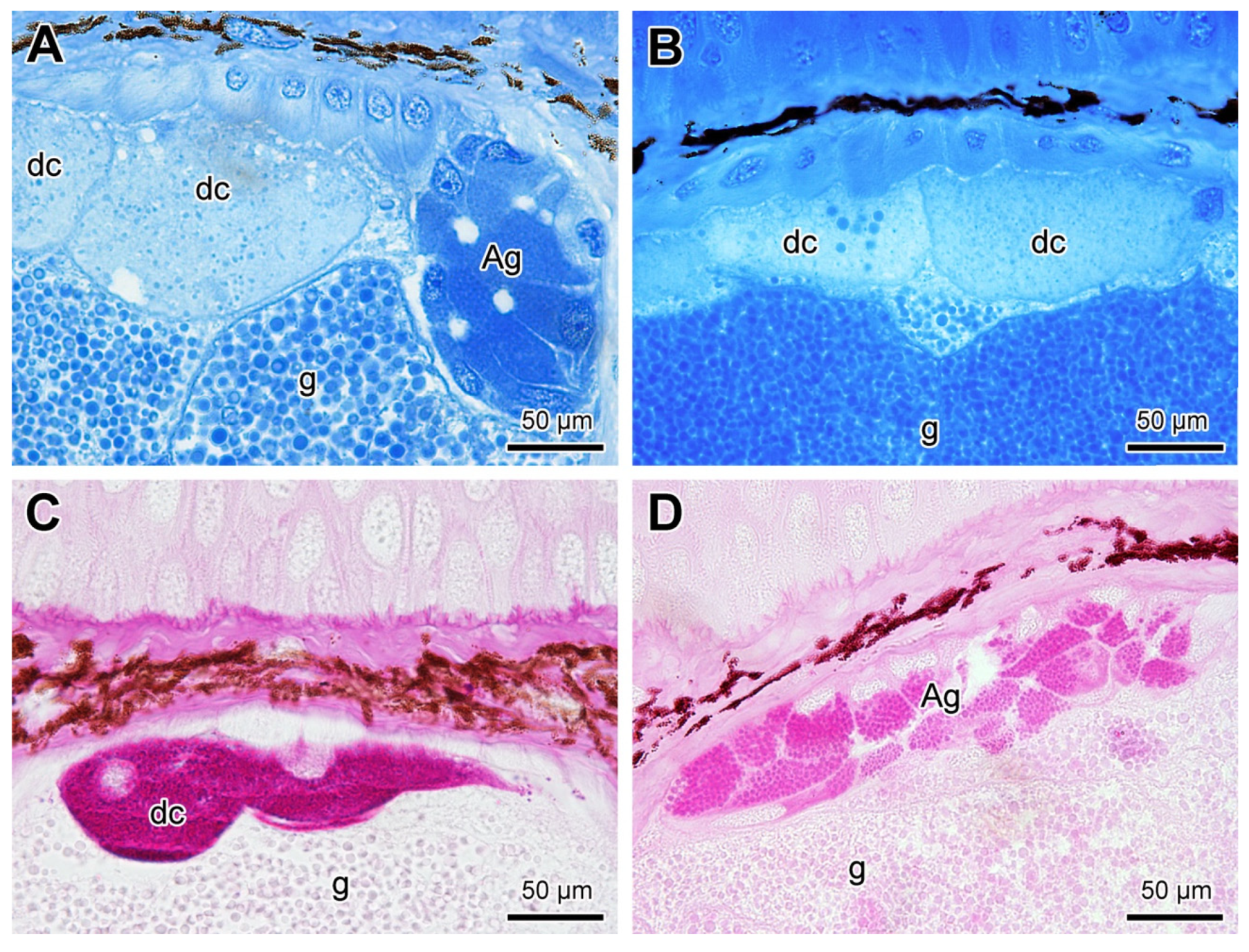
2. Results
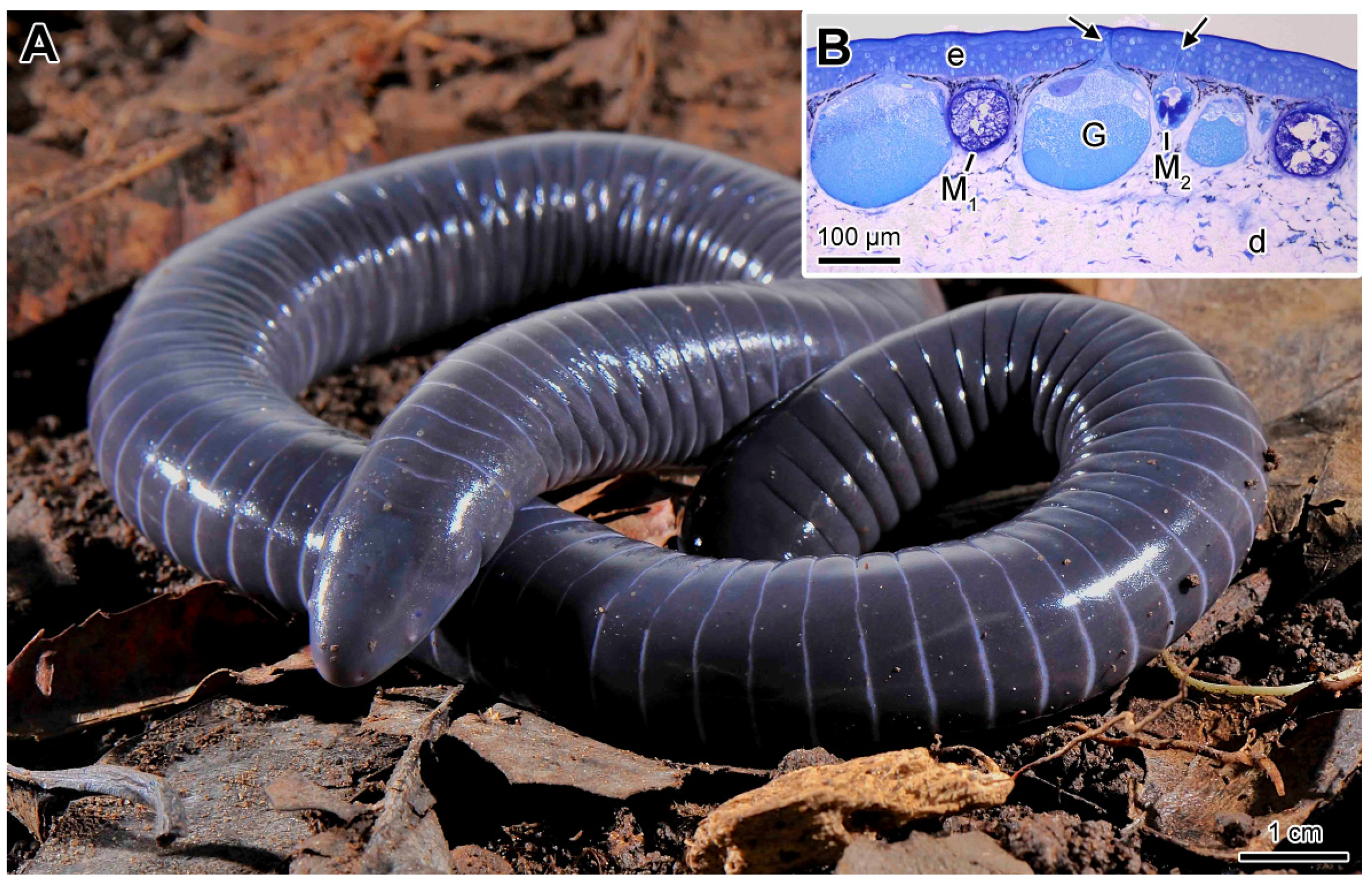
2.1. General Characteristics of the Skin
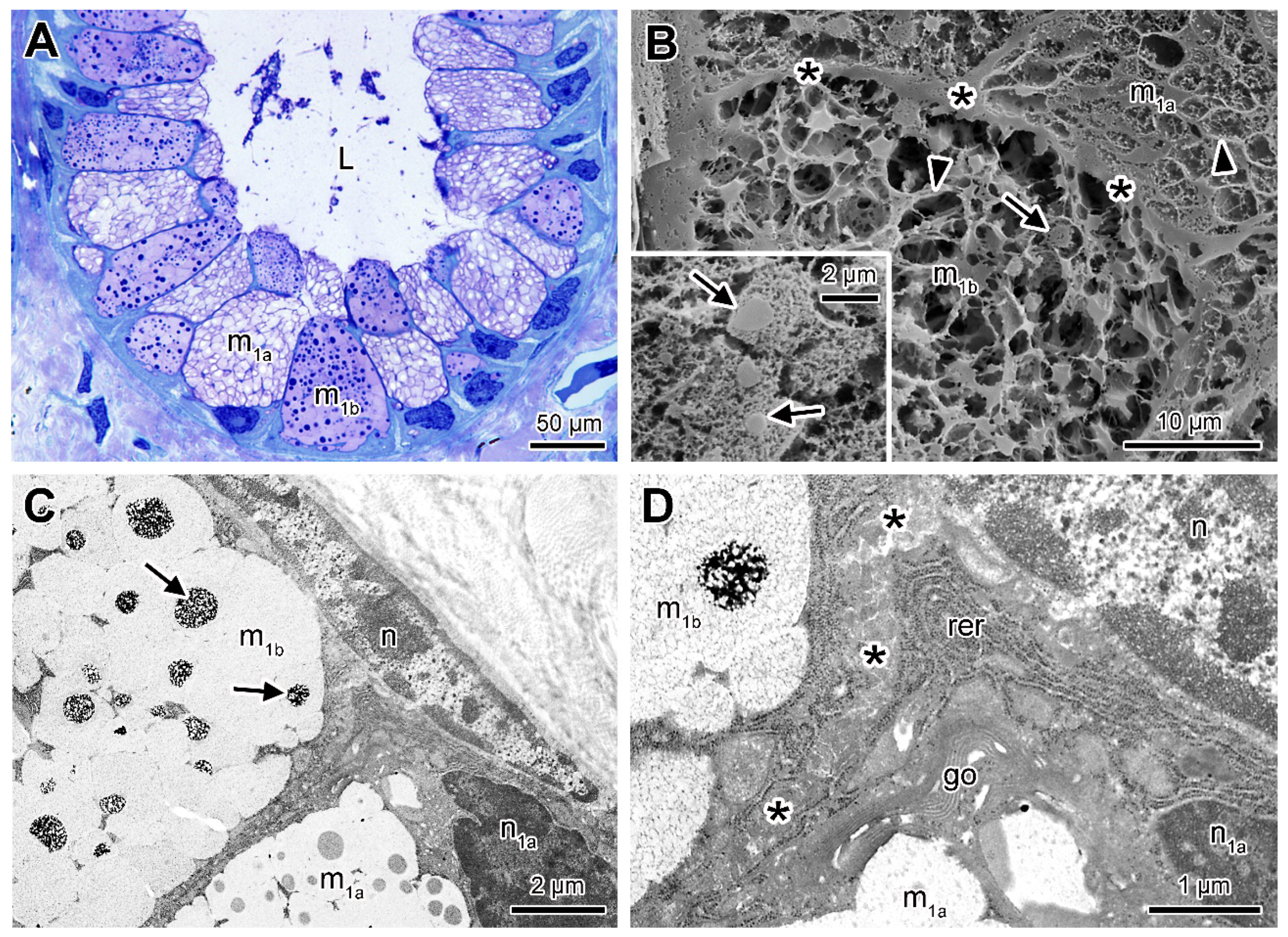
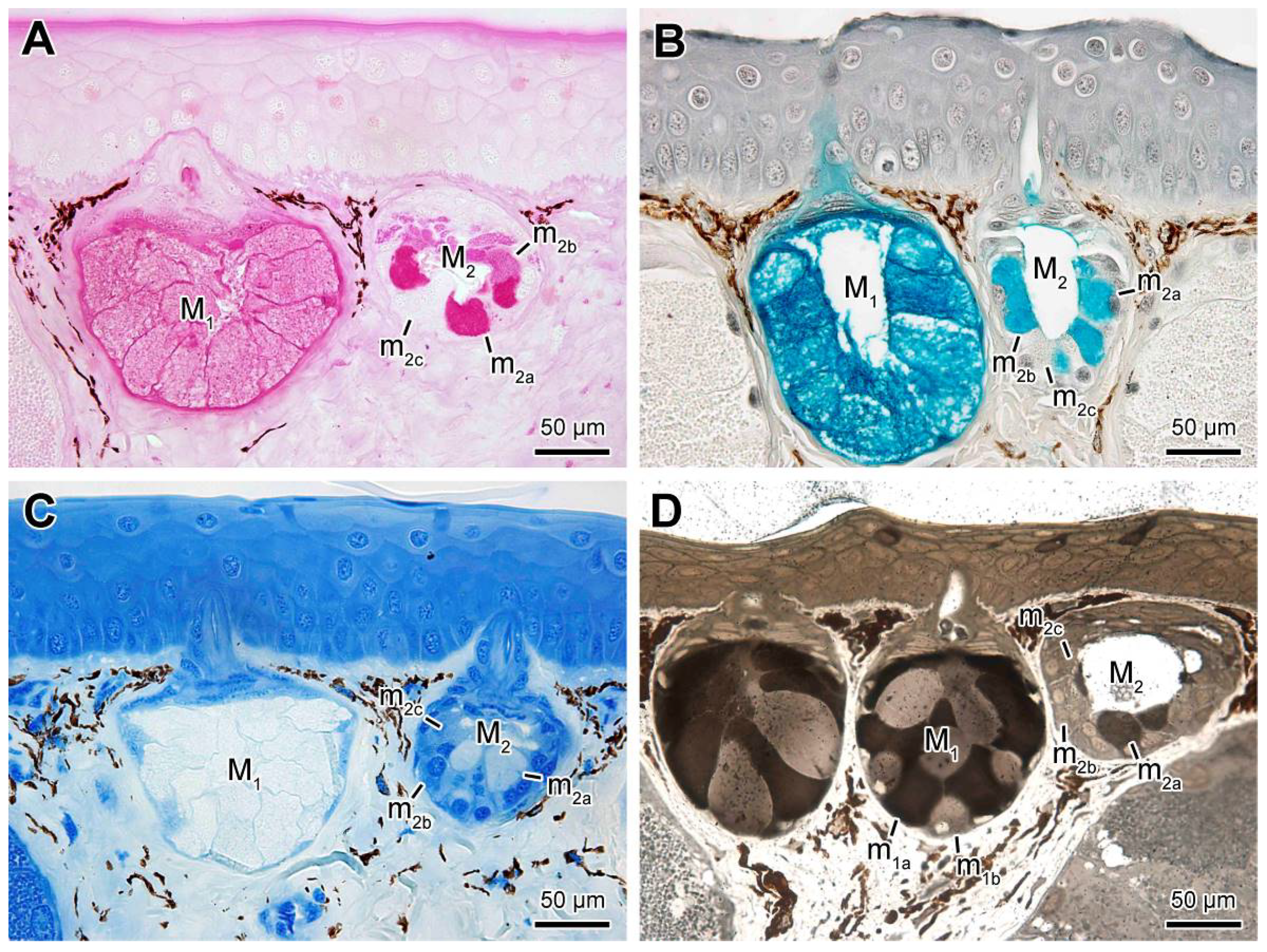
2.2. Mucous Glands
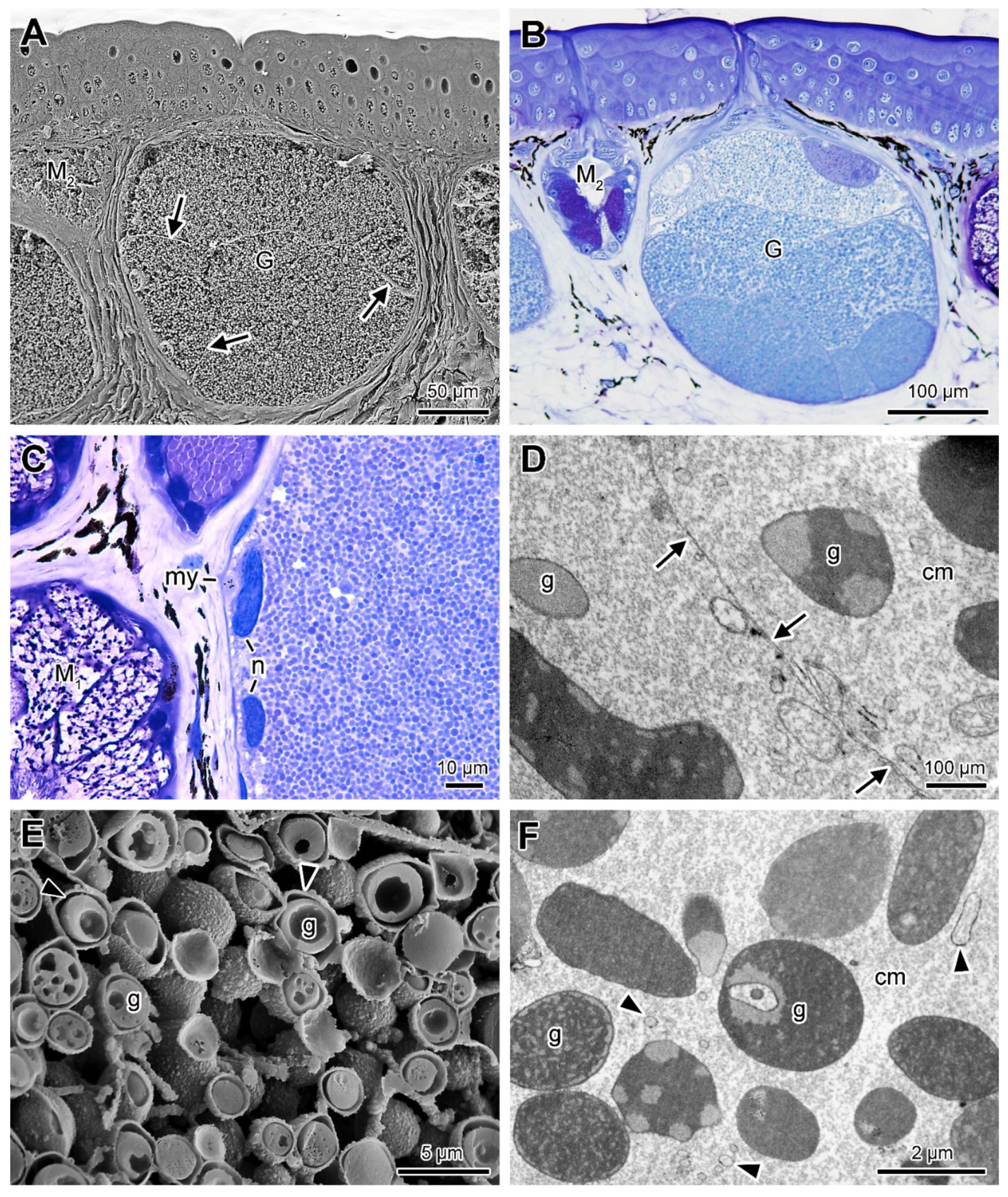
2.3. Poison Glands
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Histology
4.3. Scanning Electron Microscopy (SEM)
4.4. Transmission Electron Microscopy (TEM)
4.5. Three-Dimensional Reconstruction of the Poison Gland
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toledo, R.C.; Jared, C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol.—Part A Physiol. 1995, 111, 1–29. [Google Scholar] [CrossRef]
