Microcystin-Induced Immunotoxicity in Fishes: A Scoping Review
Abstract
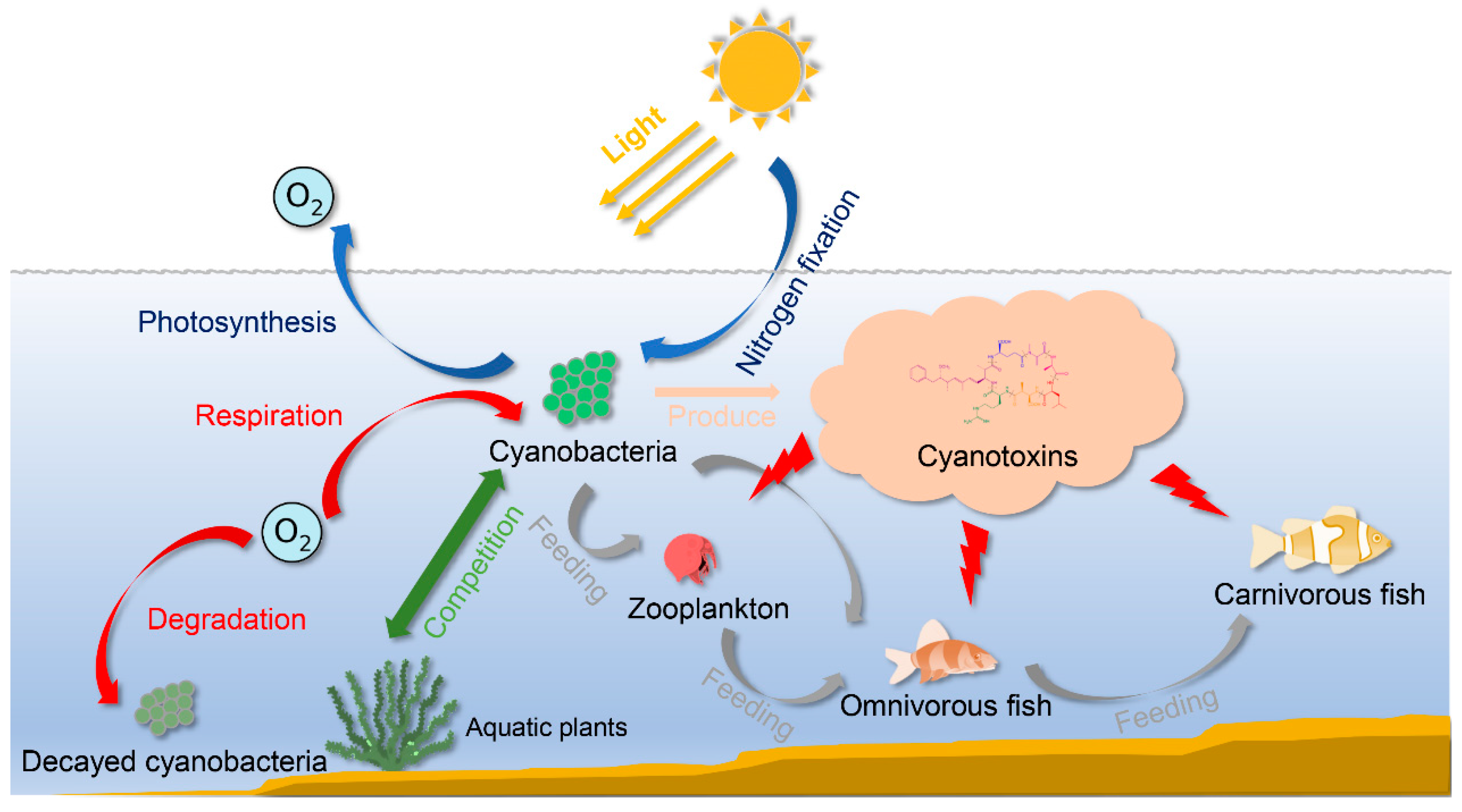
1. Introduction
2. Immune System of Fish
3. Immunotoxicity of MCs on Fish
3.1. In Vivo Studies
3.1.1. Cyanobacteria Cells
3.1.2. Cyanobacteria Extracts
3.1.3. Pure Microcystins
3.2. In Vitro Studies
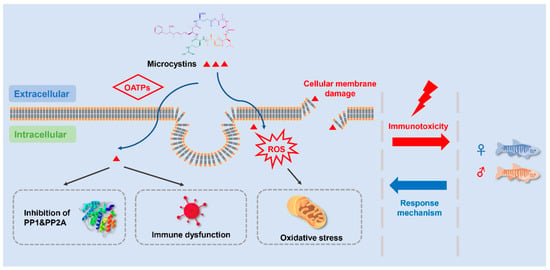
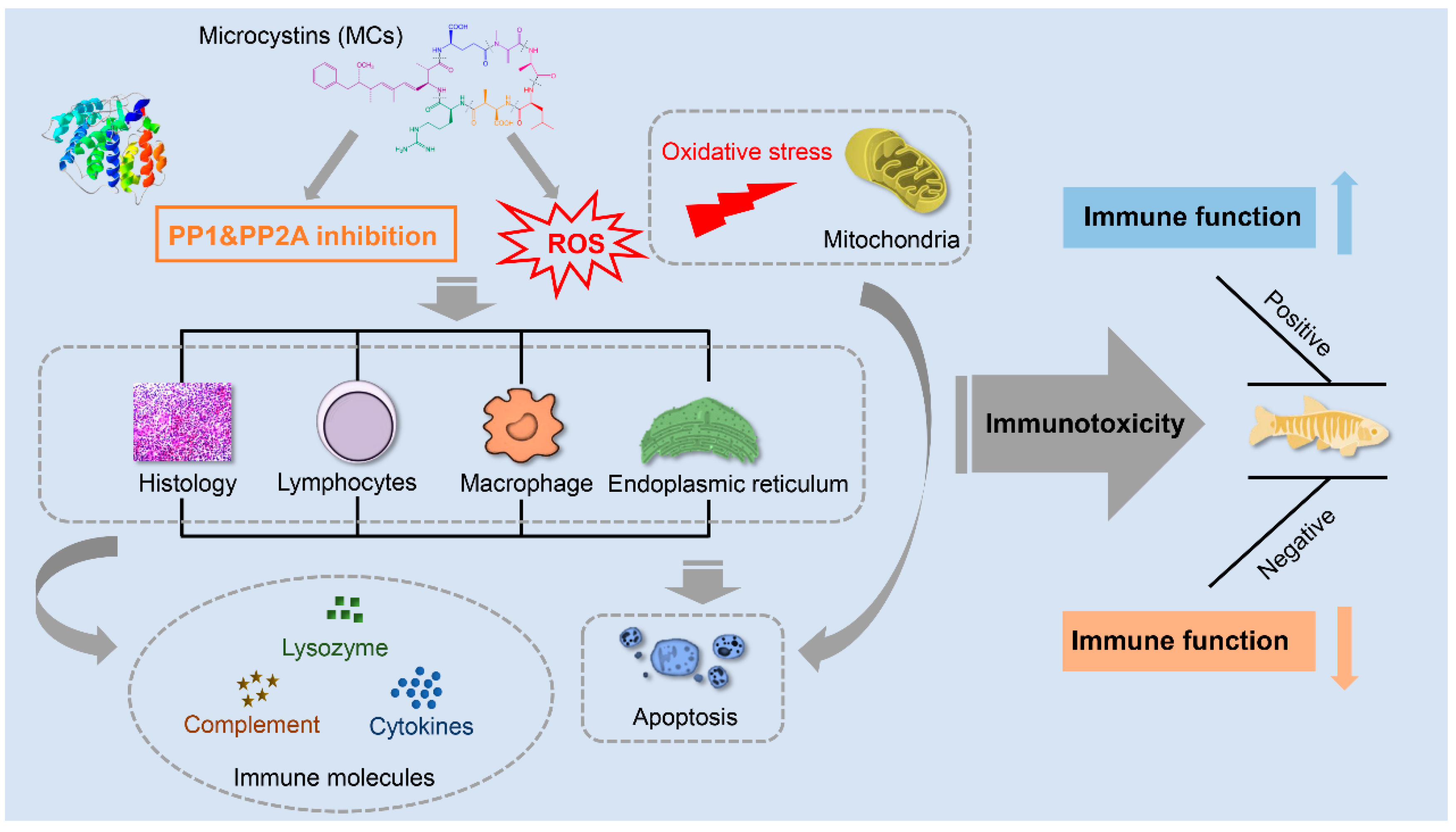
4. Potential Mechanism of MC-Induced Immunotoxicity
4.1. Adsorption and Accumulation of MCs
4.2. Inhibition of PP1 and PP2A
4.3. Oxidative Stress
4.4. Immune Cell Damages
4.5. Inflammation
4.6. Apoptosis
5. Current Research Gaps and Future Directions
5.1. Adaptive Immunity
5.2. Multi-Omics Study
5.3. Hormesis Phenomenon
5.4. Variants Other than MC-LR
5.5. Microbial Pathogens in Aquatic Ecosystems
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, B.E.; de Vos, J.M.; Antonelli, A.; Bagheri, H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Aaad. Sci. USA 2013, 110, 1791–1796. [Google Scholar] [CrossRef]
- Zehr, J.P. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011, 19, 162–173. [Google Scholar] [CrossRef]
- Osburn, F.S.; Wagner, N.D.; Scott, J.T. Biological stoichiometry and growth dynamics of a diazotrophic cyanobacteria in nitrogen sufficient and deficient conditions. Harmful Algae 2021, 103, 102011. [Google Scholar] [CrossRef]
- Backer, L.C. Cyanobacterial harmful algal blooms (CyanoHABs): Developing a public health response. Lake Reserv. Manag. 2002, 18, 20–31. [Google Scholar] [CrossRef]
- Li, R.; Hua, P.; Zhang, J.; Krebs, P. Effect of anthropogenic activities on the occurrence of polycyclic aromatic hydrocarbons in aquatic suspended particulate matter: Evidence from Rhine and Elbe Rivers. Water Res. 2020, 179, 115901. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Havens, K.E. Cyanobacteria blooms: Effects on aquatic ecosystems. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: New York, NY, USA, 2008; pp. 733–747. [Google Scholar]
- Young, N.; Sharpe, R.A.; Barciela, R.; Nichols, G.; Davidson, K.; Berdalet, E.; Fleming, L.E. Marine harmful algal blooms and human health: A systematic scoping review. Harmful Algae 2020, 98, 101901. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.D.; Beasley, V.R. One health and cyanobacteria in freshwater systems: Animal illnesses and deaths are sentinel events for human health risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of microcystins and nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Hoboken, NJ, USA, 2016; pp. 526–537. [Google Scholar]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, T.; Wang, Y.-T.; He, J.; Zhao, X.; Wang, Y.-K.; Giesy, J.P.; Chen, F.; Chen, Y.; Tuo, X.; et al. Effects of acute exposure to microcystins on hypothalamic-pituitary-adrenal (HPA), -gonad (HPG) and -thyroid (HPT) axes of female rats. Sci. Total Environ. 2021, 778, 145196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Ufelmann, H.; Krüger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef]
- Fan, H.; Cai, Y.; Xie, P.; Xiao, W.; Chen, J.; Ji, W.; Zhao, S. Microcystin-LR stabilizes c-myc protein by inhibiting protein phosphatase 2A in HEK293 cells. Toxicology 2014, 319, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020, 174, 115638. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality, 2nd ed.; Addendum to vol. 2; Health Criteria and Other Supporting Information; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Mohamed, Z.A. Breakthrough of Oscillatoria limnetica and microcystin toxins into drinking water treatment plants–examples from the Nile River, Egypt. Water SA 2016, 42, 161–165. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Carmichael, W.W.; Hussein, A.A. Estimation of microcystins in the freshwater fish Oreochromis niloticus in an Egyptian fish farm containing a Microcystis bloom. Environ. Toxicol. 2003, 18, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Sci. World. J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.A.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region, China. Environ. Health Persp. 2011, 119, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Matsushima, R.; Watanabe, M.F.; Harada, K.I.; Ichihara, A.; Carmichael, W.W.; Fujiki, H. Inhibition of protein phosphatases by microcystis and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. 1990, 116, 609–614. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of microcystin-induced cytotoxicity and apoptosis. Mini-Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm Filho, D. Fish antioxidant defenses--a comparative approach. Braz. J. Med. Biol. Res. 1996, 29, 1735–1742. [Google Scholar] [PubMed]
