Abstract
Pseudomonas (P.) aeruginosa is an opportunistic pathogen that causes serious infections and hospital-acquired pneumonia in immunocompromised patients. P. aeruginosa accounts for up to 20% of all cases of hospital-acquired pneumonia, with an attributable mortality rate of ~30–40%. The poor clinical outcome of P. aeruginosa-induced pneumonia is ascribed to its ability to disrupt lung barrier integrity, leading to the development of lung edema and bacteremia. Airway epithelial and endothelial cells are important architecture blocks that protect the lung from invading pathogens. P. aeruginosa produces a number of virulence factors that can modulate barrier function, directly or indirectly, through exploiting cytoskeleton networks and intercellular junctional complexes in eukaryotic cells. This review summarizes the current knowledge on P. aeruginosa virulence factors, their effects on the regulation of the cytoskeletal network and associated components, and molecular mechanisms regulating barrier function in airway epithelial and endothelial cells. A better understanding of these processes will help to lay the foundation for new therapeutic approaches against P. aeruginosa-induced pneumonia.
Key Contribution:
This review describes how Pseudomonas aeruginosa virulence factors disrupt lung barrier functions through modulation of cytoskeletal components in lung epithelial and endothelial cells.
1. Introduction
Pseudomonas (P.) aeruginosa is an opportunistic pathogen that causes serious infections and hospital-acquired pneumonia (HAP) in at-risk patients, such as those with compromised immune system, who are post-surgical and admitted to intensive care units (ICUs) [1,2,3,4,5]. P. aeruginosa accounts for up to 20% of all cases of HAP, with an attributable mortality rate of ~30–40% [6,7,8,9]. The devastating outcome is associated with lung barrier destruction, which permits lung edema formation and P. aeruginosa bacteremia, a poor prognostic sign [10,11]. Airway epithelium and endothelium constitute a continuous barrier that protect the lung against respiratory pathogens. P. aeruginosa disrupts this protective layer by mechanisms targeting the components involved in regulating actin cytoskeletal networks, including proteins associated with the intercellular junctional complex. Lung alveoli, composed of a single layer epithelial cells, are relatively vulnerable in response to P. aeruginosa infection compared to other epithelium, such as gut and skin epithelium. Once alveolar epithelium is breached, P. aeruginosa and its secreted effectors can get into lung interstitium and interact with endothelial cells. P. aeruginosa may then access the blood stream by crossing endothelium and can cause bacteremia and even sepsis. Figure 1 shows the representation of epithelial and endothelial barriers composing the alveolar–capillary barrier. Evidence of P. aeruginosa-mediated alteration of intercellular junctional components suggests that P. aeruginosa transmigration through cell–cell junctions may be the main route for P. aeruginosa to enter the host system [12,13]. However, the mechanisms by which P. aeruginosa directly or indirectly disrupts junction integrity are still not fully understood.
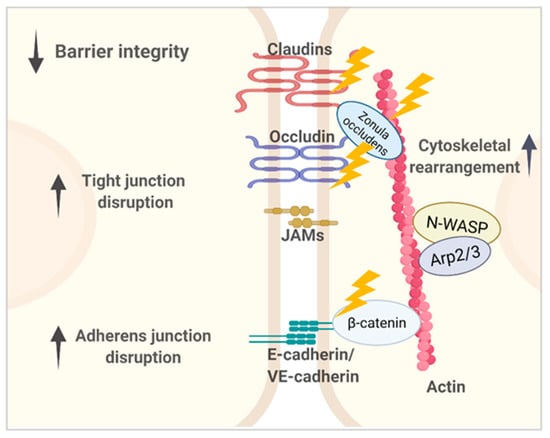
Figure 1.
Representation of lung microvascular barriers composed of the major intercellular junctional structures, including tight junctions (TJs) and adherens junctions (AJs). Epithelial AJs contain epithelial cadherin (E-cadherin), and endothelial AJs contain vascular endothelial cadherin (VE-cadherin). The main structural components and their cytoplasmic partners involved in P. aeruginosa-induced lung barrier dysregulation are shown. P. aeruginosa virulence factors impair paracellular permeability through disrupting junctional components’ expression, redistribution, and interaction with adaptors that together are key to maintain proper junctional structure and function. Compromised epithelial and endothelial barriers eventually contribute to P. aeruginosa dissemination from the lung into the bloodstream.
The actin cytoskeletal network is involved in multiple physiological functions and plays a vital role in lung barrier integrity. One important function of the actin cytoskeleton is the maintenance of intercellular junctional structures through coordinating the interactions between components contained within the junctional complex. P. aeruginosa possesses a full arsenal of virulence factors. During P. aeruginosa infection, these virulence factors interrupt lung barrier functions through modulation of cytoskeletal regulators and generating contractile forces. These contractile forces compete with cell–cell tethering forces between adjacent cells and generate tension delivered to intercellular contacts, leading to the formation of gaps between contacting cells and increasing paracellular permeability as a consequence. Adherens junctions (AJs) and tight junctions (TJs), the primary intercellular complexes that control the lung barrier functions, are linked to and coordinate with the actin cytoskeleton. By exploiting the cytoskeletal network, P. aeruginosa not only disturbs junctional stability but also disrupts signal transduction that is fundamental to cellular functions, such as those performed by Rho GTPases. Moreover, the consequences of dysregulated cytoskeleton regulators go beyond the cytoskeleton alterations and lung barrier dysfunction, as some cytoskeleton regulators are involved in modulation of immune responses. For example, aberrant Rho GTPases contribute to transcriptional regulation of important inflammatory mediators as well as cytokine expression [14,15]. Thus, the actin cytoskeleton and its associated components play important roles in fine-tuning cell–cell junction functions and signal transduction as well as providing multiple targets for P. aeruginosa virulence factors.
This review summarizes the current knowledge relating P. aeruginosa virulence factors, their interactions and effects on the cytoskeleton network and associated component proteins, as well as molecular mechanisms regulating lung barrier function in airway epithelial and endothelial cells. A thorough understanding of these processes will provide a foundation for new therapeutic approaches for P. aeruginosa-induced pneumonia and the development of therapies for lung barrier modulation.
2. P. aeruginosa Regulation of the Cytoskeletal Network in Lung Epithelial Cells
The respiratory epithelium serves as the dominant barrier against P. aeruginosa invasion [16,17,18]. Intact epithelium has strictly controlled paracellular permeability due to the presence of intercellular junctions, primarily tight junction (TJs), and adherens junctions (AJs) [19,20]. However, impaired TJs and AJs allow pathogens and large macromolecules to move through the space between adjacent cells and, thus, penetrate through the epithelium. The regulation of epithelial paracellular permeability depends on a set of specialized adhesive membrane proteins arranged to precisely coordinate with actin cytoskeletal dynamics. Thus, the cytoskeleton plays an important role in physically and functionally balancing this regulatory network, as any imbalance may result in perturbed intercellular junctional stability and permeability.
Many P. aeruginosa virulence factors modulate lung epithelial permeability through manipulation of cytoskeletal dynamics and associated regulators that alter protein expression, distribution, degradation, or phosphorylation status [21,22,23,24,25]. The Rho family of small GTPases plays a fundamental role in the regulation of actin cytoskeleton reorganization [26,27]. P. aeruginosa promotes permeability in epithelial and endothelial cells through activation of the upstream GTPase Ras homolog gene family, member A (Rho A) [28,29]. ExoS and ExoT, type 3 secretion system (T3SS) exoenzymes that are directly injected into host cells by P. aeruginosa, interfere with actin reorganization through hijacking the host eukaryotic Rho GTPase pathway [29,30,31]. Type III exoenzymes also trigger substantial redistribution of occludin (OCLN), zonula occludens-1 (ZO-1), and another TJs protein ezrin in human airway cells [25]. Besides targeting cell–cell junctions, P. aeruginosa utilizes certain integrins, such as integrin α5β1 and αvβ5, for attachment and invasion to airway epithelial cells [32,33]. TGF-β1 is a critical mediator of P. aeruginosa-induced acute lung injury [34], as integrin-mediated TGF-β1 activation contributes to signal transductions involved in cytoskeletal rearrangement and tyrosine kinase activation. For example, integrin αvβ6-mediated activation of TGF-β1 is essential for P. aeruginosa-mediated lung barrier disruption and edema formation [35,36].