- Fox, H. Dermal glands. In Biology of Integument; Bereiter Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin, Germany, 1986; pp. 78–135. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Stebbins, R.C.; Cohen, N.W. (Eds.) A Natural History of Amphibians, 1st ed.; Princeton University Press: Princeton, NJ, USA, 1997; ISBN 9780691102511. [Google Scholar]
- Clarke, B.T. The natural history of amphibian skin secretions: Their normal functioning and potencial medical applications. Biol. Rev. Camb. Philos. Soc. 1997, 72, 365–379. [Google Scholar] [CrossRef]
- Raaymakers, C.; Verbrugghe, E.; Hernot, S.; Hellebuyck, T.; Betti, C.; Peleman, C.; Claeys, M.; Bert, W.; Caveliers, V.; Ballet, S.; et al. Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, R.A.; Wilkinson, M. On the classification and phylogeny of caecilians (Amphibia: Gymnophiona), a critical review. Herpetol. Monogr. 1989, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Jared, C.; Navas, C.A.; Toledo, R.C. An appreciation of the physiology and morphology of the Caecilians (Amphibia: Gymnophiona). Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 1999, 123, 313–328. [Google Scholar] [CrossRef]
- Kupfer, A.; Müller, H.; Antoniazzi, M.M.; Jared, C.; Greven, H.; Nussbaum, R.A.; Wilkinson, M. Parental investment by skin feeding in a caecilian amphibian. Nature 2006, 440, 926–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, M.; Kupfer, A.; Marques-Porto, R.; Jeffkins, H.; Antoniazzi, M.M.; Jared, C. One hundred million years of skin feeding? Extended parental care in a Neotropical caecilian (Amphibia: Gymnophiona). Biol. Lett. 2008, 4, 358–361. [Google Scholar] [CrossRef] [Green Version]
- Jared, C.; Mailho-Fontana, P.L.; Jared, S.G.S.; Kupfer, A.; Delabie, J.H.C.; Wilkinson, M.; Antoniazzi, M.M. Life history and reproduction of the neotropical caecilian Siphonops annulatus (Amphibia, Gymnophiona, Siphonopidae), with special emphasis on parental care. Acta Zool. 2019, 100, 292–302. [Google Scholar] [CrossRef]
- Kupfer, A.; Maxwell, E.; Reinhard, S.; Kuehnel, S. The evolution of parental investment in caecilian amphibians: A comparative approach. Biol. J. Linn. Soc. 2016, 119, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Frost, D.R. Mmphibian Species of the World: An Online Reference. Version 6.0 Database; American Museum of Natural History: New York, NY, USA, 2021; Available online: http://research.amnh.org/herpetology/amphibia/index.html (accessed on 25 August 2021).