- Ding, X.S.; Li, X.Y.; Duan, H.Y.; Chung, I.K.; Lee, J.A. Toxic effects of Microcystis cell extracts on the reproductive system of male mice. Toxicon 2006, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hou, J.; Guo, H.; Qiu, Y.; Li, L.; Li, D.; Tang, R. Dualistic immunomodulation of sub-chronic microcystin-LR exposure on the innate-immune defense system in male zebrafish. Chemosphere 2017, 183, 315–322. [Google Scholar] [CrossRef]
- Wang, L.; Lin, W.; Zha, Q.; Guo, H.; Zhang, D.; Yang, L.; Li, L.; Li, D.; Tang, R. Persistent exposure to environmental levels of microcystin-LR disturbs cortisol production via hypothalamic-pituitary-interrenal (HPI) axis and subsequently liver glucose metabolism in adult male zebrafish (Danio rerio). Toxins 2020, 12, 282. [Google Scholar] [CrossRef]
- Rymuszka, A.; Sierosławska, A.; Bownik, A.; Skowroński, T. In vitro effects of pure microcystin-LR on the lymphocyte proliferation in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun. 2007, 22, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Sun, B.J.; Nie, P. Ultrastructural alteration of lymphocytes in spleen and pronephros of grass carp (Ctenopharyngodon idella) experimentally exposed to microcystin-LR. Aquaculture 2008, 280, 270–275. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Li, G.; Yan, W.; Qiao, Q.; Chen, J.; Cai, F.; He, Y.; Zhang, X. Global effects of subchronic treatment of microcystin-LR on rat splenetic protein levels. J. Proteomics 2012, 77, 383–393. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Warr, G.W. The immunoglobulin genes of fish. Dev. Comp. Immunol. 1995, 19, 1–12. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of fish immunity and the role of β-glucan in immune responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger-and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflamm. 2015, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef]
- Alejo, A.; Tafalla, C. Chemokines in teleost fish species. Dev. Comp. Immunol. 2011, 35, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M. ER Stress, UPR Activation and the Inflammatory Response to Viral Infection. Viruses 2021, 13, 798. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortes, A. Ontogeny of the immune system of fish. Fish Shellfish Immun. 2006, 20, 126–136. [Google Scholar] [CrossRef]
- Silva, A.G.; Martinez, C.B. Morphological changes in the kidney of a fish living in an urban stream. Environ. Toxicol. Phar. 2007, 23, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, S.; Glenney, G.W.; Welch, T.J.; Silverstein, J.T.; Wiens, G.D. Spleen size predicts resistance of rainbow trout to Flavobacterium psychrophilum challenge. J. Immunol. 2008, 180, 4156–4165. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Trede, N.S. Fish immunology. Curr. Biol. 2009, 19, 678–682. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Partula, S. Surface markers of fish T-cells. Fish Shellfish Immun. 1999, 9, 241–257. [Google Scholar] [CrossRef]
- Scapigliati, G.; Romano, N.; Abelli, L.; Meloni, S.; Ficca, A.G.; Buonocore, F.; Secombes, C.J. Immunopurification of T-cells from sea bass Dicentrarchus labrax (L.). Fish Shellfish Immun. 2000, 10, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Zwollo, P.; Cole, S.; Bromage, E.; Kaattari, S. B cell heterogeneity in the teleost kidney: Evidence for a maturation gradient from anterior to posterior kidney. J. Immunol. 2005, 174, 6608–6616. [Google Scholar] [CrossRef]
