Intestinal Barrier, Claudins and Mycotoxins
Abstract
1. Introduction
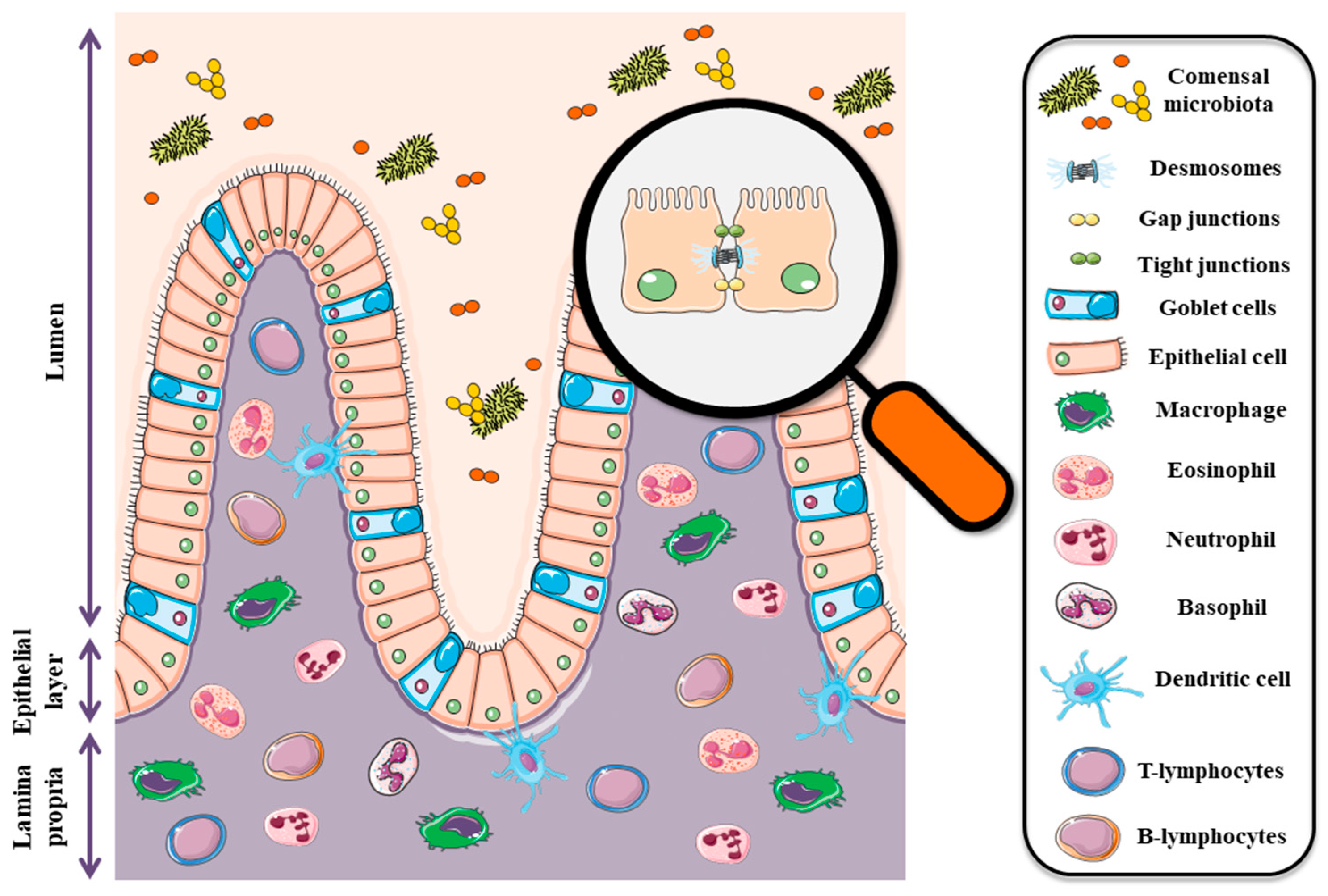
2. Intestinal Barrier: In Health and Disease
3. Claudins in Intestines-Schedule and Function in Health
4. Claudins in Gastrointestinal Cancer
4.1. Oral Cancer
4.2. Esophageal Cancer
4.3. Liver Cancer
4.4. Gastric Cancer
4.5. Colon Cancer
5. Environment Contamination by Mycotoxins and Their Occurrence in Food and Feed
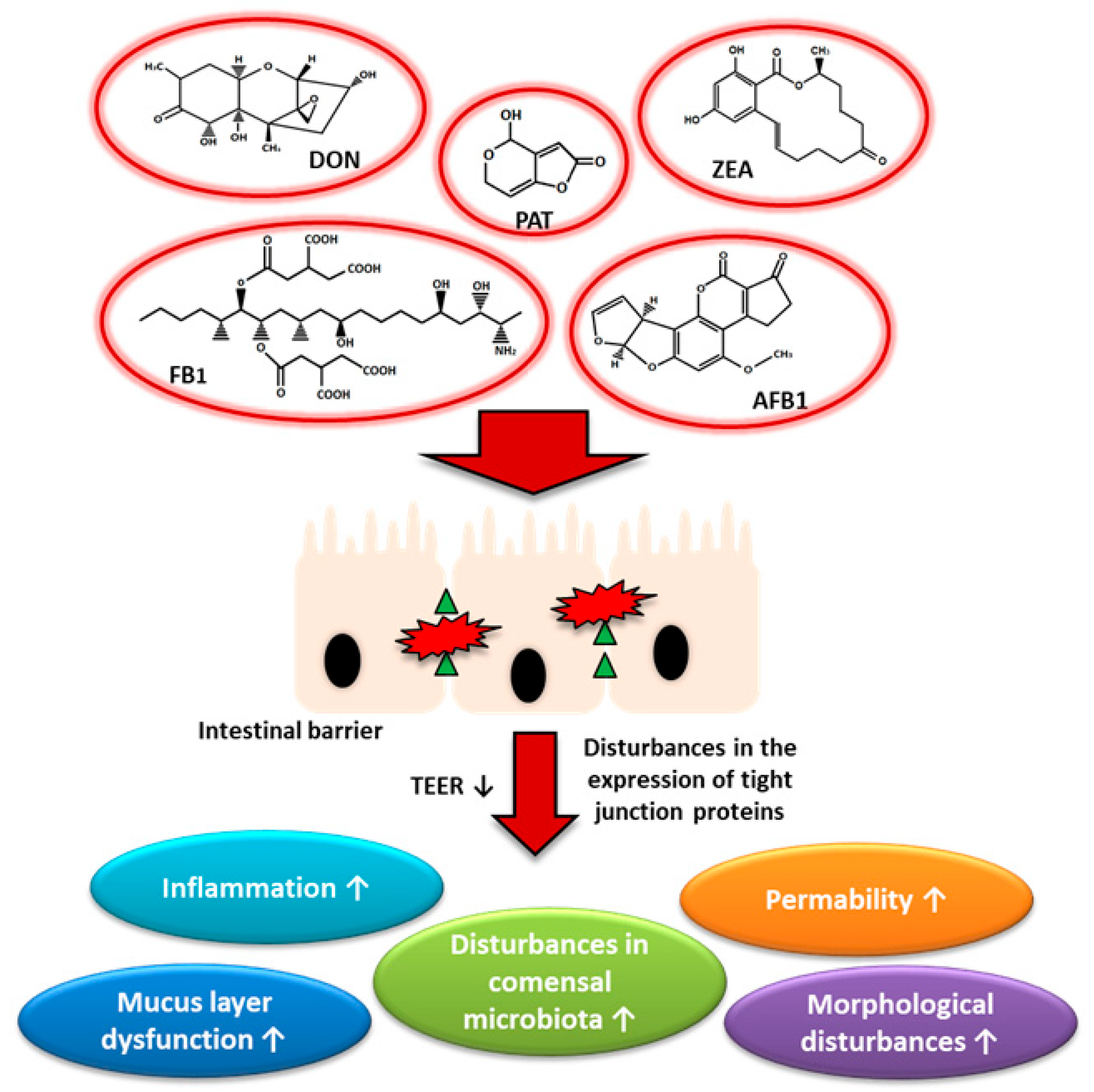
6. Mycotoxins and Human Health Especially the Health of the Gut and the Entire Digestive Tract
6.1. Aflatoxins
6.2. Fumonisins
6.3. Zearalenone
6.4. Deoxynivalenol
6.5. Patulin
7. Mycotoxins and Their Association with Claudins in Gastrointestinal Cancers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Claudins: New players in human fertility and reproductive system cancers. Cancers 2020, 12, 711. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.; Meddings, J.B.; Sharkey, K.A.; Wee Yong, V. The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 2018, 141, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Massie, I.; Meran, L.; Li, V.S.W. Extracellular Matrix Remodeling in Intestinal Homeostasis and Disease. Intest. Stem Cell Niche 2018, 2, 99–140. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, S.; Danese, S. The role of JAM-A in inflammatory bowel disease: Unrevealing the ties that bind. Ann. N. Y. Acad. Sci. 2009, 1165, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Riva, A. Intestinal barrier function in health and disease—Any role of SARS-CoV-2? Microorganisms 2020, 8, 1744. [Google Scholar] [CrossRef]
- Yang, E.; Shen, J. The roles and functions of Paneth cells in Crohn’s disease: A critical review. Cell Prolif. 2021, 54, e12958. [Google Scholar] [CrossRef]
- Nakajima, A.; Vogelzang, A.; Maruya, M.; Miyajima, M.; Murata, M.; Son, A.; Kuwahara, T.; Tsuruyama, T.; Yamada, S.; Matsuura, M.; et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018, 215, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing depression from the microbiota-gut-brain axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Zoledziewska, M. The gut microbiota perspective for interventions in MS. Autoimmun. Rev. 2019, 18, 814–824. [Google Scholar] [CrossRef]
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2015, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M. Claudin-based barrier in simple and stratified cellular sheets. Curr. Opin. Cell Biol. 2002, 14, 531–536. [Google Scholar] [CrossRef]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2, a002907. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.C.; Kamangar, F.; Kim, M.P.; Hammoud, S.; Haque, R.; Iacobuzio-Donahue, C.A.; Maitra, A.; Ashfaq, R.; Hustinx, S.; Heitmiller, R.E.; et al. Claudin-4, mitogen-activated protein kinase kinase 4, and stratifin are markers of gastric adenocarcinoma precursor lesions. Cancer Epidemiol. Biomark. Prev. 2006, 15, 281–287. [Google Scholar] [CrossRef]
- Jun, K.H.; Kim, J.H.; Jung, J.H.; Choi, H.J.; Chin, H.M. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int. J. Surg. 2014, 12, 156–162. [Google Scholar] [CrossRef]
- Soini, Y.; Tommola, S.; Helin, H.; Martikainen, P. Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch. 2006, 448, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.M.; Kallakury, B.V.S.; Sheehan, C.E.; Fisher, H.A.G.; Kaufman, R.P.; Ross, J.S. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum. Pathol. 2007, 38, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H. Recent advances in roles of G-protein coupled receptors in intestinal intraepithelial lymphocytes. Biosci. Microbiota Food Health 2020, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wiarda, J.E.; Trachsel, J.M.; Bond, Z.F.; Byrne, K.A.; Gabler, N.K.; Loving, C.L. Intraepithelial T Cells Diverge by Intestinal Location as Pigs Age. Front. Immunol. 2020, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Tabariès, S.; Siegel, P.M. The role of claudins in cancer metastasis. Oncogene 2017, 36, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Weber, C.R.; Raleigh, D.R.; Su, L.; Shen, L.; Sullivan, E.A.; Wang, Y.; Turner, J.R. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010, 285, 12037–12046. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Will, C.; Fromm, M.; Müller, D. Claudin tight junction proteins: Novel aspects in paracellular transport. Perit. Dial. Int. 2008, 28, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, C.; Furuse, K.; Mimori-Kiyosue, Y.; Furuse, M.; Tsukita, S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.L.; van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Troy, T.C.; Arabzadeh, A.; Yerlikaya, S.; Turksen, K. Claudin immunolocalization in neonatal mouse epithelial tissues. Cell Tissue Res. 2007, 330, 381–388. [Google Scholar] [CrossRef]