P. aeruginosa targets tight junction components. Tight junctions (TJs), the first line of junctional defense that gate the paracellular pathway, are composed of transmembrane proteins, including claudins (CLDN), OCLN, tricellulin, and the junctional adhesion molecule (JAM) proteins [37,38,39,40,41]. The intracellular domains of these proteins are linked to actin cytoskeleton through adaptor proteins. such as ZO-1, ZO-2, ZO-3, and cingulin. P. aeruginosa induces decreased expression of TJs proteins, including claudin-1 and OCLN, resulting in reduced transepithelial electrical resistance (TER) across cultured airway cells [42]. P. aeruginosa elastase downregulates expression of claudin-1, claudin-4, OCLN, and tricellulin [21,22,43]. In addition, P. aeruginosa has been reported to stimulate OCLN and E-cadherin redistribution and E-cadherin cleavage through toll-like receptor 2 (TLR-2)-dependent activation of calpain [44].
Furthermore, P. aeruginosa targets the adherens junction components. Actin cytoskeleton plays an important role in providing contractility for lateral adhesions between adjacent cells by forming parallel actin bundles at the AJs [45,46,47,48,49]. AJs are mainly composed of clustered E-cadherin transmembrane proteins, which are linked to the actin cytoskeleton through catenins and vinculin. P. aeruginosa virulence factors modulate AJs integrity through regulation of expression and phosphorylation status of E-cadherin and beta-catenin [24,43,50,51].
Additionally, P. aeruginosa targets the adaptor proteins that connect the actin cytoskeleton with the intercellular junctional complex. At least 40 different proteins are known to localize to TJs, and only 4 TJs proteins are known to directly bind to actin cytoskeleton, namely ZO-1, ZO-2, ZO-3, and cingulin. ZO proteins bind directly to actin filaments (F-actin) [52,53], linking actin cytoskeleton with TJs transmembrane proteins, such as CLDNs, OCLN, and JAM [54,55,56]. ZO-1 has been shown to link with regulators involved in myosin II activity and Rho GTPase signaling [57,58], which are both important mediators of cytoskeletal dynamics. Lipopolysaccharide (LPS)-induced F-actin rearrangement is essential for LPS signaling [59]. P. aeruginosa LPS increases F-actin formation in a ZO-1-dependent pathway. Overexpression of ZO-1 has been reported to increase F-actin polymerization driven by PDZ-1 domain-mediated binding to claudins [60,61].
P. aeruginosa-induced effects on epithelial barrier functions are, at least partly, regulated through cytoskeleton reorganization. such as Rho GTPases regulated actin polymerization [29,30]. Further studies are needed for a molecular-level understanding of how P. aeruginosa-induced actin cytoskeleton reorganization affects TJs and AJs assembly and its impact on paracellular permeability.
3. P. aeruginosa Targets Cytoskeletal Network in Lung Endothelial Cells
Endothelial cells are specialized cells that line the internal surface of blood vessels and are responsible for the maintenance of vascular permeability. Although serving as a barrier between blood and interstitial fluid, the lung endothelium is composed of a single layer of endothelial cells, making it vulnerable to attack by P. aeruginosa virulence factors. Following disruption of the epithelial barrier, P. aeruginosa virulence factors have access to the endothelium, where proteases and toxins released from P. aeruginosa further disrupt endothelial tight junctions [62]. As a consequence of dysregulated endothelial cell barriers, P. aeruginosa can migrate into the bloodstream and lead to bacteremia and cause a fatal outcomes [63]. However, compared to airway epithelium, a small number of studies have investigated the destructive effects of P. aeruginosa virulence factors on lung endothelium [28,50,62,64,65]. Endothelium presents similar yet distinct intercellular junctional components when compared to those of the epithelium. For example, instead of E-cadherin expressed by epithelial AJs, endothelial AJs present VE-cadherin, an endothelial-specific cadherin [66,67]. P. aeruginosa elastase cleaves VE-cadherin [21,62]. Moreover, ExoS and ExoT increase paracellular permeability across endothelial cell monolayers through integrin αvβ5 with activation of RhoA signaling [28,68,69]. In addition, compared to the junctional complex in epithelium, the endothelium presents intermingled TJs and AJs [70]. Interestingly, recent evidence suggests that actin assembly at TJs and AJs are regulated through distinctive mechanisms [71,72]. Lung endothelium and epithelium also share some similar mechanisms in the role of cytoskeleton dynamics in barrier function in response to P. aeruginosa infection. Neural Wiskott–Aldrich syndrome protein (NWASP) plays a critical role in cytoskeleton dynamics and regulates barrier integrity through Rho GTPase signaling and cytoskeletal reorganization in lung endothelial and epithelial cells in response to P. aeruginosa and transforming growth factor beta-1 [64,73]. It has recently been noted that barrier function is more strictly controlled with 10 times higher transendothelial electrical resistance and more developed intercellular junctions in lung microvascular endothelium in comparison to lung macrovascular endothelium [74,75,76,77]. Additional studies are needed to understand the molecular mechanisms by which P. aeruginosa virulence factors breach the lung microvascular endothelium by modulation of cytoskeletal structures and cytoskeletal regulatory proteins.
4. Cytoskeletal Regulation by P. aeruginosa Virulence Factors
4.1. Regulation of Lung Permeability by Virulence Factors Belonging to P. aeruginosa Type III Secretion System
Type III secretion system (T3SS) is the major contributor to P. aeruginosa-induced virulence [78,79,80,81]. Epithelial cells are especially sensitive to the effects of T3SS toxins [25,80,81,82,83]. P. aeruginosa T3SS translocates four exoenzymes (ExoS, ExoT, ExoY, and ExoU) into host cells (Figure 2). These exoenzymes have overlapping, yet distinct pathways to target cytoskeleton components and associated junctional complex, causing cell morphological changes and intercellular junction disruption, leading to a loss of barrier integrity. The interactions of these type III exoenzymes with cytoskeleton components are important in the pathogenesis of P. aeruginosa infection.
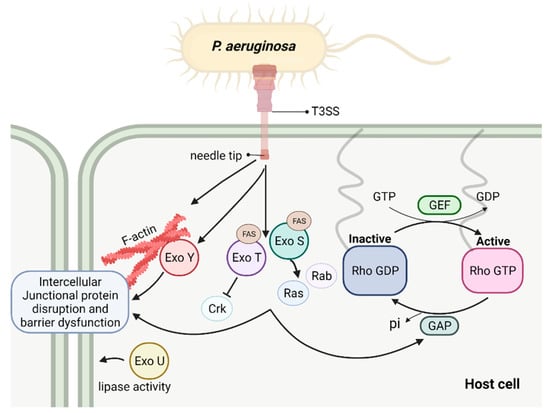
Figure 2.
Schematic depicting T3SS exoenzymes and their interaction with host intracellular pathways contributing to barrier disruption. These events result in actin stress fiber formation, cytoskeleton rearrangement, and disruption of intercellular junctions, following with increased permeability.
4.1.1. ExoS
ExoS has been studied extensively with several clearly defined eukaryotic targets [84,85,86]. ExoS has a Rho GTPase activating domain (RhoGAP) encoded within the N-terminus and a ADP-ribosyltransferase domain (ADPRT) within the C-terminus [84,87,88]. Through these two domains, ExoS targets cytoskeletal components in different host cell types, including neutrophils, leukocytes, and epithelial cells [89]. The N-terminal Rho GTPases are critical for actin polymerization and cytoskeletal dynamics [90]. The N-terminal domain of ExoS is a mimic of eukaryotic RhoGAP domain so that it can prevent small GTPases Rho, Rac, and Cdc42 from activation by keeping them in inactivate GDP-bound form [31,87,91]. Expression of the N-terminal RhoGAP domain in cultured cells stimulates reorganization of actin stress fibers, contributing to the collapse of the actin cytoskeleton and rounded cellular phenotype [31]. The C-terminus of ExoS encodes an ADP-ribosyltransferase (ADPRT) domain which becomes activated after binding to a eukaryotic cofactor (FAS, factor activating ExoS) [92]. This domain is able to ADP-ribosylate numerous substrates [84]. ADP-ribosylation of Ras and Rab proteins causes a disruption of the actin cytoskeleton, endocytosis, and vesicular trafficking [88,93,94]. In addition, this domain is also responsible for ADP-ribosylation of a set of proteins that link the plasma membrane to the actin cytoskeleton, including ezrin, radixin, and moesin proteins (ERMs), which is implied in the disruption of Rho signaling, resulting in cytoskeletal rearrangements.