- Junqueira, L.C.U.; Jared, C.; Antoniazzi, M.M. Structure of the caecilian Siphonops annulatus (Amphibia, Gymnophiona): General aspects of the body, disposition of the organs and the structure of the mouth, oesophagus and stomach. Acta Zool. 1999, 80, 75–84. [Google Scholar] [CrossRef]
- Gower, D.J.; Wilkinson, M. Conservation biology of caecilian amphibians. Conserv. Biol. 2005, 19, 45–55. [Google Scholar] [CrossRef]
- Taylor, E.H. (Ed.) The Caecilians of the World: A Taxonomic Review; University of Kansas Press: Lawrence, KS, USA, 1968. [Google Scholar]
- Jared, C.; Mailho-Fontana, P.L.; Marques-Porto, R.; Sciani, J.M.; Pimenta, D.C.; Brodie, E.D.; Antoniazzi, M.M. Skin gland concentrations adapted to different evolutionary pressures in the head and posterior regions of the caecilian Siphonops annulatus. Sci. Rep. 2018, 8, 3576. [Google Scholar] [CrossRef] [Green Version]
- Jared, C.; Antoniazzi, M.M.; Jordão, A.E.C.; Silva, J.R.M.C.; Greven, H.; Rodrigues, M.T. Parotoid macroglands in toad (Rhinella jimi): Their structure and functioning in passive defence. Toxicon 2009, 53, 197–207. [Google Scholar] [CrossRef]
- Regis-Alves, E.; Jared, S.G.S.; Maurício, B.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Fleury-Curado, M.C.; Brodie, E.D.; Jared, C. Structural cutaneous adaptations for defense in toad (Rhinella icterica) parotoid macroglands. Toxicon 2017, 137, 128–134. [Google Scholar] [CrossRef]
- Brodie, E.D., Jr. Salamander antipredator postures. Copeia 1977, 1977, 523–535. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Lourenço-de-Moraes, R.; Zocca, C.; Duca, C.; Beard, K.H.; Brodie, E.D. Antipredator mechanisms of post-metamorphic anurans: A global database and classification system. Behav. Ecol. Sociobiol. 2019, 73, 1–21. [Google Scholar] [CrossRef]
- Sawaya, A. Sobre as glandulas cutaneas de Siphonops annulatus (Mikan). Boletins da Fac. Philos. Sci. e Let. Univ. São Paulo. Zool. 1938, 2, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Delfino, G.; Nosi, D.; Giachi, F. Secretory granule-cytoplasm relationships in serous glands of anurans: Ultrastructural evidence and possible functional role. Toxicon 2001, 39, 1161–1171. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Barbaro, K.C.; Jared, C. Morphological and biochemical characterization of the cutaneous poison glands in toads (Rhinella marina group) from different environments. Front. Zool. 2018, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Heiss, E.; Natchev, N.; Rabanser, A.; Weisgram, J.; Hilgers, H. Three types of cutaneous glands in the skin of the salamandrid Pleurodeles waltl. A histological and ultrastructural study. J. Morphol. 2009, 270, 892–902. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Jared, C.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Stokes, A.N.; Grant, T.; Brodie, E.D.; Brodie, E.D. Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands. Sci. Rep. 2019, 9, 18490. [Google Scholar] [CrossRef]
- von Byern, J.; Dicke, U.; Heiss, E.; Grunwald, I.; Gorb, S.; Staedler, Y.; Cyran, N. Morphological characterization of the glandular system in the salamander Plethodon shermani (Caudata, Plethodontidae). Zoology 2015, 118, 334–347. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Rodrigues, I.; Sciani, J.M.; Pimenta, D.C.; Brodie, E.D.; Rodrigues, M.T.; Jared, C. Parotoid, radial, and tibial macroglands of the frog Odontophrynus cultripes: Differences and similarities with toads. Toxicon 2017, 129, 123–133. [Google Scholar] [CrossRef]
- Melzer, S.; Clerens, S.; Bishop, P.J. Differential polymorphism in cutaneous glands of archaic Leiopelma species. J. Morphol. 2011, 272, 1116–1130. [Google Scholar] [CrossRef]
- Sawaya, P. Sobre o veneno das glandulas cutaneas, a secreção e o coração de Siphonops annulatus. Boletins da Fac. Philos. Sci. e Let. Univ. São Paulo. Zool. 1940, 4, 207. [Google Scholar] [CrossRef]
- Schwartz, E.N.F.; Schwartz, C.A.; Sebben, A.; Largura, S.W.R.; Mendes, E.G. Indirect cardiotoxic activity of the caecilian Siphonops paulensis (Gymnophiona, Amphibia) skin secretion. Toxicon 1999, 37, 47–54. [Google Scholar] [CrossRef]