- Michlig, S.; Damak, S.; le Coutre, J. Claudin-based permeability barriers in taste buds. J. Comp. Neurol. 2007, 502, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Semba, S.; Ueda, J.; Fuku, T.; Hasuo, T.; Chiba, H.; Sawada, N.; Kuroda, Y.; Yokozaki, H. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci. 2007, 98, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Lameris, A.L.; Huybers, S.; Kaukinen, K.; Mäkelä, T.H.; Bindels, R.J.; Hoenderop, J.G.; Nevalainen, P.I. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand. J. Gastroenterol. 2013, 48, 58–69. [Google Scholar] [CrossRef]
- Markov, A.G.; Veshnyakova, A.; Fromm, M.; Amasheh, M.; Amasheh, S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2010, 180, 591–598. [Google Scholar] [CrossRef]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. 2006, 54, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Kurashige, Y.; Nishimura, M.; Yamazaki, M.; Igarashi, S.; Kaku, T.; Abiko, Y. Expression of claudin-4 and -7 in porcine gingival junctional epithelium. Med. Mol. Morphol. 2009, 42, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lioni, M.; Brafford, P.; Andl, C.; Rustgi, A.; El-Deiry, W.; Herlyn, M.; Smalley, K.S.M. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am. J. Pathol. 2007, 170, 709–721. [Google Scholar] [CrossRef]
- Pinto-Sánchez, M.I.; Nachman, F.D.; Fuxman, C.; Iantorno, G.; Hwang, H.J.; Ditaranto, A.; Costa, F.; Longarini, G.; Wang, X.Y.; Huang, X.; et al. Altered Esophageal Mucosal Structure in Patients with Celiac Disease. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1980686. [Google Scholar] [CrossRef]
- Lu, Y.; Jing, J.; Sun, L.; Gong, Y.; Chen, M.; Wang, Z.; Sun, M.; Yuan, Y. Expression of claudin-11, -23 in different gastric tissues and its relationship with the risk and prognosis of gastric cancer. PLoS ONE 2017, 12, e0174476. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, W.; Wang, H.; Wang, G. The distinct expression patterns of claudin-10, -14, -17 and E-cadherin between adjacent non-neoplastic tissues and gastric cancer tissues. Diagn. Pathol. 2013, 8, 205. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hussain, Z.; Huh, C.W.; Lee, Y.J.; Park, H. Inflammation, impaired motility, and permeability in a Guinea Pig model of postoperative ileus. J. Neurogastroenterol. Motil. 2018, 24, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, L.M.; Liu, Y.L.; Xie, C.; Cai, L.; Xu, K.; Zhou, X.H. Maternal dietary uridine supplementation reduces diarrhea incidence in piglets by regulating the intestinal mucosal barrier and cytokine profiles. J. Sci. Food Agric. 2020, 100, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Deluco, B.; Fourie, K.R.; Simko, O.M.; Wilson, H.L. Localization of Claudin-3 and Claudin-4 within the Small Intestine of newborn piglets. Physiol. Rep. 2021, 9, e14717. [Google Scholar] [CrossRef]
- Zong, Q.F.; Huang, Y.J.; Wu, L.S.; Wu, Z.C.; Wu, S.L.; Bao, W.B. Effects of porcine epidemic diarrhea virus infection on tight junction protein gene expression and morphology of the intestinal mucosa in pigs. Pol. J. Vet. Sci. 2019, 22, 345–353. [Google Scholar] [CrossRef]
- Martínez, C.; Rodinõ-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; González-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. MiR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017, 66, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sang, Z.; Zhuo, Y.; Wang, X.; Guo, Z.; He, L.; Zeng, C.; Dai, H. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1521–1529. [Google Scholar] [CrossRef]
- Yong, Y.; Li, J.; Gong, D.; Yu, T.; Wu, L.; Hu, C.; Liu, X.; Yu, Z.; Ma, X.; Gooneratne, R.; et al. ERK1/2 mitogen-activated protein kinase mediates downregulation of intestinal tight junction proteins in heat stress-induced IBD model in pig. J. Therm. Biol. 2021, 101, 103103. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.C.; Reis, P.P.; Jurisica, I.; Sultan, M.; Arora, S.; Macmillan, C.; Makitie, A.A.; Grénman, R.; Reid, N.; Sukhai, M.; et al. Molecular classification of oral cancer by cDNA microarrays identifies overexpressed genes correlated with nodal metastasis. Int. J. Cancer 2004, 110, 857–868. [Google Scholar] [CrossRef]
- Sappayatosok, K.; Phattarataratip, E. Overexpression of Claudin-1 is Associated with Advanced Clinical Stage and Invasive Pathologic Characteristics of Oral Squamous Cell Carcinoma. Head Neck Pathol. 2015, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ouban, A.; Ahmed, A. Analysis of the Distribution and Expression of Claudin-1 Tight Junction Protein in the Oral Cavity. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 444–448. [Google Scholar] [CrossRef]
- Upadhaya, P.; Barhoi, D.; Giri, A.; Bhattacharjee, A.; Giri, S. Joint detection of claudin-1 and junctional adhesion molecule-A as a therapeutic target in oral epithelial dysplasia and oral squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 18117–18127. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, P.P.; Bharadwaj, R.R.; Machado, J.; MacMillan, C.; Pintilie, M.; Sukhai, M.A.; Perez-Ordonez, B.; Gullane, P.; Irish, J.; Kamel-Reid, S.; et al. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer 2008, 113, 3169–3180. [Google Scholar] [CrossRef]