4.1.2. ExoT
Closely related to ExoS, ExoT is also a bifunctional exoenzyme, possessing a RhoGAP domain on its N-terminus and an ADP-ribosylation domain on its C-terminus [95]. The RhoGAP activities of ExoT appear to be biochemically and biologically similar to that of ExoS, targeting substrate such as Rho, Rac, and Cdc42 [29,91,95]. Similar to ExoS, the overexpression of the ExoT RhoGAP domain induces the actin cytoskeleton disruption through a Rho-dependent pathway. On the other hand, while ExoS can ADP-ribosylate a wide range of host proteins, ExoT possesses limited ADP-ribosyltransferase activity. When overexpressed in cultured cells, ExoT ADPRT affects the host cell phagocytic activity while ExoS ADPRT has a cytotoxic effect. One substrate of ExoT ADPRT are Crk adaptor proteins that are essential in signal transduction and are involved in actin reorganization [96,97]. ADP-ribosylation of Crk proteins prevents their interaction with focal adhesion proteins and with DOCK180 which is a guanine nucleotide exchange factors (GEF) for Rac, thus inhibiting Rac-dependent phagocytosis [98]. The ADPRT domain is required for ExoT-induced inhibition of migration and wound healing in epithelial cells [99]. ExoT has been suggested in the regulation of cytoskeletal reorganization as different ExoT mutants differentially affect the subcellular localization of paxillin and focal adhesion kinase [100]. It is noteworthy that, although ExoT ADP-ribosylates a more restricted subset of substrates, it has been suggested that ExoT is important for P. aeruginosa to achieve full virulence in a mouse pneumonia model [30]. A prevalence study of type III secretion genes suggests that nearly all clinical isolated P. aeruginosa strains encode ExoT, while exoS, exoU, and exoY genes were variably expressed [101], suggesting that ExoT may have a more conserved role in the context of P. aeruginosa pathogenesis.
4.1.3. ExoY
ExoY is a nucleotidyl cyclase that synthesizes cyclic nucleotides including cGMP, cAMP, cUMP, and cCMP [102,103,104,105]. ExoY is highly prevalent in clinically isolated strains and has been indicated as an important edema factor which significantly contributes to end-organ dysfunction in critically ill patients with P. aeruginosa lung infection [101,106,107]. Excessively generated cyclic nucleotides can alter cell morphology through disrupting signaling involved in cytoskeletal organization. However, ExoY, to prevent toxicity to bacterial cells, is produced in its inactive form by P. aeruginosa. The mechanisms by which ExoY produces large quantity of cyclic nucleotides in eukaryotic cells are poorly understood. Recent studies reveal ExoY activation upon binding to F-actin in host cells [108]. Binding with F-actin drives ExoY go through a con-formational change which is critical to increase ExoY catalytic activity and generate excessive cyclic. These data indicate that binding to actin filaments (F-actin), but not globular actin (G-actin), activates ExoY, which in turn helps to stabilize actin filaments [108,109,110]. In addition, it has been shown that ExoY promotes Tau phosphorylation which dissociates from microtubule and results in microtubule breakdown, leading to gap formation and increased permeability [103,106].
4.1.4. ExoU
ExoU is mutually exclusively expressed with ExoS by P. aeruginosa [111,112,113]. ExoU is a phospholipase that induces acute cytotoxic effects and is capable to destroy cell monolayers in a short time period [114]. ExoU production is associated with accelerated lung injury and is often associated with the most severe pathological outcomes in experimental animals and in patients [111,115]. ExoU has been reported to associate with membrane fractions and resides on cell–cell junctions in A549 cells [116]. ExoU presents a high affinity to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). PI(4,5)P2 is a multifunctional phosphoinositide located in the eukaryotic plasma membrane and is involved in the regulation of focal adhesion formation and cytoskeletal dynamics. Studies have shown that a high affinity of ExoU with PI(4,5)P2 and subsequent cleavage of PI(4,5)P2 at focal adhesion complexes contribute to the collapse of the cytoskeleton network in human epithelial cells [117]. Although ExoU lipase activity appears to have a large range of substrates in cell cytosol where it may damage plasma membrane components, whether there is physical association between ExoU and junctional proteins or with cytoskeletal components still require further investigation.
4.1.5. T3SS Needle Tip Complex
The needle tip complex is important in early infection stages as it functions to inject P. aeruginosa effectors into host cells upon contact. In addition to its needle-like function, the presence of this needle complex has been reported to induce actin stress fiber formation in cultured murine pulmonary microvascular endothelial cells [118,119].
4.2. Regulation of Lung Permeability by P. aeruginosa Secreted Virulence Factors
4.2.1. Elastase
P. aeruginosa elastase (PE) is a secreted metalloproteinase with highly efficient proteolytic activity on a number of host structural proteins in airway epithelium [120,121,122,123,124,125]. It has been reported that PE can transiently disintegrate and redistribute tight junction proteins OCLN and ZO-1, induce cleavage of VE-cadherin, and cause actin cytoskeleton reorganization [22,23,62,126,127,128]. By using the B.V strain that is known for its high elastase activity, it has been shown that PE is capable of completely degrading ZO-1 and significantly degrading OCLN [127]. Besides targeting on tight junction proteins, PE has tissue-damaging activities. In addition, PE can degrade lung elastin, an important structural protein for maintaining blood vessel integrity [123,129], as well as matrix proteins including laminin and collagen (type III and type IV), leading to basement membrane impairment [130,131,132].
4.2.2. Exotoxin A
P. aeruginosa produces a highly toxic virulence factor exotoxin A (ExoA) which is released into extracellular medium by type 2 secretion system (T2SS) [133,134]. It has ADP-ribosylation activity and affects the protein synthesis processes in host cells. ExoA has been shown to delay wound repair in the animal cutaneious injury model through its effects on cytoskeleton remodeling [135]. Treatment with ExoA reduces TJs proteins ZO-1 and ZO-2 and increases paracellular permeability in type II pneumocyte cultures [23]. However, the exact mechanism undergoing ExoA-mediated epithelial barrier damage still need further studies.
4.3. Regulation of Lung Permeability by P. aeruginosa Surface-Bound Virulence Factors
4.3.1. Pilus and Flagellum
Type IV pilus and flagellum are important surface structural components for P. aeruginosa attachment to cell surface and are critical in preparation for T3SS toxin injection [136,137]. Due to the nature as P. aeruginosa surface structure, pilus and flagellum are likely to have roles beyond mediating an initial attachment to the host surface. Evidence show that pilus and flagellum are required for transmigration across epithelial cell junctions [136,137]. Recently, pilus has been shown to preferentially interact with the cell basolateral domain and T3SS effectors are only injected into host cells through their basolateral membrane domain [136,137,138]. Internalization of P. aeruginosa in the epithelial basolateral surface requires flagellum binding to heparan sulfate, with subsequent signaling activation of epidermal growth factor receptor (EGFR), phosphoinositide 3-kinases (PI3K), and protein kinase B (AKT) [138]. These findings suggest these surface-bound virulence factors may play an important role in mediating P. aeruginosa transmigration through paracellular route.
4.3.2. Lipopolysccharide
Lipopolysccharide (LPS) is a major structure component which is integrated in the P. aeruginosa cell wall and plays an important role in bacterium–host interactions [139]. LPS is a pro-inflammatory mediator which can increase airway epithelial permeability [140]. LPS-induced F-actin rearrangement and actin assembly are important for LPS signaling [59]. However, molecular mechanisms for LPS-induced endothelial cell permeability are still not well understood.