- Doggett, T.A.; Wrathmell, A.B.; Harris, J.E. A cytochemical and light microscopical study of the peripheral blood leucocytes of Oreochromis mossambicus, Cichlidae. J Fish Biol. 1987, 31, 147–153. [Google Scholar] [CrossRef]
- Secombes, C.J.; Mannig, M.J. Comparative studies on the immune system of fishes and amphibians: Antigen localization in the carp Cyprinus carpio L. J. Fish Dis. 1980, 3, 399–412. [Google Scholar] [CrossRef]
- Evans, D.L.; Taylor, S.L.; Leary III, J.H.; Bishop, G.R.; Eldar, A.; Jaso-Friedmann, L. In vivo activation of tilapia nonspecific cytotoxic cells by Streptococcus iniaeand amplification with apoptosis regulatory factor (s). Fish Shellfish Immun. 2000, 10, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immun. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immun. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Ellison, R.; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Kong, X.; Wang, S.; Jiang, H.; Nie, G.; Li, X. Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat. Toxicol. 2012, 120, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef]
- Holland, M.C.H.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immun. 2002, 12, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Gasque, P. Complement: A unique innate immune sensor for danger signals. Mol. Immunol. 2004, 41, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Tsujikura, M.; Ichiki, S.; Vo, T.K.; Somamoto, T. The complement system in teleost fish: Progress of post-homolog-hunting researches. Dev. Comp. Immunol. 2011, 35, 1296–1308. [Google Scholar] [CrossRef]
- Atencio, L.; Moreno, I.; Jos, Á.; Prieto, A.I.; Moyano, R.; Blanco, A.; Cameán, A.M. Effects of dietary selenium on the oxidative stress and pathological changes in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Toxicon 2009, 53, 269–282. [Google Scholar] [CrossRef]
- Qiao, Q.; Liang, H.; Zhang, X. Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 2013, 90, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Song, T.; Wang, L.; Jiang, L.; Zhou, Q.; Wang, W.; Liu, L.; Yang, P.; Zhang, X. Effects of dietary toxic cyanobacteria and ammonia exposure on immune function of blunt snout bream (Megalabrama amblycephala). Fish Shellfish Immun. 2018, 78, 383–391. [Google Scholar] [CrossRef]
- Kopp, R.; Heteša, J. Changes of haematological indices of juvenile carp (Cyprinus carpio L.) under the influence of natural populations of cyanobacterial water blooms. Acta Vet. Brno 2000, 69, 131–137. [Google Scholar] [CrossRef]
- Kopp, R.; Palíková, M.; Navrátil, S.; Kubíček, Z.; Ziková, A.; Mareš, J. Modulation of biochemical and haematological indices of silver carp (Hypophthalmichthys molitrix Val.) exposed to toxic cyanobacterial water bloom. Acta Vet. Brno 2010, 79, 135–146. [Google Scholar] [CrossRef]
- Li, L.; Xie, P.; Chen, J. Biochemical and ultrastructural changes of the liver and kidney of the phytoplanktivorous silver carp feeding naturally on toxic Microcystis blooms in Taihu Lake, China. Toxicon 2007, 49, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, P.; Wang, W.; Li, D.; Shi, Z. Plasma biochemical responses of the omnivorous crucian carp (Carassius auratus) to crude cyanobacterial extracts. Fish Physiol. Biochem. 2008, 34, 323–329. [Google Scholar] [CrossRef]
- Rymuszka, A.; Adaszek, Ł. Cytotoxic effects and changes in cytokine gene expression induced by microcystin-containing extract in fish immune cells–An in vitro and in vivo study. Fish Shellfish Immun. 2013, 34, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Palíková, M.; Navratil, S.; Krejčí, R.; Štěrba, F.; Tichý, F.; Kubala, L.; Blaha, L. Outcomes of repeated exposure of the carp (Cyprinus carpio L.) to cyanobacteria extract. ACTA Vet. Brno 2004, 73, 259–265. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Li, J.; Meng, F.; Yao, L. Hepatotoxicity and immunotoxicity of MC-LR on silver carp. Ecotox. Environ. Safe. 2019, 169, 28–32. [Google Scholar] [CrossRef]