- Babkair, H.; Yamazaki, M.; Uddin, M.S.; Maruyama, S.; Abé, T.; Essa, A.; Sumita, Y.; Ahsan, M.S.; Swelam, W.; Cheng, J.; et al. Aberrant expression of the tight junction molecules claudin-1 and zonula occludens-1 mediates cell growth and invasion in oral squamous cell carcinoma. Hum. Pathol. 2016, 57, 51–60. [Google Scholar] [CrossRef]
- Lourenço, S.V.; Coutinho-Camillo, C.M.; Buim, M.E.C.; de Carvalho, A.C.; Lessa, R.C.; Pereira, C.M.; Vettore, A.L.; Carvalho, A.L.; Fregnani, J.H.; Kowalski, L.P.; et al. Claudin-7 down-regulation is an important feature in oral squamous cell carcinoma. Histopathology 2010, 57, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Nozaki, S.; Kato, A.; Hirai, M.; Yanase, M.; Yoshimoto, T.; Kimura, I.; Sugiura, S.; Okamune, A.; Kitahara, H.; et al. Loss of claudin-7 is a negative prognostic factor for invasion and metastasis in oral squamous cell carcinoma. Oncol. Rep. 2013, 29, 445–450. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.X.; Xu, T.; Li, J.; Hu, Z.; Wang, C.; Long, Y.; He, X.M.; Deng, X.; Ren, D.L.; et al. CLDN1 induces autophagy to promote proliferation and metastasis of esophageal squamous carcinoma through AMPK/STAT1/ULK1 signaling. J. Cell. Physiol. 2020, 235, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, Z.; Song, L.; Wang, D.; Sun, Z. Low expression of claudin-4: An indicator of recurrence in esophageal squamous cell carcinoma after Ivor Lewis esophagectomy? Med. Oncol. 2014, 31, 951. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Chiba, H.; Nakayama, F.; Ueda, J.; Matsuda, Y.; Sawada, N.; Komori, T.; Ito, A.; Yokozaki, H. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum. Pathol. 2006, 37, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Suzuki, S.; Sakaguchi, T.; Nakamura, T.; Baba, S.; Reinecker, H.C.; Nakamura, S.; Konno, H. Loss of Claudin-1 Expression Correlates with Malignancy of Hepatocellular Carcinoma. J. Surg. Res. 2007, 139, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, Y.D.; Fu, L.; Xu, W.; Liu, D.; Liang, Q.; Zhang, X.; Xu, L.; Guan, X.Y.; Wu, B.; et al. CLDN3 inhibits cancer aggressiveness via Wnt-EMT signaling and is a potential prognostic biomarker for hepatocellular carcinoma. Oncotarget 2014, 5, 7663–7676. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.W.; Ding, X.; Chen, S.L.; Zeng, L. Expression of claudin 10 protein in hepatocellular carcinoma: Impact on survival. J. Cancer Res. Clin. Oncol. 2011, 137, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Eftang, L.L.; Esbensen, Y.; Tannæs, T.M.; Blom, G.P.; Bukholm, I.R.K.; Bukholm, G. Up-regulation of CLDN1 in gastric cancer is correlated with reduced survival. BMC Cancer 2013, 13, 586. [Google Scholar] [CrossRef]
- Shiozaki, A.; Shimizu, H.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; Okamoto, K.; Iitaka, D.; Nakashima, S.; et al. Claudin 1 mediates tumor necrosis factor alpha-induced cell migration in human gastric cancer cells. World J. Gastroenterol. 2014, 20, 17863–17876. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; He, C.; Qu, Y.; Li, J.; Zhang, J.; Du, T.; Chen, X.; Yu, Y.; Liu, B.; et al. Claudin-1 enhances tumor proliferation and metastasis by regulating cell anoikis in gastric cancer. Oncotarget 2015, 6, 1652–1665. [Google Scholar] [CrossRef]
- Hwang, T.L.; Lee, L.Y.; Wang, C.C.; Liang, Y.; Huang, S.F.; Wu, C.M. Claudin-4 expression is associated with tumor invasion, MMP-2 and MMP-9 expression in gastric cancer. Exp. Ther. Med. 2010, 1, 789–797. [Google Scholar] [CrossRef]
- Ohtani, S.; Terashima, M.; Satoh, J.; Soeta, N.; Saze, Z.; Kashimura, S.; Ohsuka, F.; Hoshino, Y.; Kogure, M.; Gotoh, M. Expression of tight-junction-associated proteins in human gastric cancer: Downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer 2009, 12, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, H.; Chen, H.; Gan, G.; Zheng, Y. CLDN4 silencing promotes proliferation and reduces chemotherapy sensitivity of gastric cancer cells through activation of the PI3K/Akt signalling pathway. Exp. Physiol. 2020, 105, 979–988. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Li, Q.; Zhang, Z.; Zhao, G.; Xu, J. CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer. Cell Death Dis. 2019, 10, 949. [Google Scholar] [CrossRef]