4.4. Regulation of Lung Permeability by Quorum Sensing and Other P. aeruginosa Virulence Factors
Quorum sensing (QS) is a specialized cell density-dependent regulation system in bacteria [141,142,143]. These bacterial signals also modulate mammalian airway epithelial cell responses to the pathogen in a process called interkingdom signaling. N-(3-Oxododecanoyl)-L-homoserine lactone (C12) is a small molecule quorum-sensing signal produced by a P. aeruginosa lasR-lasI QS system [144,145]. In addition to the regulation of P. aeruginosa population behavior, C12 also regulates a range of complex biological processes in host cells. In human epithelial Caco-2 cells, C12 induces a decrease in transepithelial electrical resistance (TER), an increase in paracellular flux, a reduction in the expression and distribution of ZO-1 and OCLN, and reorganization of F-actin through activation of p38 and p42/44 pathways [146]. In intestinal epithelial cells, C12 alters the phosphorylation status of cell junctional components, including E-cadherin, beta-catenin, OCLN, ZO-1, and ZO-3, and JAM-A. In addition, the changes in phosphorylation status of regulatory proteins disrupt the association between junctional components and result in a loss of epithelial barrier and increased paracellular permeability [24,147]. C12 also induces degradation and de-location of TJs proteins (OCLN and tricellulin) in intestinal epithelial Caco-2 cells [148]. These findings collectively indicate that C12 induces epithelial paracellular permeability possibly through a mechanism that mediates the disassembly of intercellular links. C12 induces myofibroblast differentiation in vitro and in vivo for accelerated wound healing [149]. In cultured nonpolarized airway epithelial cells, C12 induces massive morphological changes of cell structure with perturbed gap junction shortly after application [150]. C12 may also facilitate dissemination of virus into bloodstream [151].
Rhamnolipids
P. aeruginosa produces biosurfactants called rhamnolipids [152,153]. Rhamnolipids act as a potent detergent and have been reported to disrupt intercellular junctions in sheep tracheal epithelium at high concentrations [154]. Rhamnolipids induce ciliostasis of airway epithelial cells and may disrupt their barrier function, allowing invasion of pseudomonas [12]. Alzheimer’s disease (AD) has been attributed to chronic bacterial infections, and the levels of rhamnolipids in sera and cerebrospinal fluid of AD patients are significantly increased when compared to controls [155]. However, the meaning of the increased rhamnolipids levels in AD patients and AD pathogenesis is unclear so far.
5. Conclusions
P. aeruginosa virulence factors have a significant impact on host biological functions by targeting different cellular components. Figure 3 shows the major virulence factors involved in P. aeruginosa-induced cytoskeleton rearrangement and impaired barrier integrity. The actin cytoskeleton plays an important role in coordinating junctional components with cytosolic signaling regulators. Pathological modulation of regulators involved in actin cytoskeleton reorganization links to lung barrier dysfunction. Rho GTPases play critical roles in the regulation of cytoskeleton contractility and dynamics. Virulence factors with the capacity to interfere host Rho GTPases activities can thus disrupt junctional functions due to altered cytoskeleton contractility. For example, ExoS and ExoT can hijack Rho GTPase signaling pathway, by mimicking the eukaryotic Rho GTPases at its N terminal domain, directly altering the function of host cytoskeletal regulation. In addition to delivering cytotoxic type III secretion exoenzymes into eukaryotic cells, other virulence factors, such as surface bound structure LPS, also induce stress fiber formation and cytoskeletal protein reorganization. Furthermore, P. aeruginosa produces a large number of exo-products, of which the quorum-sensing molecule, C12 transkingdomly, interferes with host junctional protein expression and redistribution. Hence, as cytoskeleton alteration can be induced at each stage of P. aeruginosa infection, a barrier breach can also occur at different levels, through initial bacteria attachment, to toxin injection into cytosol that interferes host cytoskeletal components. Figure 4 shows an overview of effects of P. aeruginosa virulence factors on intercellular junctional impairment, cytoskeleton rearrangement, and lung barrier dysfunction. Future studies are needed to elucidate the mechanisms by which virulence factors disturb the mammalian cytoskeleton network and modulate invasion, and to highlight how those activities contribute to the pathogenesis of P. aeruginosa infection.
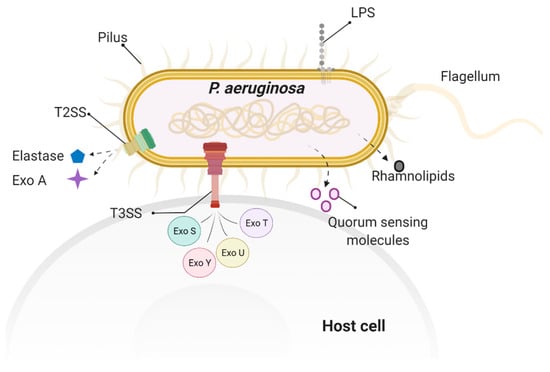
Figure 3.
Virulence factors involved in P. aeruginosa-induced cytoskeleton rearrangement and impaired barrier integrity. These P. aeruginosa virulence factors include surface factors, such as flagellum, pilus, and LPS; secreted factors, such as type III secretion system (T3SS) exoenzymes (ExoS, ExoT, ExoY, ExoU) and rhamnolipid; and quorum-sensing factor, such as N-(3-Oxododecanoyl)-L-homoserine lactone.
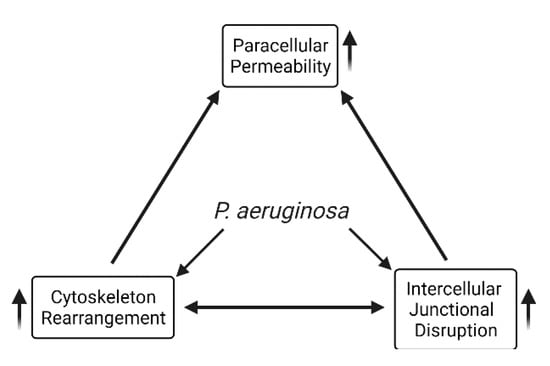
Figure 4.
Overview of effects of P. aeruginosa virulence factors on intercellular junctional impairment, cytoskeleton rearrangement, and lung barrier dysfunction.
Although P. aeruginosa virulence factors use the cytoskeleton network as a common route to modulate barrier integrity and signal transduction, different strategies have been adopted due to distinct features present in epithelial and endothelial cells. The diversified virulence factor types further complicate the pathogenic pathways. Therefore, each virulence factor establishes a unique pathogenic strategy to penetrate lung barrier, and a number of molecular mechanisms have been proposed regarding how P. aeruginosa virulent factors breach lung barrier functions. However, appropriate in vivo models are not easily applicable, and most of these mechanisms were based on observations using in vitro cell cultures. One pitfall of in vitro studies is the use of relatively high concentration of cytotoxins, which may not adequately reflect the exact situations in the course of infection. The in vivo condition is confounded with abundant immune cells and fluid flow, which in turn further complicates the interactions between P. aeruginosa virulence factors, cytoskeleton, and its associated components. Moreover, the biological effects of virulence factors vary depending upon the route of bacterial delivery and the nature of the host cell types, and different route of bacterial delivery can further affect the biological effects of P. aeruginosa virulence factors.
It is currently not well understood by what mechanisms, and to what degree, TJs or AJs proteins are affected by the destructive effects of P. aeruginosa virulence factors. Recent evidence suggests that actin assembly at TJs and AJs are regulated through distinctive mechanisms [71,72]. This is further complicated by the fact that many cytoskeletal regulatory proteins involved in P. aeruginosa infection have other roles in cell biology. Future studies will be needed to understand the underlying mechanisms. Furthermore, tight junctions contain at least 40 different proteins [155]. Besides the aforementioned transmembrane proteins (such as CLDNs and OCLN) and adaptor protein (such as ZO-1), other intracellular proteins (such as cingulin, MAGI-1, Pals1, and TATJ) also form scaffolds between transmembrane junctional proteins and the actin cytoskeleton. Whether these proteins are manipulated by P. aeruginosa and associated consequences on barrier regulation is unknown. While this review focuses on the role of actin cytoskeleton in regulating lung barrier and permeability, other cytoskeleton structures, such as microtubules, may also play a role in P. aeruginosa-induced barrier dysregulation. Additional research and a better understanding of the effects of P. aeruginosa virulence factors on lung epithelial and endothelial barrier functions will be important for uncovering novel strategies to reduce P. aeruginosa-induced edema and bacteremia. A better understanding of how actin cytoskeleton controls intercellular junction assembly will provide new insights to selectively modulate the paracellular flux between airway epithelial and endothelial cells, which would, in turn, benefit the development of small molecule and proteins for novel therapeutics against P. aeruginosa-induced lung complications.