- Chen, X.; Guo, G.; Sun, L.; Yang, Q.; Wang, G.; Zhang, D. Modulatory role of L-carnitine against microcystin-LR-induced immunotoxicity and oxidative stress in common carp. Fish Physiol. Biochem. 2017, 43, 1081–1093. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Xie, P.; Li, G.; Hao, L.; Xiong, Q. Identification and expression profiles of IL-8 in bighead carp (Aristichthys nobilis) in response to microcystin-LR. Arch. Environ. Con. Tox. 2013, 65, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, Y.; Zhong, S.; Wu, H.; Ruan, J.; Liu, M.; Zhou, Q.; Zhong, Q. Transcriptome analysis of grass carp provides insights into the immune-related genes and pathways in response to MC-LR induction. Aquaculture 2018, 488, 207–216. [Google Scholar] [CrossRef]
- Okogwu, O.I.; Xie, P.; Zhao, Y.; Fan, H. Organ-dependent response in antioxidants, myoglobin and neuroglobin in goldfish (Carassius auratus) exposed to MC-RR under varying oxygen level. Chemosphere 2014, 112, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.I.; Jos, Á.; Pichardo, S.; Moreno, I.; Cameán, A.M. Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat. Toxicol. 2006, 77, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, B.; Wu, H.; Nie, P. Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio). Aquaculture 2009, 289, 154–160. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, L.; Li, J.; Chen, Y.; Jin, J.; Atufa, K.; Zhang, X. Pathological damage and immunomodulatory effects of zebrafish exposed to microcystin-LR. Toxicon 2016, 118, 13–20. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Waterborne microcystin-LR exposure induced chronic inflammatory response via MyD88-dependent toll-like receptor signaling pathway in male zebrafish. Sci. Total Environ. 2020, 702, 134969. [Google Scholar] [CrossRef]
- Falfushynska, H.; Horyn, O.; Osypenko, I.; Rzymski, P.; Wejnerowski, Ł.; Dziuba, M.K.; Sokolova, I.M. Multibiomarker-based assessment of toxicity of central European strains of filamentous cyanobacteria Aphanizomenon gracile and Raphidiopsis raciborskii to zebrafish Danio rerio. Water Res. 2021, 194, 116923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Chen, Y. Sensitive apoptosis induced by microcystins in the crucian carp (Carassius auratus) lymphocytes in vitro. Toxicol. Vitr. 2006, 20, 560–566. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, Y.; Zhu, Y. Influence of intracellular Ca2+, mitochondria membrane potential, reactive oxygen species, and intracellular ATP on the mechanism of microcystin-LR induced apoptosis in Carassius auratus lymphocytes in vitro. Environ. Toxicol. 2007, 22, 559–564. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, Y.; Zhu, Y. Microcystin-RR induces apoptosis in fish lymphocytes by generating reactive oxygen species and causing mitochondrial damage. Fish Physiol. Biochem. 2008, 34, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Y.; Fang, W.; Wang, D. Regulatory effect of quercetin on hazardous microcystin-LR-induced apoptosis of Carassius auratus lymphocytes in vitro. Fish Shellfish Immun. 2014, 37, 278–285. [Google Scholar] [CrossRef]
- Sierosławska, A.; Rymuszka, A.; Bownik, A.; Skowroński, T. The influence of microcystin-LR on fish phagocytic cells. Hum. Exp. Toxicol. 2007, 26, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Liu, W.; Qiao, Q.; Wu, K.; Wen, J.; Huang, C.; Tang, R.; Zhang, X. Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat. Toxicol. 2015, 165, 41–50. [Google Scholar] [CrossRef]
- Rymuszka, A.; Sierosławska, A.; Bownik, A.; Skowroński, T. Microcystin-LR modulates selected immune parameters and induces necrosis/apoptosis of carp leucocytes. Environ. Toxicol. Chem. 2010, 29, 569–574. [Google Scholar] [CrossRef]
- Rymuszka, A. Microcystin-LR induces cytotoxicity and affects carp immune cells by impairment of their phagocytosis and the organization of the cytoskeleton. J. Appl. Toxicol. 2013, 33, 1294–1302. [Google Scholar] [CrossRef]