- Zavala-Zendejas, V.E.; Torres-Martinez, A.C.; Salas-Morales, B.; Fortoul, T.I.; Montaño, L.F.; Rendon-Huerta, E.P. Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Investig. 2011, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, J.; Song, Y.; Zhao, J.; Sun, J.; Chen, X.; Gao, P.; Wang, Z. Claudin-7 (CLDN7) is overexpressed in gastric cancer and promotes gastric cancer cell proliferation, invasion and maintains mesenchymal state. Neoplasma 2018, 65, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Mori, Y.; Cheng, Y.; Jin, Z.; Olaru, A.V.; Hamilton, J.P.; David, S.; Selaru, F.M.; Yang, J.; Abraham, J.M.; et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE 2009, 4, e8002. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.R.; Shiou, S.R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Sharma, A.; Smith, J.J.; Krishnan, M.; Chen, X.; Eschrich, S.; Washington, M.K.; Yeatman, T.J.; Beauchamp, R.D.; Dhawan, P. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology 2011, 141, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Ahmad, R.; Chaturvedi, R.; Smith, J.J.; Midha, R.; Mittal, M.K.; Krishnan, M.; Chen, X.; Eschrich, S.; Yeatman, T.J.; et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: Role of epidermal growth factor receptor activation. Oncogene 2011, 30, 3234–3247. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, B.; Chen, Z.; Chen, X.; Müller, D.; Lele, S.M.; Washington, M.K.; Batra, S.K.; Dhawan, P.; Singh, A.B. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/β-catenin signaling. Oncogene 2017, 36, 6592–6604. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Pope, J.L.; Smith, J.J.; Ahmad, R.; Chen, X.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene 2015, 34, 4570–4580. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, T.; Xu, C.; Ding, Y.; Li, W.; Ding, L. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via the promotion of epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019, 508, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.C.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef]
- Oku, N.; Sasabe, E.; Ueta, E.; Yamamoto, T.; Osaki, T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 γ2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006, 66, 5251–5257. [Google Scholar] [CrossRef] [PubMed]
- Melchers, L.J.; Bruine De Bruin, L.; Schnell, U.; Slagter-Menkema, L.; Mastik, M.F.; de Bock, G.H.; van Dijk, B.A.C.; Giepmans, B.N.G.; van der Laan, B.F.A.M.; van der Wal, J.E.; et al. Lack of claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2013, 49, 998–1005. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kusumi, T.; Sato, F.; Kawasaki, H.; Shibata, S.; Ohashi, M.; Hakamada, K.; Sasaki, M.; Kijima, H. Decreased expression of claudin-1 is correlated with recurrence status in Esophageal squamous cell carcinoma. Biomed. Res. 2008, 29, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farsakh, S.; Wu, T.; Lalonde, A.; Sun, J.; Zhou, Z. High expression of Claudin-2 in esophageal carcinoma and precancerous lesions is significantly associated with the bile salt receptors VDR and TGR5. BMC Gastroenterol. 2017, 17, 33. [Google Scholar] [CrossRef]
- Sung, C.O.; Han, S.Y.; Kim, S.H. Low expression of claudin-4 is associated with poor prognosis in esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2011, 18, 273–281. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, N.K.; Kim, J.H.; Kang, M.S.; Yoo, H.Y.; Kim, H.H.; Um, S.H.; Kim, S.H. Twist1 causes the transcriptional repression of claudin-4 with prognostic significance in esophageal cancer. Biochem. Biophys. Res. Commun. 2012, 423, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bouchagier, K.A.; Assimakopoulos, S.F.; Karavias, D.D.; Maroulis, I.; Tzelepi, V.; Kalofonos, H.; Karavias, D.D.; Kardamakis, D.; Scopa, C.D.; Tsamandas, A.C. Expression of claudins-1, -4, -5, -7 and occludin in hepatocellular carcinoma and their relation with classic clinicopathological features and patients’ survival. In Vivo 2014, 28, 315–326. [Google Scholar] [PubMed]
- Suh, Y.; Yoon, C.H.; Kim, R.K.; Lim, E.J.; Oh, Y.S.; Hwang, S.G.; An, S.; Yoon, G.; Gye, M.C.; Yi, J.M.; et al. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene 2013, 32, 4873–4882. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, T.; Akagi, Y.; Ochi, T.; Tanaka, N.; Kawahara, A.; Ishibashi, Y.; Gotanda, Y.; Yamaguchi, K.; Shiratuchi, I.; Oka, Y.; et al. Increased claudin-1 protein expression in hepatic metastatic lesions of colorectal cancer. Anticancer Res. 2012, 32, 2309–2314. [Google Scholar] [PubMed]
- Tong, H.; Li, T.; Qiu, W.; Zhu, Z. Claudin-1 silencing increases sensitivity of liver cancer HepG2 cells to 5-fluorouracil by inhibiting autophagy. Oncol. Lett. 2019, 18, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Brokalaki, E.I.; Weber, F.; Sotiropoulos, G.C.; Daoudaki, M.; Cicinnati, V.R.; Beckebaum, S. Claudin-7 expression in hepatocellular carcinoma. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2012; Volume 44, pp. 2737–2740. [Google Scholar]
- Ono, Y.; Hiratsuka, Y.; Murata, M.; Takasawa, A.; Fukuda, R.; Nojima, M.; Tanaka, S.; Osanai, M.; Hirata, K.; Sawada, N. Claudins-4 and -7 might be valuable markers to distinguish hepatocellular carcinoma from cholangiocarcinoma. Virchows Arch. 2016, 469, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Jakab, C.; Kiss, A.; Schaff, Z.; Szabó, Z.; Rusvai, M.; Gálfi, P.; Szabára, A.; Sterczer, Á.; Kulka, J. Claudin-7 protein differentiates canine cholangiocarcinoma from hepatocellular carcinoma. Histol. Histopathol. 2010, 25, 857–864. [Google Scholar] [CrossRef]