- Schwartz, E.N.F.; Schwartz, C.A.; Sebben, A. Occurrence of hemolytic activity in the skin secretion of the caecilian Siphonops paulensis. Nat. Toxins 1998, 6, 179–182. [Google Scholar] [CrossRef]
- Pinto, E.; Antoniazzi, M.; Jared, C.; Tempone, A. Antileishmanial and antitrypanosomal activity of the cutaneous secretion of Siphonops annulatus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 50. [Google Scholar] [CrossRef] [Green Version]
- McManus, M.I. A cytological study of the skin glands of the dusky salamander. J. Elisha Mitchell Sci. Soc. 1937, 53, 101–113. [Google Scholar]
- Damodaran, A.; Reston Saroja, B.; Kotharambath, R.; Mohammad Abdulkader, A.; Oommen, O.V.; Lekha, D. Light and electron microscopic observations on the organization of skin and associated glands of two caecilian amphibians from Western Ghats of India. Micron 2018, 106, 59–68. [Google Scholar] [CrossRef]
- Arun, D.; Akbarsha, M.A.; Oommen, O.V.; Divya, L. Light and transmission electron microscopic structure of skin glands and dermal scales of a caecilian amphibian Gegeneophis ramaswamii, with a note on antimicrobial property of skin gland secretion. Microsc. Res. Tech. 2019, 82, 1267–1276. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Wilkinson, M.; Delabie, J.H.C. Conservation of the caecilian Siphonops annulatus (Amphibia, Gymnophiona) in brazilian cacao plantations: A successful relationship between a fossorial animal and an agrosystem. Agrotrópica 2015, 27, 233–238. [Google Scholar] [CrossRef]
- Delfino, G.; Brizzi, R.; Alvarez, B.B.; Taddei, L. Secretory polymorphism and serous cutaneous gland heterogeneity in Bufo granulosus (Amphibia, Anura). Toxicon 1999, 37, 1281–1296. [Google Scholar] [CrossRef]
- Sever, D.M.; Siegel, D.S. Histology and ultrastructure of the caudal courtship glands of the red-backed salamander, Plethodon cinereus (Amphibia: Plethodontidae). J. Morphol. 2015, 276, 319–330. [Google Scholar] [CrossRef]
- Bingol-Ozakpinar, O.; Murathanoglu, O. The morphology of the dorsal and ventral skin of Triturus karelinii (Caudata: Salamandridae). Biologia 2011, 66, 349–356. [Google Scholar] [CrossRef]
- Fontana, M.F.; Ask, K.A.; Macdonald, R.J.; Carnes, A.M.; Staub, N.L. Loss of traditional mucous glands and presence of a novel mucus-producing granular gland in the plethodontid salamander Ensatina eschscholtzii. Biol. J. Linn. Soc. 2006, 87, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- Hillman, S.S.; Withers, P.C.; Drewes, R.C.; Hillyard, S.D. Ecological and Environmental Physiology of Amphibians; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Alexandre, C.; Pimenta, D.C.; Sciani, J.M.; Brodie, E.D.; Jared, C. Morphological evidence for an oral venom system in caecilian amphibians. iScience 2020, 23, 101234. [Google Scholar] [CrossRef]
- Antoniazzi, M.M.; Neves, P.R.; Mailho-Fontana, P.L.; Rodrigues, M.T.; Jared, C. Morphology of the parotoid macroglands in Phyllomedusa leaf frogs. J. Zool. 2013, 291, 42–50. [Google Scholar] [CrossRef]
- Brodie, E.D.; Smatresk, N.J. The antipredator arsenal of fire salamanders: Spraying of secretions from highly pressurized dorsal skin glands. Herpetologica 1990, 46, 1–7. [Google Scholar]
- Heiss, E.; Natchev, N.; Salaberger, D.; Gumpenberger, M.; Rabanser, A.; Weisgram, J. Hurt yourself to hurt your enemy: New insights on the function of the bizarre antipredator mechanism in the salamandrid Pleurodeles waltl. J. Zool. 2010, 280, 156–162. [Google Scholar] [CrossRef]
- Sarasin, P.B. Ergebnisse Naturwissenschaftlicher Forschungen auf Ceylon (in … 1884–86); CW Kreidel: Wiesbaden, Germany, 1908. [Google Scholar]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Barros-Battesti, D.M.; Jared, C.; Campbell, J.A.; Brodie, E.D. Toad Parotoid pores shelter tick larvae. S. Am. J. Herpetol. 2016, 11, 110–113. [Google Scholar] [CrossRef]
- Larson, A.; Wilson, A.C. Patterns of ribosomal RNA evolution in salamanders. Mol. Biol. Evol. 1989, 6, 131–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, A.E.; Hedges, S.B. Molecular evidence for the early history of living amphibians. Mol. Phylogenet. Evol. 1998, 9, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Frost, D.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; de Sá, R.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 297, 1–291. [Google Scholar] [CrossRef]