- Rymuszka, A.; Adaszek, Ł. Pro-and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress—An in vitro study. Fish Shellfish Immun. 2012, 33, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhang, X.Z.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Toivola, D.; Meriluoto, J.A.O.; Karaki, H.; Han, Y.G.; Hartshorne, D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem. Bioph. Res. Co. 1990, 173, 1347–1353. [Google Scholar] [CrossRef]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. 2006, 25, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharm. 2005, 203, 257–263. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.P.; Davis, M.A.; Ryan, T.P.; Searfoss, G.H.; Hooser, S.B. Hepatic gene expression changes in mice associated with prolonged sublethal microcystin exposure. Toxicol. Pathol. 2007, 35, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Steiner, K.; Zimmermann, L.; Hagenbuch, B.; Dietrich, D. Zebrafish Oatp-mediated transport of microcystin congeners. Arch. Toxicol. 2016, 90, 1129–1139. [Google Scholar] [CrossRef]
- Sontag, E. Protein phosphatase 2A: The Trojan Horse of cellular signaling. Cell. Signal. 2011, 13, 7–16. [Google Scholar] [CrossRef]
- Lechward, K.; Awotunde, O.S.; Swiatek, W.; Muszyńska, G. Protein phosphatase 2A: Variety of forms and diversity of functions. Acta Biochim. Pol. 2001, 48, 921–933. [Google Scholar] [CrossRef]
- Liang, J.; Li, T.; Zhang, Y.L.; Guo, Z.L.; Xu, L.H. Effect of microcystin-LR on protein phosphatase 2A and its function in human amniotic epithelial cells. J. Zhejiang Univ-SC B 2011, 12, 951–960. [Google Scholar] [CrossRef]
- Meng, G.; Sun, Y.; Fu, W.; Guo, Z.; Xu, L. Microcystin-LR induces cytoskeleton system reorganization through hyperphosphorylation of tau and HSP27 via PP2A inhibition and subsequent activation of the p38 MAPK signaling pathway in neuroendocrine (PC12) cells. Toxicology 2011, 290, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tu, W.W.; Lazar, L.; Chen, D.N.; Zhao, J.S.; Xu, J. Hyperphosphorylation of microfilament-associated proteins is involved in microcystin-LR-induced toxicity in HL7702 cells. Environ. Toxicol. 2015, 30, 981–988. [Google Scholar] [CrossRef]
- Amado, L.L.; Monserrat, J.M. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ. Int. 2010, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Gong, Z.; Hande, M.P.; Dionysiou, D.D.; Armah, A.; Balasubramanian, R. Biochemical response of diverse organs in adult Danio rerio (zebrafish) exposed to sub-lethal concentrations of microcystin-LR and microcystin-RR: A balneation study. Aquat. Toxicol. 2012, 109, 1–10. [Google Scholar] [CrossRef]
- Lin, W.; Hou, J.; Guo, H.; Li, L.; Wang, L.; Zhang, D.; Li, D.; Tang, R. The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish. Environ. Pollut. 2018, 235, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Wu, D.; Santos, M.S.; Hayek, M.G. Antioxidants and immune response in aged persons: Overview of present evidence. Am. J. Clin. Nutr. 1995, 62, 1462S–1476S. [Google Scholar] [CrossRef]
- Baier-Bitterlich, G.; Fuchs, D.; Wachter, H. Chronic immune stimulation, oxidative stress, and apoptosis in HIV infection. Biochem. Pharmacol. 1997, 53, 755–763. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Li, D.; Tang, R. Nitrite enhances MC-LR-induced changes on splenic oxidation resistance and innate immunity in male zebrafish. Toxins 2018, 10, 512. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. BBA-Gen. Subjects 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Wang, H.; Ji, Y.L.; Ning, H.; Wang, S.; Zhang, C.; Lu, J.; Duan, H.; Xu, D. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol. Sci. 2008, 103, 149–157. [Google Scholar] [CrossRef]
- Fischer, W.J.; Dietrich, D.R. Pathological and biochemical characterization of microcystin-induced hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharm. 2000, 164, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Oral toxicity of the microcystin-containing cyanobacterium Planktothrix rubescens in European whitefish (Coregonus lavaretus). Aquat. Toxicol. 2006, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Djediat, C.; Malécot, M.; de Luze, A.; Bernard, C.; Puiseux-Dao, S.; Edery, M. Localization of microcystin-LR in medaka fish tissues after cyanotoxin gavage. Toxicon 2010, 55, 531–535. [Google Scholar] [CrossRef]