- Lódi, C.; Szabó, E.; Holczbauer, A.; Batmunkh, E.; Szíjártó, A.; Kupcsulik, P.; Kovalszky, I.; Paku, S.; Illyés, G.; Kiss, A.; et al. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod. Pathol. 2006, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.I.; Siu, T.C.; Yuk, T.L.; Ho, J.C.; Sheung, T.F. Inhibition of hepatocellular carcinoma invasion by suppression of claudin-10 in HLE cells. Mol. Cancer Ther. 2007, 6, 2858–2867. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.; Qu, Y.; Zhang, J.; Zhang, L.; Chen, X.; Liu, B.; Zhu, Z. The expression of Claudin 1 correlates with β-catenin and is a prognostic factor of poor outcome in gastric cancer. Int. J. Oncol. 2014, 44, 1293–1301. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Liu, Z.; Liu, Q.; Wang, L.; Lu, Y.; Liu, Y.; Wang, M.; Yang, M.; Jin, X.; et al. The distinct expression patterns of claudin-2, -6, and -11 between human gastric neoplasms and adjacent non-neoplastic tissues. Diagn. Pathol. 2013, 8, 133. [Google Scholar] [CrossRef]
- Yang, L.; Sun, X.; Meng, X. Differences in the expression profiles of claudin proteins in human gastric carcinoma compared with non-neoplastic mucosa. Mol. Med. Rep. 2018, 18, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Changchien, T.T.; Wang, C.C.; Wu, C.M. Claudin-4 expression in gastric cancer cells enhances the invasion and is associated with the increased level of matrix metalloproteinase-2 and -9 expression. Oncol. Lett. 2014, 8, 1367–1371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohmoto, T.; Masuda, K.; Shoda, K.; Takahashi, R.; Ujiro, S.; Tange, S.; Ichikawa, D.; Otsuji, E.; Imoto, I. Claudin-6 is a single prognostic marker and functions as a tumor-promoting gene in a subgroup of intestinal type gastric cancer. Gastric Cancer 2020, 23, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, A.C.; Gallardo-Vera, J.F.; Lara-Holguin, A.N.; Montaño, L.F.; Rendón-Huerta, E.P. Claudin-6 enhances cell invasiveness through claudin-1 in AGS human adenocarcinoma gastric cancer cells. Exp. Cell Res. 2017, 350, 226–235. [Google Scholar] [CrossRef]
- Matsuda, M.; Sentani, K.; Noguchi, T.; Hinoi, T.; Okajima, M.; Matsusaki, K.; Sakamoto, N.; Anami, K.; Naito, Y.; Oue, N.; et al. Immunohistochemical analysis of colorectal cancer with gastric phenotype: Claudin-18 is associated with poor prognosis. Pathol. Int. 2010, 60, 673–680. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, T.; Sheng, Y.; Dai, Y.; Xia, B.; Xue, Y. Correlation between Claudin-18 expression and clinicopathological features and prognosis in patients with gastric cancer. J. Gastrointest. Oncol. 2020, 11, 1253–1260. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Hu, D.; Gong, T.; Xu, R.; Gao, J. Analysis of the expression and genetic alteration of CLDN18 in gastric cancer. Aging 2020, 12, 14271–14284. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. Public Health England—National Cancer Registration and Analysis Service; Office for National Statistics: London, UK, 2017.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Miwa, N.; Furuse, M.; Tsukita, S.; Niikawa, N.; Nakamura, Y.; Furukawa, Y. Involvement of claudin-1 in the β-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol. Res. 2000, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Qun, H.; Kinugasa, T.; Lin, W.; Huang, J.; Jun, Z.; Shibaguchi, H.; Kuroki, M.; Tanaka, T.; Yamashita, Y.; Nabeshima, K.; et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 2009, 29, 851–858. [Google Scholar]
- Pope, J.L.; Bhat, A.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 2014, 63, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.H.; Hwang, D.; An, J.; Chung, H.S.; Yang, E.G.; Kim, S.Y. Extracellular pyruvate kinase M2 facilitates cell migration by upregulating claudin-1 expression in colon cancer cells. Biochem. Cell Biol. 2020, 98, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hollandsworth, H.M.; Lwin, T.M.; Amirfakhri, S.; Filemoni, F.; Batra, S.K.; Hoffman, R.M.; Dhawan, P.; Bouvet, M. Anti-Claudin-1 Conjugated to a Near-Infrared Fluorophore Targets Colon Cancer in PDOX Mouse Models. J. Surg. Res. 2019, 242, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Duan, X.; Chen, J.; Gao, Z.; Zhou, J.; Wu, X.; Chang, T.S.; Lee, M.; Li, G.; Nusrat, A.; et al. Integrated Imaging Methodology Detects Claudin-1 Expression in Premalignant Nonpolypoid and Polypoid Colonic Epithelium in Mice. Clin. Transl. Gastroenterol. 2020, 11, e00089. [Google Scholar] [CrossRef]
- Resnick, M.B.; Konkin, T.; Routhier, J.; Sabo, E.; Pricolo, V.E. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: A tissue microarray study. Mod. Pathol. 2005, 18, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Kunisaki, C.; Yoshihara, K.; Yamada, R.; Yamamoto, N.; Sato, T.; Makino, H.; Yamagishi, S.; Nagano, Y.; Fujii, S.; et al. Reduced expression of the claudin-7 gene correlates with venous invasion and liver metastasis in colorectal cancer. Oncol. Rep. 2008, 19, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- World Health Organization. Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 13 July 2021).