- Roelants, K.; Gower, D.J.; Wilkinson, M.; Loader, S.P.; Biju, S.D.; Guillaume, K.; Moriau, L.; Bossuyt, F. Global patterns of diversification in the history of modern amphibians. Proc. Natl. Acad. Sci. USA 2007, 104, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.J.; Brown, J.M.; Fujita, M.K.; Boussau, B. A phylogenomic approach to vertebrate phylogeny supports a turtle-archosaur affinity and a possible paraphyletic lissamphibia. PLoS ONE 2012, 7, e48990. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Liang, D.; Zhang, P. Selecting question-specific genes to reduce incongruence in phylogenomics: A case study of jawed vertebrate backbone phylogeny. Syst. Biol. 2015, 64, 1104–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jetz, W.; Pyron, R.A. The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat. Ecol. Evol. 2018, 2, 850–858. [Google Scholar] [CrossRef]
- Siu-Ting, K.; Torres-Sánchez, M.; San Mauro, D.; Wilcockson, D.; Wilkinson, M.; Pisani, D.; O’Connell, M.J.; Creevey, C.J. Inadvertent paralog inclusion drives artifactual topologies and timetree estimates in phylogenomics. Mol. Biol. Evol. 2019, 36, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Hime, P.M.; Lemmon, A.R.; Lemmon, E.C.M.; Prendini, E.; Brown, J.M.; Thomson, R.C.; Kratovil, J.D.; Noonan, B.P.; Pyron, R.A.; Peloso, P.L.V.; et al. Phylogenomics reveals ancient gene tree discordance in the amphibian tree of life. Syst. Biol. 2021, 70, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S. Focal Review: The origin(s) of modern amphibians. Evol. Biol. 2008, 35, 231–247. [Google Scholar] [CrossRef]
- Pyron, A.R.; Wiens, J.J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- Haeckel, E.H.P.A. Generelle Morphologie der Organismen. Allgemeine Grundzüge der Organischen Formen-Wissenschaft, Mechanisch Begründet Durch die von Charles Darwin Reformirte Descendenztheorie, von Ernst Haeckel; G. Reimer: Berlin, Germany, 1866. [Google Scholar]
- Zardoya, R.; Meyer, A. On the origin of and phylogenetic relationships among living amphibians. Proc. Natl. Acad. Sci. USA 2001, 98, 7380–7383. [Google Scholar] [CrossRef] [Green Version]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: Amsterdam, The Neatherlands, 1995. [Google Scholar]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- Fiala, J.C. Reconstruct: A free editor for serial section microscopy. J. Microsc. 2005, 218, 52–61. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauricio, B.; Mailho-Fontana, P.L.; Sato, L.A.; Barbosa, F.F.; Astray, R.M.; Kupfer, A.; Brodie, E.D., Jr.; Jared, C.; Antoniazzi, M.M. Morphology of the Cutaneous Poison and Mucous Glands in Amphibians with Particular Emphasis on Caecilians (Siphonops annulatus). Toxins 2021, 13, 779. https://doi.org/10.3390/toxins13110779
Mauricio B, Mailho-Fontana PL, Sato LA, Barbosa FF, Astray RM, Kupfer A, Brodie ED Jr., Jared C, Antoniazzi MM. Morphology of the Cutaneous Poison and Mucous Glands in Amphibians with Particular Emphasis on Caecilians (Siphonops annulatus). Toxins. 2021; 13(11):779. https://doi.org/10.3390/toxins13110779
Chicago/Turabian StyleMauricio, Beatriz, Pedro Luiz Mailho-Fontana, Luciana Almeida Sato, Flavia Ferreira Barbosa, Renato Mancini Astray, Alexander Kupfer, Edmund D. Brodie, Jr., Carlos Jared, and Marta Maria Antoniazzi. 2021. "Morphology of the Cutaneous Poison and Mucous Glands in Amphibians with Particular Emphasis on Caecilians (Siphonops annulatus)" Toxins 13, no. 11: 779. https://doi.org/10.3390/toxins13110779
APA StyleMauricio, B., Mailho-Fontana, P. L., Sato, L. A., Barbosa, F. F., Astray, R. M., Kupfer, A., Brodie, E. D., Jr., Jared, C., & Antoniazzi, M. M. (2021). Morphology of the Cutaneous Poison and Mucous Glands in Amphibians with Particular Emphasis on Caecilians (Siphonops annulatus). Toxins, 13(11), 779. https://doi.org/10.3390/toxins13110779