Author Contributions
Conceptualization, B.M.W., R.H., Q.D. and P.C.; writing—original draft preparation, B.M.W., R.H., Q.D. and P.C.; writing—review and editing, B.M.W., R.H., S.W., J.-F.P., Q.D. and P.C.; supervision, Q.D. and P.C.; funding acquisition, B.M.W., J.-F.P., Q.D. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants R01HL143017, R01HL127338, 1R01HL152183, and R01GM127584 to Q. Ding, J. Pittet, and B. Wagener, and American Heart Association Career Development Award to P. Che.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review and does not include any data.
Acknowledgments
Schematic diagrams were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hatipoglu, M.; Mutluoglu, M.; Uzun, G.; Karabacak, E.; Turhan, V.; Lipsky, B.A. The microbiologic profile of diabetic foot infections in Turkey: A 20-year systematic review: Diabetic foot infections in Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Lund-Palau, H.; Turnbull, A.R.; Bush, A.; Bardin, E.; Cameron, L.; Soren, O.; Wierre-Gore, N.; Alton, E.W.; Bundy, J.G.; Connett, G.; et al. Pseudomonas aeruginosa infection in cystic fibrosis: Pathophysiological mechanisms and therapeutic approaches. Expert Rev. Respir. Med. 2016, 10, 685–697. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- Wilson, M.G.; Pandey, S. Pseudomonas Aeruginosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cook, D.J.; Griffith, L.; Keenan, S.P.; Brun-Buisson, C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am. J. Respir. Crit. Care Med. 1999, 159, 1249–1256. [Google Scholar] [CrossRef]
- Rello, J.; Gallego, M.; Mariscal, D.; Sonora, R.; Valles, J. The value of routine microbial investigation in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 1997, 156, 196–200. [Google Scholar] [CrossRef]
- Fagon, J.Y.; Chastre, J.; Domart, Y.; Trouillet, J.L.; Pierre, J.; Darne, C.; Gibert, C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am. Rev. Respir. Dis. 1989, 139, 877–884. [Google Scholar] [CrossRef]
- Magret, M.; Lisboa, T.; Martin-Loeches, I.; Manez, R.; Nauwynck, M.; Wrigge, H.; Cardellino, S.; Diaz, E.; Koulenti, D.; Rello, J.; et al. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: A prospective and observational multicenter study. Crit. Care 2011, 15, R62. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Matthay, M.A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu. Rev. Physiol. 2013, 75, 593–615. [Google Scholar] [CrossRef]
- Zulianello, L.; Canard, C.; Kohler, T.; Caille, D.; Lacroix, J.S.; Meda, P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 2006, 74, 3134–3147. [Google Scholar] [CrossRef]
- Golovkine, G.; Faudry, E.; Bouillot, S.; Elsen, S.; Attree, I.; Huber, P. Pseudomonas aeruginosa Transmigrates at Epithelial Cell-Cell Junctions, Exploiting Sites of Cell Division and Senescent Cell Extrusion. PLoS Pathog. 2016, 12, e1005377. [Google Scholar] [CrossRef]
- Dreikhausen, U.; Varga, G.; Hofmann, F.; Barth, H.; Aktories, K.; Resch, K.; Szamel, M. Regulation by rho family GTPases of IL-1 receptor induced signaling: C3-like chimeric toxin and Clostridium difficile toxin B inhibit signaling pathways involved in IL-2 gene expression. Eur. J. Immunol. 2001, 31, 1610–1619. [Google Scholar] [CrossRef]
- Croker, B.A.; Tarlinton, D.M.; Cluse, L.A.; Tuxen, A.J.; Light, A.; Yang, F.C.; Williams, D.A.; Roberts, A.W. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J. Immunol. 2002, 168, 3376–3386. [Google Scholar] [CrossRef]
- Page, L.K.; Staples, K.J.; Spalluto, C.M.; Watson, A.; Wilkinson, T.M.A. Influence of Hypoxia on the Epithelial-Pathogen Interactions in the Lung: Implications for Respiratory Disease. Front. Immunol. 2021, 12, 653969. [Google Scholar] [CrossRef]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef]
- Bossardi Ramos, R.; Adam, A.P. Molecular Mechanisms of Vascular Damage During Lung Injury. Adv. Exp. Med. Biol. 2021, 1304, 95–107. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004, 19, 331–338. [Google Scholar] [CrossRef]
- Beaufort, N.; Corvazier, E.; Mlanaoindrou, S.; de Bentzmann, S.; Pidard, D. Disruption of the endothelial barrier by proteases from the bacterial pathogen Pseudomonas aeruginosa: Implication of matrilysis and receptor cleavage. PLoS ONE 2013, 8, e75708. [Google Scholar] [CrossRef]
- Nomura, K.; Obata, K.; Keira, T.; Miyata, R.; Hirakawa, S.; Takano, K.; Kohno, T.; Sawada, N.; Himi, T.; Kojima, T. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir. Res. 2014, 15, 21. [Google Scholar] [CrossRef]
- Azghani, A.O. Pseudomonas aeruginosa and epithelial permeability: Role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol. 1996, 15, 132–140. [Google Scholar] [CrossRef]
- Vikstrom, E.; Bui, L.; Konradsson, P.; Magnusson, K.E. The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp. Cell Res. 2009, 315, 313–326. [Google Scholar] [CrossRef]
- Soong, G.; Parker, D.; Magargee, M.; Prince, A.S. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 2008, 190, 2814–2821. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Ganter, M.T.; Roux, J.; Su, G.; Lynch, S.V.; Deutschman, C.S.; Weiss, Y.G.; Christiaans, S.C.; Myazawa, B.; Kipnis, E.; Wiener-Kronish, J.P.; et al. Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am. J. Respir. Cell Mol. Biol. 2009, 40, 108–118. [Google Scholar] [CrossRef]
- Kazmierczak, B.I.; Engel, J.N. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 2002, 70, 2198–2205. [Google Scholar] [CrossRef]
- Garrity-Ryan, L.; Kazmierczak, B.; Kowal, R.; Comolli, J.; Hauser, A.; Engel, J.N. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 2000, 68, 7100–7113. [Google Scholar] [CrossRef]
- Pederson, K.J.; Vallis, A.J.; Aktories, K.; Frank, D.W.; Barbieri, J.T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol. Microbiol. 1999, 32, 393–401. [Google Scholar] [CrossRef]
- Roger, P.; Puchelle, E.; Bajolet-Laudinat, O.; Tournier, J.M.; Debordeaux, C.; Plotkowski, M.C.; Cohen, J.H.; Sheppard, D.; de Bentzmann, S. Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 1999, 13, 1301–1309. [Google Scholar]
- Leroy-Dudal, J.; Gagniere, H.; Cossard, E.; Carreiras, F.; Di Martino, P. Role of alphavbeta5 integrins and vitronectin in Pseudomonas aeruginosa PAK interaction with A549 respiratory cells. Microbes Infect. 2004, 6, 875–881. [Google Scholar] [CrossRef]
- Pittet, J.F.; Griffiths, M.J.; Geiser, T.; Kaminski, N.; Dalton, S.L.; Huang, X.; Brown, L.A.; Gotwals, P.J.; Koteliansky, V.E.; Matthay, M.A.; et al. TGF-beta is a critical mediator of acute lung injury. J. Clin. Investig. 2001, 107, 1537–1544. [Google Scholar] [CrossRef]
- Pittet, J.F.; Koh, H.; Fang, X.; Iles, K.; Christiaans, S.; Anjun, N.; Wagener, B.M.; Park, D.W.; Zmijewski, J.W.; Matthay, M.A.; et al. HMGB1 accelerates alveolar epithelial repair via an IL-1beta- and alphavbeta6 integrin-dependent activation of TGF-beta1. PLoS ONE 2013, 8, e63907. [Google Scholar] [CrossRef]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef]
- McCarthy, K.M.; Skare, I.B.; Stankewich, M.C.; Furuse, M.; Tsukita, S.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996, 109 Pt 9, 2287–2298. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Patkee, W.R.; Carr, G.; Baker, E.H.; Baines, D.L.; Garnett, J.P. Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. J. Cell Mol. Med. 2016, 20, 758–764. [Google Scholar] [CrossRef]