- Massart, F.; Saggese, G. Oestrogenic mycotoxin exposures and precocious pubertal development. Int. J. Androl. 2010, 33, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Massart, F.; Meucci, V.; Saggese, G.; Soldani, G. High Growth Rate of Girls with Precocious Puberty Exposed to Estrogenic Mycotoxins. J. Pediatr. 2008, 152, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of Aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, Z.; Jankovic, S.; Jevremovic, I. Incidence of tumors of urinary organs in a focus of Balkan endemic nephropathy. Kidney Int. 1991, 40, S75–S76. [Google Scholar]
- Gill, S.; Kumara, V.M.R. Detecting neurodevelopmental toxicity of domoic acid and ochratoxin a using rat fetal neural stem cells. Mar. Drugs 2019, 17, 566. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Brera, C.; Mezzelani, A.; Soricelli, S.; Ciceri, F.; Moretti, G.; Debegnach, F.; Bonaglia, M.C.; Villa, L.; Molteni, M.; et al. Role of mycotoxins in the pathobiology of autism: A first evidence. Nutr. Neurosci. 2019, 22, 132–144. [Google Scholar] [CrossRef]
- De Santis, B.; Raggi, M.E.; Moretti, G.; Facchiano, F.; Mezzelani, A.; Villa, L.; Bonfanti, A.; Campioni, A.; Rossi, S.; Camposeo, S.; et al. Study on the association among mycotoxins and other variables in children with autism. Toxins 2017, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, Z.; Eeza, M.N.H.; Matysik, J.; Berry, J.P.; Alia, A. NMR-based metabolic profiles of intact zebrafish embryos exposed to aflatoxin b1 recapitulates hepatotoxicity and supports possible neurotoxicity. Toxins 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Cheng, Y.C.; Chen, P.J.; Huang, Y.T.; Yu, F.Y.; Liu, B.H. Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere 2019, 217, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, e05172. [Google Scholar] [CrossRef] [PubMed]
- Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [CrossRef]
- Food, E.; Authority, S. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of intestinal bacterial flora to the long-term feeding of aflatoxin B1 (AFB1) in mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar]
- WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide); IARC Publications: Lyon, France, 2008; Volume 97, pp. 3–471. [Google Scholar]
- Kolenda, M.; Mroczkowski, S. Fusarium mycotoxins and methods of assessing the mycotoxicity: A review. J. Cent. Eur. Agric. 2013, 14, 169–180. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, Q.; Yan, H.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of chronic exposure to low levels of dietary aflatoxin b1 on growth performance, apparent total tract digestibility and intestinal health in pigs. Animals 2021, 11, 336. [Google Scholar] [CrossRef]
- Breves, G.; Kock, J.; Schröder, B. Transport of nutrients and electrolytes across the intestinal wall in pigs. Livest. Sci. 2007, 109, 4–13. [Google Scholar] [CrossRef]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, M.; Fernandez, J.; Yaman, I.; Closs, E. Regulation of cationic amino acid transport: The story of the CAT-1 transporter. Annu. Rev. Nutr. 2004, 24, 377–399. [Google Scholar] [CrossRef]
- Yang, Z.; Venardos, K.; Jones, E.; Morris, B.J.; Chin-Dusting, J.; Kaye, D.M. Identification of a novel polymorphism in the 3′UTR of the L-arginine transporter gene SLC7A1: Contribution to hypertension and endothelial dysfunction. Circulation 2007, 115, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bao, X.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity through Clathrin-Mediated Endocytosis. Toxins 2021, 13, 184. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.H.; Wyatt, R.D. Environment and Health: The toxicity of fumonisin B1, B2, and B3, individually and in combination, in chicken embryos. Poult. Sci. 2001, 80, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sydenham, E.W.; Shephard, G.S.; Thiel, P.G.; Marasas, W.F.O.; Stockenström, S. Fumonisin Contamination of Commercial Corn-Based Human Foodstuffs. J. Agric. Food Chem. 1991, 39, 2014–2018. [Google Scholar] [CrossRef]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Concentration-dependent effect of fumonisin B1 on apoptosis in oesophageal cancer cells. Hum. Exp. Toxicol. 2018, 37, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Richard, J.L.; Meerdink, G.; Maragos, C.M.; Tumbleson, M.; Bordson, G.; Rice, L.G.; Ross, P.F. Absence of detectable fumonisins in the milk of cows fed Fusarium proliferatum (Matsushima) Nirenberg culture material. Mycopathologia 1996, 133, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Heutte, N.; Bouchart, V.; Garon, D. Evaluation of fungal contamination and mycotoxin production in maize silage. Anim. Feed Sci. Technol. 2009, 148, 309–320. [Google Scholar] [CrossRef]
- Marasas, W.F.O. Fumonisins: Their implications for human and animal health. Nat. Toxins 1995, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 2001, 109, 283–289. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Li, X.; Yuan, Q.; Su, J.; Yang, L.; Ning, L.; Lei, H. Fumonisin B1 damages the barrier functions of porcine intestinal epithelial cells in vitro. J. Biochem. Mol. Toxicol. 2019, 33, e22397. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, Y.; Fan, Y.; Ma, Y.; Lei, H.; Su, J. Fumonisin B1 induces oxidative stress and breaks barrier functions in pig iliac endothelium cells. Toxins 2019, 11, 387. [Google Scholar] [CrossRef]
- Mateos, I.; Combes, S.; Pascal, G.; Cauquil, L.; Barilly, C.; Cossalter, A.M.; Laffitte, J.; Botti, S.; Pinton, P.; Oswald, I.P. Fumonisin-exposure impairs age-related ecological succession of bacterial species in weaned pig gut microbiota. Toxins 2018, 10, 230. [Google Scholar] [CrossRef]