- Neumann, N.F.; Stafford, J.L.; Barreda, D.; Ainsworth, A.J.; Belosevic, M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev. Comp. Immunol. 2001, 25, 807–825. [Google Scholar] [CrossRef]
- Palíková, M.; Kovářů, F.; Navratil, S.; Kubala, L.; Pešák, S.; Vajcová, V. The effect of pure microcystin LR and biomass of blue-green algae on selected immunological indices of carp (Cyprinus carpio L.) and silver carp (Hypophthalmichthys molitrix Val.). ACTA Vet. Brno 1998, 67, 265–272. [Google Scholar] [CrossRef]
- Salazar-Mather, T.P.; Hokeness, K.L. Cytokine and chemokine networks: Pathways to antiviral defense. In Chemokines and Viral Infection; Springer: Berlin/Heidelberg, Germany, 2006; pp. 29–46. [Google Scholar]
- Savan, R.; Sakai, M. Genomics of fish cytokines. Comp. Biochem. Phys. D 2006, 1, 89–101. [Google Scholar] [CrossRef]
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immun. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. The interleukins of fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef]
- Wang, T.; Huang, W.; Costa, M.M.; Secombes, C.J. The gamma-chain cytokine/receptor system in fish: More ligands and receptors. Fish Shellfish Immun. 2011, 31, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Pelegrín, P.; Chaves-Pozo, E.; Mulero, V.; Meseguer, J. Production and mechanism of secretion of interleukin-1β from the marine fish gilthead seabream. Dev. Comp. Immunol. 2004, 28, 229–237. [Google Scholar] [CrossRef]
- Angosto, D.; López-Castejón, G.; López-Muñoz, A.; Sepulcre, M.P.; Arizcun, M.; Meseguer, J.; Mulero, V. Evolution of inflammasome functions in vertebrates: Inflammasome and caspase-1 trigger fish macrophage cell death but are dispensable for the processing of IL-1β. Innate Immun. 2012, 18, 815–824. [Google Scholar] [CrossRef]
- Wei, L.; Sun, B.; Chang, M.; Liu, Y.; Nie, P. Effects of cyanobacterial toxin microcystin-LR on the transcription levels of immune-related genes in grass carp Ctenopharyngodon idella. Environ. Biol. Fish. 2009, 85, 231–238. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Yu, F.; Xiang, Z.; Li, J.; Thorpe, K.L.; Yu, Z. Characteristic and functional analysis of toll-like receptors (TLRs) in the lophotrocozoan, Crassostrea gigas, reveals ancient origin of TLR-mediated innate immunity. PLoS ONE 2013, 8, e76464. [Google Scholar]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution. Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Saraste, A. Morphologic criteria and detection of apoptosis. Herz 1999, 24, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Acilan, C.; Yilmaz, Y. Apoptosis: Why and how does it occur in biology? Cell Biochem. Funct. 2011, 29, 468–480. [Google Scholar] [CrossRef]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes. Hepatology 2000, 32, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Q.; Cui, J.; Yang, W.; Shi, Q.; Hua, Z.; Ji, J.; Shen, P. Induction of apoptosis in mouse liver by microcystin-LR: A combined transcriptomic, proteomic, and simulation strategy. Mol. Cell. Proteomics 2005, 4, 958–974. [Google Scholar] [CrossRef]
- Qin, W.; Xu, L.; Zhang, X.; Wang, Y.; Meng, X.; Miao, A.; Yang, L. Endoplasmic reticulum stress in murine liver and kidney exposed to microcystin-LR. Toxicon 2010, 56, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, J.T. Biomarkers of immunotoxicity in fish and other non-mammalian sentinel species: Predictive value for mammals? Toxicology 1998, 129, 63–71. [Google Scholar] [CrossRef]
- Secombes, C.J.; Bird, S.; Zou, J. Adaptive immunity in teleosts: Cellular immunity. Dev. Biol. 2005, 121, 25–32. [Google Scholar]