- Azghani, A.O.; Gray, L.D.; Johnson, A.R. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 1993, 61, 2681–2686. [Google Scholar] [CrossRef]
- Chun, J.; Prince, A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 2009, 5, 47–58. [Google Scholar] [CrossRef]
- Hull, B.E.; Staehelin, L.A. The terminal web. A reevaluation of its structure and function. J. Cell Biol. 1979, 81, 67–82. [Google Scholar] [CrossRef]
- Harris, A.R.; Daeden, A.; Charras, G.T. Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J. Cell Sci. 2014, 127, 2507–2517. [Google Scholar] [CrossRef]
- Radeva, M.Y.; Waschke, J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol. (Oxf.) 2018, 222. [Google Scholar] [CrossRef]
- Cerutti, C.; Ridley, A.J. Endothelial cell-cell adhesion and signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef]
- Yonemura, S. Actin filament association at adherens junctions. J. Med. Investig. 2017, 64, 14–19. [Google Scholar] [CrossRef]
- Huber, P.; Bouillot, S.; Elsen, S.; Attree, I. Sequential inactivation of Rho GTPases and Lim kinase by Pseudomonas aeruginosa toxins ExoS and ExoT leads to endothelial monolayer breakdown. Cell. Mol. Life Sci. 2014, 71, 1927–1941. [Google Scholar] [CrossRef]
- Cott, C.; Thuenauer, R.; Landi, A.; Kuhn, K.; Juillot, S.; Imberty, A.; Madl, J.; Eierhoff, T.; Romer, W. Pseudomonas aeruginosa lectin LecB inhibits tissue repair processes by triggering beta-catenin degradation. Biochim. Biophys. Acta 2016, 1863, 1106–1118. [Google Scholar] [CrossRef]
- Itoh, M.; Nagafuchi, A.; Moroi, S.; Tsukita, S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 1997, 138, 181–192. [Google Scholar] [CrossRef]
- Wittchen, E.S.; Haskins, J.; Stevenson, B.R. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 1999, 274, 35179–35185. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 2008, 1778, 588–600. [Google Scholar] [CrossRef]
- Fanning, A.S.; Anderson, J.M. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 113–120. [Google Scholar] [CrossRef]
- Huo, L.; Wen, W.; Wang, R.; Kam, C.; Xia, J.; Feng, W.; Zhang, M. Cdc42-dependent formation of the ZO-1/MRCKbeta complex at the leading edge controls cell migration. EMBO J. 2011, 30, 665–678. [Google Scholar] [CrossRef]
- Etournay, R.; Zwaenepoel, I.; Perfettini, I.; Legrain, P.; Petit, C.; El-Amraoui, A. Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J. Cell Sci. 2007, 120, 2838–2850. [Google Scholar] [CrossRef]
- Chakravortty, D.; Nanda Kumar, K.S. Bacterial lipopolysaccharide induces cytoskeletal rearrangement in small intestinal lamina propria fibroblasts: Actin assembly is essential for lipopolysaccharide signaling. Biochim. Biophys. Acta 2000, 1500, 125–136. [Google Scholar] [CrossRef]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef]
- Lee, T.J.; Choi, Y.H.; Song, K.S. The PDZ motif peptide of ZO-1 attenuates Pseudomonas aeruginosa LPS-induced airway inflammation. Sci. Rep. 2020, 10, 19644. [Google Scholar] [CrossRef]
- Golovkine, G.; Faudry, E.; Bouillot, S.; Voulhoux, R.; Attree, I.; Huber, P. VE-cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog. 2014, 10, e1003939. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Blackwell, T.S.; Christman, J.W.; Prince, A.S. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 1209–1223. [Google Scholar] [CrossRef]
- Che, P.; Wagener, B.M.; Zhao, X.; Brandon, A.P.; Evans, C.A.; Cai, G.Q.; Zhao, R.; Xu, Z.X.; Han, X.; Pittet, J.F.; et al. Neuronal Wiskott-Aldrich syndrome protein regulates Pseudomonas aeruginosa-induced lung vascular permeability through the modulation of actin cytoskeletal dynamics. FASEB J. 2020, 34, 3305–3317. [Google Scholar] [CrossRef]
- Elsen, S.; Huber, P.; Bouillot, S.; Coute, Y.; Fournier, P.; Dubois, Y.; Timsit, J.F.; Maurin, M.; Attree, I. A type III secretion negative clinical strain of Pseudomonas aeruginosa employs a two-partner secreted exolysin to induce hemorrhagic pneumonia. Cell Host. Microbe 2014, 15, 164–176. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Resnati, M.; Raiteri, M.; Pigott, R.; Pisacane, A.; Houen, G.; Ruco, L.P.; Dejana, E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J. Cell Biol. 1992, 118, 1511–1522. [Google Scholar] [CrossRef]
- Breier, G.; Breviario, F.; Caveda, L.; Berthier, R.; Schnurch, H.; Gotsch, U.; Vestweber, D.; Risau, W.; Dejana, E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 1996, 87, 630–641. [Google Scholar] [CrossRef]
- Ganter, M.T.; Roux, J.; Miyazawa, B.; Howard, M.; Frank, J.A.; Su, G.; Sheppard, D.; Violette, S.M.; Weinreb, P.H.; Horan, G.S.; et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ. Res. 2008, 102, 804–812. [Google Scholar] [CrossRef]
- Su, G.; Hodnett, M.; Wu, N.; Atakilit, A.; Kosinski, C.; Godzich, M.; Huang, X.Z.; Kim, J.K.; Frank, J.A.; Matthay, M.A.; et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am. J. Respir. Cell Mol. Biol. 2007, 36, 377–386. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 2008, 1778, 794–809. [Google Scholar] [CrossRef]
- Belardi, B.; Hamkins-Indik, T.; Harris, A.R.; Kim, J.; Xu, K.; Fletcher, D.A. A Weak Link with Actin Organizes Tight Junctions to Control Epithelial Permeability. Dev. Cell 2020, 54, 792–804.e7. [Google Scholar] [CrossRef]
- Hansen, S.D.; Kwiatkowski, A.V.; Ouyang, C.Y.; Liu, H.; Pokutta, S.; Watkins, S.C.; Volkmann, N.; Hanein, D.; Weis, W.I.; Mullins, R.D.; et al. alphaE-catenin actin-binding domain alters actin filament conformation and regulates binding of nucleation and disassembly factors. Mol. Biol. Cell 2013, 24, 3710–3720. [Google Scholar] [CrossRef]
- Wagener, B.M.; Hu, M.; Zheng, A.; Zhao, X.; Che, P.; Brandon, A.; Anjum, N.; Snapper, S.; Creighton, J.; Guan, J.L.; et al. Neuronal Wiskott-Aldrich syndrome protein regulates TGF-beta1-mediated lung vascular permeability. FASEB J. 2016, 30, 2557–2569. [Google Scholar] [CrossRef]
- Blum, M.S.; Toninelli, E.; Anderson, J.M.; Balda, M.S.; Zhou, J.; O’Donnell, L.; Pardi, R.; Bender, J.R. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am. J. Physiol. 1997, 273, H286–H294. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Siflinger-Birnboim, A.; Del Vecchio, P.J.; Malik, A.B. Segmental differentiation of permeability, protein glycosylation, and morphology of cultured bovine lung vascular endothelium. Biochem. Biophys. Res. Commun. 1994, 199, 11–19. [Google Scholar] [CrossRef]
- Saguil, A.; Fargo, M. Acute respiratory distress syndrome: Diagnosis and management. Am. Fam Physician 2012, 85, 352–358. [Google Scholar]
- Stevens, T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc. Am. Thorac. Soc. 2011, 8, 453–457. [Google Scholar] [CrossRef]
- Horna, G.; Ruiz, J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021, 246, 126719. [Google Scholar] [CrossRef]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef]
- Williams McMackin, E.A.; Djapgne, L.; Corley, J.M.; Yahr, T.L. Fitting Pieces into the Puzzle of Pseudomonas aeruginosa Type III Secretion System Gene Expression. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef]
- Bohn, E.; Sonnabend, M.; Klein, K.; Autenrieth, I.B. Bacterial adhesion and host cell factors leading to effector protein injection by type III secretion system. Int. J. Med. Microbiol. 2019, 309, 344–350. [Google Scholar] [CrossRef]