- Adibnia, E.; Razi, M.; Malekinejad, H. Zearalenone and 17 β-estradiol induced damages in male rats reproduction potential; evidence for ERα and ERβ receptors expression and steroidogenesis. Toxicon 2016, 120, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Briones-Reyes, D.; Gómez-Martinez, L.; Cueva-Rolón, R. Zearalenone contamination in corn for human consumption in the state of Tlaxcala, Mexico. Food Chem. 2007, 100, 693–698. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Yumbe-Guevara, B.E.; Imoto, T.; Yoshizawa, T. Effects of heating procedures on deoxynivalenol, nivalenol and zearalenone levels in naturally contaminated barley and wheat. Food Addit. Contam. 2003, 20, 1132–1140. [Google Scholar] [CrossRef]
- Wang, Y.C.; Deng, J.L.; Xu, S.W.; Peng, X.; Zuo, Z.C.; Cui, H.M.; Wang, Y.; Ren, Z.H. Effects of zearalenone on IL-2, IL-6, and IFN-γ mRNA levels in the splenic lymphocytes of chickens. Sci. World J. 2012, 2012, 567327. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Maaroufi, K.; Chekir, L.; Creppy, E.E.; Ellouz, F.; Bacha, H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon 1996, 34, 535–540. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Feng, Q.; Huang, L.; Zhang, G.; Liu, F.; Jiang, S.; Yang, Z. Effects of purified zearalenone on selected immunological measurements of blood in post-weaning gilts. Anim. Nutr. 2016, 2, 142–148. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Wang, S.W.; Kuang, S.Y.; Tang, L.; Feng, L. Effects of dietary zearalenone on oxidative stress, cell apoptosis, and tight junction in the intestine of juvenile grass carp (Ctenopharyngodon idella). Toxins 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-Toxic Activity Relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef]
- Urbanek, K.A.; Habrowska-Górczyńska, D.E.; Kowalska, K.; Stańczyk, A.; Domińska, K.; Piastowska-Ciesielska, A.W. Deoxynivalenol as potential modulator of human steroidogenesis. J. Appl. Toxicol. 2018, 38, 1450–1459. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.T.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol-induced proinflammatory gene expression: Mechanisms and pathological sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef]
- Ranzenigo, G.; Caloni, F.; Cremonesi, F.; Aad, P.Y.; Spicer, L.J. Effects of Fusarium mycotoxins on steroid production by porcine granulosa cells. Anim. Reprod. Sci. 2008, 107, 115–130. [Google Scholar] [CrossRef]
- Habrowska-Górczyńska, D.E.; Kowalska, K.; Urbanek, K.A.; Domińska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. Deoxynivalenol modulates the viability, ROS production and apoptosis in prostate cancer cells. Toxins 2019, 11, 265. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.P.; del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Springler, A.; Hessenberger, S.; Schatzmayr, G.; Mayer, E. Early activation of MAPK p44/42 is partially involved in DON-induced disruption of the intestinal barrier function and tight junction network. Toxins 2016, 8, 264. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Wang, X.; Yang, J.; Wu, K.; Zhang, J.; Zhang, B.; Yang, A.; Qi, D. Deoxynivalenol inhibits porcine intestinal trefoil factors expression in weanling piglets and IPEC-J2 cells. Toxins 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Szabó, O.; Czimmermann, Á.E.; Babiczky, Á.; Jerzsele, Á.; Pászti-Gere, E. Investigation of the inflammatory and oxidative stress-inducing effects of deoxynivalenol and T-2 toxin exposure in non-tumorigenic human intestinal cell model. Toxicon 2021, 200, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Lai, Y.H.; Hsiao, F.S.H.; Cheng, Y.H. Effects of deoxynivalenol and mycotoxin adsorbent agents on mitogen-activated protein kinase signaling pathways and inflammation-associated gene expression in porcine intestinal epithelial cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.E.; Jeong, J.Y.; Song, J.; Lee, Y.; Lee, H.J.; Kim, D.W.; Jung, H.J.; Kim, K.H.; Kim, M.; Oh, Y.K.; et al. Colon microbiome of pigs fed diet contaminated with commercial purified deoxynivalenol and zearalenone. Toxins 2018, 10, 347. [Google Scholar] [CrossRef]
- Li, X.G.; Zhu, M.; Chen, M.X.; Fan, H.B.; Fu, H.L.; Zhou, J.Y.; Zhai, Z.Y.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicol. Lett. 2019, 305, 19–31. [Google Scholar] [CrossRef]
- Hanyu, H.; Yokoi, Y.; Nakamura, K.; Ayabe, T.; Tanaka, K.; Uno, K.; Miyajima, K.; Saito, Y.; Iwatsuki, K.; Shimizu, M.; et al. Mycotoxin deoxynivalenol has different impacts on intestinal barrier and stem cells by its route of exposure. Toxins 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; He, J.; Young, J.C.; Zhu, H.; Li, X.Z.; Ji, C.; Zhou, T. Transformation of trichothecene mycotoxins by microorganisms from fish digesta. Aquaculture 2009, 290, 290–295. [Google Scholar] [CrossRef]
- Khezri, A.; Herranz-Jusdado, J.G.; Ropstad, E.; Fraser, T.W. Mycotoxins induce developmental toxicity and behavioural aberrations in zebrafish larvae. Environ. Pollut. 2018, 242, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Ma, Y.; Chai, Y.; Li, Y. Biodegradation of patulin by a Byssochlamys nivea strain. Food Control. 2016, 64, 142–150. [Google Scholar] [CrossRef]
- Joshi, V.K.; Lakhanpal, P.; Kumar, V. Occurrence of Patulin its Dietary Intake through Consumption of Apple and Apple Products and Methods of its Removal. Int. J. Food Ferment. Technol. 2013, 3, 15. [Google Scholar] [CrossRef]