- Dixon, B.; Stet, R.J.M. The relationship between major histocompatibility receptors and innate immunity in teleost fish. Dev. Comp. Immunol. 2001, 25, 683–699. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Balasubramanian, R. Toxicological evaluation of microcystins in aquatic fish species: Current knowledge and future directions. Aquat. Toxicol. 2013, 142, 1–16. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; He, J.; Chen, J.; Giesy, J.P.; Xie, P. Responses of the proteome and metabolome in livers of zebrafish exposed chronically to environmentally relevant concentrations of microcystin-LR. Environ. Sci. Technol. 2017, 51, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Hu, M.; Shang, Y.; Pan, L.; Jia, P.; Fu, C.; Liu, Q.; Wang, Y. Liver transcriptome and miRNA analysis of silver carp (Hypophthalmichthys molitrix) intraperitoneally injected with microcystin-LR. Front. Physiol. 2018, 9, 381. [Google Scholar] [CrossRef]
- Marie, B. Disentangling of the ecotoxicological signal using “omics” analyses, a lesson from the survey of the impact of cyanobacterial proliferations on fishes. Sci. Total Environ. 2020, 736, 139701. [Google Scholar] [CrossRef]
- Slikker Jr, W.; Andersen, M.E.; Bogdanffy, M.S.; Bus, J.S.; Cohen, S.D.; Conolly, R.B.; Hattis, D. Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharm. 2004, 201, 203–225. [Google Scholar] [CrossRef]
- Calabrese, E.J. Cancer biology and hormesis: Human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit. Rev. Toxicol. 2005, 35, 463–582. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Applications of hormesis in toxicology, risk assessment and chemotherapeutics. Trends Pharmacol. Sci. 2002, 23, 331–337. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 998E. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Palikova, M.; Navratil, S.; Papezikova, I.; Ambroz, P.; Vesely, T.; Pokorova, D.; Mares, J.; Adamovsky, P.; Navratil, L.; Kopp, R. Combined exposure of carps (Cyprinus carpio L.) to cyanobacterial biomass and white spot disease. Neuroendocrinol. Lett. 2012, 33, 77–83. [Google Scholar]
- Palikova, M.; Kopp, R.; Kohoutek, J.; Blaha, L.; Mares, J.; Ondrackova, P.; Papezikova, I.; Minarova, H.; Pojezdal, L.; Adamovsky, O. Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp. Toxins 2021, 13, 601. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Parental transfer of microcystin-LR-induced Innate Immune dysfunction of zebrafish: A cross-generational study. Environ. Sci. Technol. 2019, 54, 1014–1023. [Google Scholar] [CrossRef] [PubMed]

| Test Objects | Toxicant | Exposure | Doses/Concentrations | Time Points | Biological Responses | References |
|---|---|---|---|---|---|---|
| Nile tilapia | Cyanobacterial cells | Orally | 120 μg MC-LR/fish | 24 h | Kidney: ultrastructural damages, LPO ↑, GSH/GSSG ratio ↓, CAT ↓, SOD ↓, GR ↑, GPx ↑, GST ↓ | [66] |
| Crucian carp | Cyanobacteria lyophilized powder | Orally | 20% and 40% of cyanobacteria (1.41 mg/g MCs) | 30 d | Spleen and head kidney: histopathological damages, macrophage bactericidal activity ↑, lysozyme activity ↑↓, blood nitroblue tetrazolium activity ↑ | [67] |
| Blunt snout bream | Cyanobacteria lyophilized powder | Orally | 1.41 mg/g MCs (Dry weight) | 30 d | Head kidney: ultrastructural damages, white blood cells numbers↓, phagocytosis activity ↓, sIgM ↓, sIgD ↓, sIgZ ↓ | [68] |
| Carp | Cyanobacterial cells | Immersion | 5.6 × 104–3.2 × 105; 2.6 × 105–3.6 × 106 cells/mL | 96, 168 h | Plasma: LDH ↑, TP ↑, ALT ↑, AST ↑ | [69] |
| Silver carp | Cyanobacterial cells | Immersion | 2.8–7.4 μg/L MCs | 7, 14, 21, 28 d | Plasma: ALB ↓, ALP ↓, CHOL ↓, TP ↓, CRE ↓, LACT ↓, LDH ↓, P ↓, Fe ↓, CHE ↓, ALT ↑ | [70] |
| Silver carp | Toxic Microcystis blooms | Immersion | 0–15.58 μg/L MCs (averaged 4.16 μg/L) | Monthly (1 year) | Kidney: ultrastructural damages, CAT ↑, GST ↑, GSH ↓ | [71] |
| Crucian Carp | Cyanobacteria extract | IP injection | 50, 200 μg MC-LR equiv kg−1 BW | 12, 24, 48, 60 h | Plasma: ALT ↑, ALP ↑, AST ↑, LDH ↑, GLU ↑↓, CHO ↓, TG ↓, TP ↓ | [72] |
| Common Carp | Cyanobacteria extract | Immersion | 25 μg/L MCs | 1, 3, 5 d | Blood and head kidney: Intracellular O2− production ↑, ROS ↑, lymphocyte proliferation ↓, IL-1β ↑, TNF-α ↑, IL-10 ↑ | [73] |
| Common carp | Cyanobacteria extract | Immersion | 1.3 μg/L, 13 μg/L MCs | 8, 30 d | Blood: hematocrit value ↑, hemoglobin concentration ↑, phagocytic activity ↓, total plasma protein ↑, AST ↓, LDH ↓ | [74] |