- Rucks, E.A.; Fraylick, J.E.; Brandt, L.M.; Vincent, T.S.; Olson, J.C. Cell line differences in bacterially translocated ExoS ADP-ribosyltransferase substrate specificity. Microbiology (Reading) 2003, 149, 319–331. [Google Scholar] [CrossRef]
- Kurahashi, K.; Kajikawa, O.; Sawa, T.; Ohara, M.; Gropper, M.A.; Frank, D.W.; Martin, T.R.; Wiener-Kronish, J.P. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 1999, 104, 743–750. [Google Scholar] [CrossRef]
- Barbieri, J.T. Pseudomonas aeruginosa exoenzyme S, a bifunctional type-III secreted cytotoxin. Int. J. Med. Microbiol 2000, 290, 381–387. [Google Scholar] [CrossRef]
- Berube, B.J.; Rangel, S.M.; Hauser, A.R. Pseudomonas aeruginosa: Breaking down barriers. Curr. Genet. 2016, 62, 109–113. [Google Scholar] [CrossRef]
- Belmadi, N.; Wu, Y.; Touqui, L. Immuno-modulatory functions of the type-3 secretion system and impacts on the pulmonary host defense: A role for ExoS of Pseudomonas aeruginosa in cystic fibrosis. Toxicon 2018, 143, 68–73. [Google Scholar] [CrossRef]
- Goehring, U.M.; Schmidt, G.; Pederson, K.J.; Aktories, K.; Barbieri, J.T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 1999, 274, 36369–36372. [Google Scholar] [CrossRef]
- Pederson, K.J.; Barbieri, J.T. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas exoenzyme S is cytotoxic to eukaryotic cells. Mol. Microbiol. 1998, 30, 751–759. [Google Scholar] [CrossRef]
- Rangel, S.M.; Diaz, M.H.; Knoten, C.A.; Zhang, A.; Hauser, A.R. The Role of ExoS in Dissemination of Pseudomonas aeruginosa during Pneumonia. PLoS Pathog. 2015, 11, e1004945. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Sun, J.; Barbieri, J.T. ExoS Rho GTPase-activating protein activity stimulates reorganization of the actin cytoskeleton through Rho GTPase guanine nucleotide disassociation inhibitor. J. Biol. Chem. 2004, 279, 42936–42944. [Google Scholar] [CrossRef]
- Fu, H.; Coburn, J.; Collier, R.J. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc. Natl. Acad. Sci. USA 1993, 90, 2320–2324. [Google Scholar] [CrossRef]
- Barbieri, A.M.; Sha, Q.; Bette-Bobillo, P.; Stahl, P.D.; Vidal, M. ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect. Immun. 2001, 69, 5329–5334. [Google Scholar] [CrossRef]
- Fraylick, J.E.; La Rocque, J.R.; Vincent, T.S.; Olson, J.C. Independent and coordinate effects of ADP-ribosyltransferase and GTPase-activating activities of exoenzyme S on HT-29 epithelial cell function. Infect. Immun. 2001, 69, 5318–5328. [Google Scholar] [CrossRef][Green Version]
- Krall, R.; Schmidt, G.; Aktories, K.; Barbieri, J.T. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 2000, 68, 6066–6068. [Google Scholar] [CrossRef]
- Yahr, T.L.; Barbieri, J.T.; Frank, D.W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol. 1996, 178, 1412–1419. [Google Scholar] [CrossRef]
- Sun, J.; Barbieri, J.T. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 2003, 278, 32794–32800. [Google Scholar] [CrossRef]
- Barbieri, J.T.; Sun, J. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Garrity-Ryan, L.; Shafikhani, S.; Balachandran, P.; Nguyen, L.; Oza, J.; Jakobsen, T.; Sargent, J.; Fang, X.; Cordwell, S.; Matthay, M.A.; et al. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 2004, 72, 546–558. [Google Scholar] [CrossRef]
- Geiser, T.K.; Kazmierczak, B.I.; Garrity-Ryan, L.K.; Matthay, M.A.; Engel, J.N. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell Microbiol. 2001, 3, 223–236. [Google Scholar] [CrossRef]
- Feltman, H.; Schulert, G.; Khan, S.; Jain, M.; Peterson, L.; Hauser, A.R. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology (Reading) 2001, 147, 2659–2669. [Google Scholar] [CrossRef]
- Beckert, U.; Wolter, S.; Hartwig, C.; Bahre, H.; Kaever, V.; Ladant, D.; Frank, D.W.; Seifert, R. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem. Biophys. Res. Commun. 2014, 450, 870–874. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Alexeyev, M.; Pastukh, V.; Balczon, R.; Stevens, T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J. Biol. Chem. 2012, 287, 25407–25418. [Google Scholar] [CrossRef]
- Belyy, A.; Mechold, U.; Renault, L.; Ladant, D. ExoY, an actin-activated nucleotidyl cyclase toxin from P. aeruginosa: A minireview. Toxicon 2018, 149, 65–71. [Google Scholar] [CrossRef]
- Khanppnavar, B.; Datta, S. Crystal structure and substrate specificity of ExoY, a unique T3SS mediated secreted nucleotidyl cyclase toxin from Pseudomonas aeruginosa. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2090–2103. [Google Scholar] [CrossRef]
- Morrow, K.A.; Frank, D.W.; Balczon, R.; Stevens, T. The Pseudomonas aeruginosa Exoenzyme Y: A Promiscuous Nucleotidyl Cyclase Edema Factor and Virulence Determinant. Handb. Exp. Pharmacol. 2017, 238, 67–85. [Google Scholar] [CrossRef]
- Wagener, B.M.; Anjum, N.; Christiaans, S.C.; Banks, M.E.; Parker, J.C.; Threet, A.T.; Walker, R.R.; Isbell, K.D.; Moser, S.A.; Stevens, T.; et al. Exoenzyme Y Contributes to End-Organ Dysfunction Caused by Pseudomonas aeruginosa Pneumonia in Critically Ill Patients: An Exploratory Study. Toxins 2020, 12, 369. [Google Scholar] [CrossRef]
- Belyy, A.; Raoux-Barbot, D.; Saveanu, C.; Namane, A.; Ogryzko, V.; Worpenberg, L.; David, V.; Henriot, V.; Fellous, S.; Merrifield, C.; et al. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat. Commun. 2016, 7, 13582. [Google Scholar] [CrossRef]
- Belyy, A.; Santecchia, I.; Renault, L.; Bourigault, B.; Ladant, D.; Mechold, U. The extreme C terminus of the Pseudomonas aeruginosa effector ExoY is crucial for binding to its eukaryotic activator, F-actin. J. Biol. Chem. 2018, 293, 19785–19796. [Google Scholar] [CrossRef]
- Mancl, J.M.; Suarez, C.; Liang, W.G.; Kovar, D.R.; Tang, W.J. Pseudomonas aeruginosa exoenzyme Y directly bundles actin filaments. J. Biol. Chem. 2020, 295, 3506–3517. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Foulkes, D.M.; McLean, K.; Haneef, A.S.; Fernig, D.G.; Winstanley, C.; Berry, N.; Kaye, S.B. Pseudomonas aeruginosa Toxin ExoU as a Therapeutic Target in the Treatment of Bacterial Infections. Microorganisms 2019, 7, 707. [Google Scholar] [CrossRef]
- Sawa, T.; Hamaoka, S.; Kinoshita, M.; Kainuma, A.; Naito, Y.; Akiyama, K.; Kato, H. Pseudomonas aeruginosa Type III Secretory Toxin ExoU and Its Predicted Homologs. Toxins 2016, 8, 307. [Google Scholar] [CrossRef]
- Finck-Barbancon, V.; Goranson, J.; Zhu, L.; Sawa, T.; Wiener-Kronish, J.P.; Fleiszig, S.M.; Wu, C.; Mende-Mueller, L.; Frank, D.W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 1997, 25, 547–557. [Google Scholar] [CrossRef]
- Juan, C.; Pena, C.; Oliver, A. Host and Pathogen Biomarkers for Severe Pseudomonas aeruginosa Infections. J. Infect. Dis. 2017, 215, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Vincent Deruelle, S.B. Viviana Job, Emmanuel Taillebourg, Marie-Odile Fauvarque, Ina Attrée, Philippe Huber. The bacterial toxin ExoU requires a host trafficking chaperone for transportation and to induce necrosis. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Sato, H.; Frank, D.W. Intoxication of host cells by the T3SS phospholipase ExoU: PI(4,5)P2-associated, cytoskeletal collapse and late phase membrane blebbing. PLoS ONE 2014, 9, e103127. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.P.; Lindsey, A.S.; Housley, N.A.; Ochoa, C.R.; Zhou, C.; Toba, M.; Oka, M.; Annamdevula, N.S.; Fitzgerald, M.S.; Frank, D.W.; et al. In the absence of effector proteins, the Pseudomonas aeruginosa type three secretion system needle tip complex contributes to lung injury and systemic inflammatory responses. PLoS ONE 2013, 8, e81792. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, W.; Shao, X.; Zhang, W.; Deng, X. Signal transduction schemes in Pseudomonas syringae. Comput. Struct. Biotechnol. J. 2020, 18, 3415–3424. [Google Scholar] [CrossRef]
- Janda, J.M.; Bottone, E.J. Pseudomonas aeruginosa enzyme profiling: Predictor of potential invasiveness and use as an epidemiological tool. J. Clin. Microbiol. 1981, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bleves, S.; Viarre, V.; Salacha, R.; Michel, G.P.; Filloux, A.; Voulhoux, R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int. J. Med. Microbiol. 2010, 300, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.E.; Cryz, S.J.; Friedman, R.L.; Iglewski, B.H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect. Immun. 1982, 36, 1223–1228. [Google Scholar] [CrossRef]
- Azghani, A.O.; Connelly, J.C.; Peterson, B.T.; Gray, L.D.; Collins, M.L.; Johnson, A.R. Effects of Pseudomonas aeruginosa elastase on alveolar epithelial permeability in guinea pigs. Infect. Immun. 1990, 58, 433–438. [Google Scholar] [CrossRef]
- Peters, J.E.; Galloway, D.R. Purification and characterization of an active fragment of the LasA protein from Pseudomonas aeruginosa: Enhancement of elastase activity. J. Bacteriol. 1990, 172, 2236–2240. [Google Scholar] [CrossRef]
- Everett, M.J.; Davies, D.T. Pseudomonas aeruginosa elastase (LasB) as a therapeutic target. Drug Discov. Today 2021, 26, 2108–2123. [Google Scholar] [CrossRef]
- Azghani, A.O.; Miller, E.J.; Peterson, B.T. Virulence factors from Pseudomonas aeruginosa increase lung epithelial permeability. Lung 2000, 178, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ramezanpour, M.; Fong, S.A.; Cooksley, C.; Murphy, J.; Suzuki, M.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Pseudomonas aeruginosa Exoprotein-Induced Barrier Disruption Correlates With Elastase Activity and Marks Chronic Rhinosinusitis Severity. Front. Cell Infect. Microbiol. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Thomas, L.K.; Azghani, A.O. Inhibition of protein kinase C attenuates Pseudomonas aeruginosa elastase-induced epithelial barrier disruption. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1263–1271. [Google Scholar] [CrossRef]
- Saulnier, J.M.; Curtil, F.M.; Duclos, M.C.; Wallach, J.M. Elastolytic activity of Pseudomonas aeruginosa elastase. Biochim. Biophys. Acta 1989, 995, 285–290. [Google Scholar] [CrossRef]
- Bejarano, P.A.; Langeveld, J.P.; Hudson, B.G.; Noelken, M.E. Degradation of basement membranes by Pseudomonas aeruginosa elastase. Infect. Immun. 1989, 57, 3783–3787. [Google Scholar] [CrossRef]
- Heck, L.W.; Morihara, K.; Abrahamson, D.R. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect. Immun. 1986, 54, 149–153. [Google Scholar] [CrossRef]
- Heck, L.W.; Morihara, K.; McRae, W.B.; Miller, E.J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect. Immun. 1986, 51, 115–118. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef]
- Heggers, J.P.; Haydon, S.; Ko, F.; Hayward, P.G.; Carp, S.; Robson, M.C. Pseudomonas aeruginosa exotoxin A: Its role in retardation of wound healing: The 1992 Lindberg Award. J. Burn Care Rehabil. 1992, 13, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Heiniger, R.W.; Winther-Larsen, H.C.; Pickles, R.J.; Koomey, M.; Wolfgang, M.C. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 2010, 12, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Nishizawa, H.; Kitao, S.; Deguchi, S.; Nakamura, T.; Fujimoto, A.; Shikata, M.; Gotoh, N. Pseudomonas aeruginosa injects type III effector ExoS into epithelial cells through the function of type IV pili. FEBS Lett. 2015, 589, 890–896. [Google Scholar] [CrossRef]
- Bucior, I.; Pielage, J.F.; Engel, J.N. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012, 8, e1002616. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef]
- Eutamene, H.; Theodorou, V.; Schmidlin, F.; Tondereau, V.; Garcia-Villar, R.; Salvador-Cartier, C.; Chovet, M.; Bertrand, C.; Bueno, L. LPS-induced lung inflammation is linked to increased epithelial permeability: Role of MLCK. Eur. Respir. J. 2005, 25, 789–796. [Google Scholar] [CrossRef]
- Schuster, M.; Greenberg, E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006, 296, 73–81. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: A chronicle through the perspective of quorum sensing. Environ. Microbiol. 2021. [Google Scholar] [CrossRef]
- Kariminik, A.; Baseri-Salehi, M.; Kheirkhah, B. Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article. Immunol. Lett. 2017, 190, 1–6. [Google Scholar] [CrossRef]
- Pearson, J.P.; Gray, K.M.; Passador, L.; Tucker, K.D.; Eberhard, A.; Iglewski, B.H.; Greenberg, E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 1994, 91, 197–201. [Google Scholar] [CrossRef]
- Guo, J.; Yoshida, K.; Ikegame, M.; Okamura, H. Quorum sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone: An all-rounder in mammalian cell modification. J. Oral. Biosci. 2020, 62, 16–29. [Google Scholar] [CrossRef]
- Vikstrom, E.; Tafazoli, F.; Magnusson, K.E. Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett. 2006, 580, 6921–6928. [Google Scholar] [CrossRef]
- Vikstrom, E.; Bui, L.; Konradsson, P.; Magnusson, K.E. Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur. J. Cell Biol. 2010, 89, 584–597. [Google Scholar] [CrossRef]
- Eum, S.Y.; Jaraki, D.; Bertrand, L.; Andras, I.E.; Toborek, M. Disruption of epithelial barrier by quorum-sensing N-3-(oxododecanoyl)-homoserine lactone is mediated by matrix metalloproteinases. Am. J. Physiol. Gastrointest Liver Physiol. 2014, 306, G992–G1001. [Google Scholar] [CrossRef]
- Nakagami, G.; Minematsu, T.; Asada, M.; Nagase, T.; Akase, T.; Huang, L.; Morohoshi, T.; Ikeda, T.; Ohta, Y.; Sanada, H. The Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl) homoserine lactone can accelerate cutaneous wound healing through myofibroblast differentiation in rats. FEMS Immunol. Med. Microbiol. 2011, 62, 157–163. [Google Scholar] [CrossRef]
- Losa, D.; Kohler, T.; Bacchetta, M.; Saab, J.B.; Frieden, M.; van Delden, C.; Chanson, M. Airway Epithelial Cell Integrity Protects from Cytotoxicity of Pseudomonas aeruginosa Quorum-Sensing Signals. Am. J. Respir. Cell Mol. Biol. 2015, 53, 265–275. [Google Scholar] [CrossRef]
- Qiao, J.; Cao, Y.; Zabaleta, J.; Yang, L.; Dai, L.; Qin, Z. Regulation of Virus-Associated Lymphoma Growth and Gene Expression by Bacterial Quorum-Sensing Molecules. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Jensen, P.O.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar] [CrossRef]
- Soberon-Chavez, G.; Gonzalez-Valdez, A.; Soto-Aceves, M.P.; Cocotl-Yanez, M. Rhamnolipids produced by Pseudomonas: From molecular genetics to the market. Microb. Biotechnol. 2021, 14, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Steel, D.M.; Wilson, R.; Cole, P.J.; Alton, E.W.; Geddes, D.M. Effects of purified Pseudomonas rhamnolipids on bioelectric properties of sheep tracheal epithelium. Exp. Lung Res. 1993, 19, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, E.; Pantazaki, A.A.; Daniilidou, M.; Tsolaki, M. Rhamnolipids, Microbial Virulence Factors, in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 209–222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).