| Test Objects | Toxicant | Exposure | Doses/Concentrations | Time Points | Biological Responses | References |
|---|---|---|---|---|---|---|
| Silver carp. | MC-LR | IP | 104.9 μg/kg, 262.1 μg/kg | 6, 9, 12, 24, 72, 168 h | Serum: ALT ↑, AST ↑, lysozyme activity ↑, complement C3 ↑, TNF-α ↑, IL-1β ↑, IFN-γ ↑ | [75] |
| Common carp | MC-LR | IP | 150 μg/kg BW | 28 d | Serum: CAT ↑, SOD ↑, GSH ↑, GPx ↑, LPO ↑, complement C3 ↓, lysozyme activity ↓ | [76] |
| Grass carp | MC-LR | IP | 50 μg/kg | 1, 2, 7, 14, 12 d | Spleen and head kidney: mitochondrial edema, chromatin condensation, apoptotic lymphocytes | [34] |
| Bighead carp | MC-LR | IP | 50, 200, 500 μg MC-LR/kg BW | 3, 24 h | Liver and kidney: temporal- and dose-dependent increase in interleukin-8 | [77] |
| Grass carp | MC-LR | IP | 25, 75, 100 μg/kg BW | 96 h | Liver: complement and coagulation cascades pathway ↑, soc3 ↑, hsp70 ↑, | [78] |
| Gold fish | MC-RR | IP | 50, 200 μg/kg BW | 6, 12, 24, 48 h | Kidney: T-AOC ↓, SOD ↑, GPx ↓ | [79] |
| Tilapia | MC-LR, -RR | IP | 500 μg/kg MC-LR or MC-RR | 7 d | Kidney: SOD ↑, CAT ↑, GPx ↑, LPO ↑, | [80] |
| Zebrafish | MC-LR | Immersion | 200, 800 μg/L | 12, 24, 48, 96, 168 h | Larvae: Rag1 ↑, Rag2 ↑, Ikaros ↑, GATA1↑, Lck↑, TCRα↑ | [81] |
| Zebrafish | MC-LR | Immersion | 0, 1, 5, 20 μg/L | 30 d | Spleen: ultrastructural damages, ifn1 ↑, il8 ↑, il1β ↑, tnfα ↑ | [82] |
| Zebrafish | MC-LR | Immersion | 0.3, 1, 3, 10, 30 μg/L | 30 d | Spleen: histopathological lesions, complement C3 ↑↓; | [31] |
| Zebrafish | MC-LR | Immersion | 0, 0.4, 2, 10 μg/L | 30 d | Spleen: histopathological lesions, apoptosis, TNF-α ↑, IL-1β ↑, MYD88 ↑, complement C3 ↑↓ | [83] |
| Zebrafish | MC-LR | Immersion | 20 μg/L | 14 d | Liver: TBARS ↑, GSH ↑, LDH ↑, GST ↓, CAT ↓, cas3a ↑, cas3b ↑ | [84] |
| Test Objects | Toxicant | Doses/Concentrations | Time Points | BiologicalResponses | References |
|---|---|---|---|---|---|
| Crucian carp lymphocytes | MC-LR, MC-RR | 1, 5, 10 nM | 2, 4, 6, 8 h | Apoptosis, nuclear chromatin condensation | [85] |
| Crucian carp lymphocytes | MC-LR | 10 nM | 0.5, 1, 3, 6 h | Apoptosis, intracellular Ca2+ ↑, ROS ↑, MMP↓, ATP ↓, | [86] |
| Crucian carp lymphocytes | MC-RR | 10 nM | 0.25, 0.5, 1, 3, 6 h | Apoptosis, MMP ↓, ROS ↑, intercellular ATP ↓ | [87] |
| Crucian carp lymphocytes | MC-LR | 1 μg/L | 24 h | Apoptosis, MMP ↓, ROS ↑, GSH ↓, SOD ↓, CAT ↓, MDA ↑ | [88] |
| Rainbow trout lymphocytes | MC-LR | 1, 5, 10, 20, 40 μg/mL | 4, 24, 48, 72, 96, 120 h | Cell viability ↓, lymphocytes proliferation ↑↓ | [33] |
| Rainbow trout phagocytic cells | MC-LR | 1, 5, 10, 20 μg/mL | 2, 4, 24 h | Time- and concentration-dependent cell viability decrease, phagocytic cell ability ↑↓, respiratory burst activity ↑↓ | [89] |
| CIK cells | MC-LR | 1, 10, 100 μg/L | 24, 48 h | Apoptosis, cytoskeleton disruption, cell viability ↑↓, ROS ↑, MDA ↑, GSH ↓, GST ↑, SOD ↓ | [90] |
| Carp leucocytes | MC-LR | 0.01, 0.1, 0.5, 1 μg/mL | 24, 72 h | Respiratory burst activity ↑↓, B lymphocytes proliferation ↑, necrosis of leucocytes ↑ | [91] |
| Common carp lymphocytes and phagocytes | MC-LR | 0.01, 0.05, 0.1, 1 μg/mL | 2, 6, 24 h | Phagocytosis ↓, LDH ↑, GSH ↓, apoptosis, necrosis | [92] |
| Common carp leukocytes | MC-LR | 0.01, 0.1 μg/mL | 4 h | il1β ↑↓, tnfα ↑↓, il10 ↑, tgfβ ↑ | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Hung, T.-C.; Kurobe, T.; Wang, Y.; Yang, P. Microcystin-Induced Immunotoxicity in Fishes: A Scoping Review. Toxins 2021, 13, 765. https://doi.org/10.3390/toxins13110765
Lin W, Hung T-C, Kurobe T, Wang Y, Yang P. Microcystin-Induced Immunotoxicity in Fishes: A Scoping Review. Toxins. 2021; 13(11):765. https://doi.org/10.3390/toxins13110765
Chicago/Turabian StyleLin, Wang, Tien-Chieh Hung, Tomofumi Kurobe, Yi Wang, and Pinhong Yang. 2021. "Microcystin-Induced Immunotoxicity in Fishes: A Scoping Review" Toxins 13, no. 11: 765. https://doi.org/10.3390/toxins13110765
APA StyleLin, W., Hung, T.-C., Kurobe, T., Wang, Y., & Yang, P. (2021). Microcystin-Induced Immunotoxicity in Fishes: A Scoping Review. Toxins, 13(11), 765. https://doi.org/10.3390/toxins13110765