- Zhai, Q.; Gong, X.; Wang, C.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Food-borne patulin toxicity is related to gut barrier disruption and can be prevented by docosahexaenoic acid and probiotic supplementation. Food Funct. 2019, 10, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Alvito, P.; Kleiveland, C.R.; Lea, T.E. Characterization of in vitro effects of patulin on intestinal epithelial and immune cells. Toxicol. Lett. 2016, 250–251, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bansal, M.; Pal, S.; Alam, S.; Jagdale, P.; Ayanur, A.; Ansari, K.M. COX-2/EP2-EP4/β-catenin signaling regulates patulin-induced intestinal cell proliferation and inflammation. Toxicol. Appl. Pharmacol. 2018, 356, 224–234. [Google Scholar] [CrossRef]
- Maidana, L.; Gerez, J.R.; El Khoury, R.; Pinho, F.; Puel, O.; Oswald, I.P.; Bracarense, A.P.F.R.L. Effects of patulin and ascladiol on porcine intestinal mucosa: An ex vivo approach. Food Chem. Toxicol. 2016, 98, 189–194. [Google Scholar] [CrossRef]
- McKinley, E.R.; Carlton, W.W. Patulin mycotoxicosis in Swiss ICR mice. Food Cosmet. Toxicol. 1980, 18, 181–187. [Google Scholar] [CrossRef]
- McKinley, E.R.; Carlton, W.W.; Boon, G.D. Patulin mycotoxicosis in the rat: Toxicology, pathology and clinical pathology. Food Chem. Toxicol. 1982, 20, 289–300. [Google Scholar] [CrossRef]
- McLaughlin, J.; Lambert, D.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. The mycotoxin patulin, modulates tight junctions in caco-2 cells. Toxicol. Vitr. 2009, 23, 83–89. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Woo, C.S.J.; Turner, P.C.; Wan, J.M.F.; El-Nezami, H. Individual and combined effects of Fusarium toxins on the mRNA expression of pro-inflammatory cytokines in swine jejunal epithelial cells. Toxicol. Lett. 2013, 220, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, F.; Kara-Kudo, Y.; Saito, N.; Kumagai, S.; Sugita-Konishi, Y. In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia 1998, 142, 161–167. [Google Scholar] [CrossRef]
- Van de Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kawauchiya, T.; Takumi, R.; Kudo, Y.; Takamori, A.; Sasagawa, T.; Takahashi, K.; Kikuchi, H. Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol. Lett. 2011, 205, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Q.; Gao, Y.N.; Li, S.L.; Huang, X.; Bao, X.Y.; Wang, J.Q.; Zheng, N. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A, and zearalenone individually or collectively. Toxicol. Lett. 2019, 309, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Li, S.; Wu, C.; Wang, J.; Zheng, N. Modulation of Mucin (MUC2, MUC5AC and MUC5B) mRNA Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-Cultures Following Exposure to Individual and Combined Aflatoxin M1 and Ochratoxin A. Toxins 2019, 11, 132. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of intestinal epithelial permeability in differentiated caco-2 cells exposed to aflatoxin M1 and ochratoxin a individually or collectively. Toxins 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Abassi, H.; Ayed-Boussema, I.; Shirley, S.; Abid, S.; Bacha, H.; Micheau, O. The mycotoxin zearalenone enhances cell proliferation, colony formation and promotes cell migration in the human colon carcinoma cell line HCT116. Toxicol. Lett. 2016, 254, 1–7. [Google Scholar] [CrossRef]
- Yip, K.Y.; Wan, M.L.Y.; Wong, A.S.T.; Korach, K.S.; El-Nezami, H. Combined low-dose zearalenone and aflatoxin B1 on cell growth and cell-cycle progression in breast cancer MCF-7 cells. Toxicol. Lett. 2017, 281, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Piastowska-Ciesielska, A.W. The dose-dependent effect of zearalenone on mitochondrial metabolism, plasma membrane permeabilization and cell cycle in human prostate cancer cell lines. Chemosphere 2017, 180, 455–466. [Google Scholar] [CrossRef]
- McLaughlin, J.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Pfohl-Leszkowicz, A.; Fantini, J. The mycotoxin ochratoxin A alters intestinal barrier and absorption functions but has no effect on chloride secretion. Toxicol. Appl. Pharmacol. 2001, 176, 54–63. [Google Scholar] [CrossRef]

| Part of GI Tract | Claudins | References | |||
|---|---|---|---|---|---|
| Human | Mouse | Rat | Pig | ||
| Mouth | 1, 4, 7, 8, 17 | 1, 2, 3, 4, 6, 7, 10, 11, 12, 17, 18, 23 | - | 4, 7 | [35,36,41] |
| Esophagus | 2, 3, 4, 7, 8, 12, 15, 18 | - | - | - | [38,42,43] |
| Stomach | 10, 11, 14, 17, 18, 23 | 1, 3, 5, 6, 11, 18 | - | 1 | [35,37,38,44,45,46] |
| Duodenum | 1, 2, 3, 4, 7, 8, 12, 15, 18 | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 14, 15, 18 | 1, 2, 3, 4, 5, 7, 8, 12 | 1, 3, 4, 5 | [34,38,39,40,47,48,49] |
| Jejunum | 2 | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 18 | 1, 2, 3, 5, 7, 12 | 1, 3, 4, 5 | [34,38,39,40,46,47,48,49,50,51] |
| Ileum | 2, 3, 4, 7, 8, 12, 15, 18 | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15 | 1, 2, 3, 5, 7, 8, 12 | 1, 3, 4, 5 | [34,38,39,40,46,47,48,49] |
| Cecum | - | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 14, 15 | - | - | [34] |
| Colon | - | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 14, 15 | 1, 2, 3, 4, 5, 7, 8, 9, 12 | 1, 4 | [34,39,40,46,52] |
| Ascending * | 2, 3, 4, 7, 8, 12, 15, 18 | - | - | - | [38] |
| Transverse * | 2, 3, 4, 7, 8, 12, 15, 18 | - | - | - | [38] |
| Descending * | 2, 3, 4, 7, 8, 12, 15, 18 | - | - | - | [38] |
| Sigmoid * | 2, 3, 4, 7, 8, 12, 15, 18 | - | - | - | [38] |
| rectum | 1, 2, 3, 4, 7, 8, 12, 15, 18 | - | 3 | - | [26,38,42] |
| Cancer | Claudin | Expression | Described Effects on Cells | References |
|---|---|---|---|---|
| Oral | 1 | ↑ | Invasiveness ↑ Proliferation ↑ | [54,55,56,57,58,59] |
| 7 | ↓ | Invasiveness ↑ | [60,61] | |
| Oesophageal | 1 | ↑ | Proliferation ↑ Metastasis ↑ Invasiveness ↑ | [62] |
| 4 | ↓ | Growth ↑ Colony formation ↑ Invasiveness ↑ | [63] | |
| 7 | ↓ | Invasiveness ↑ Metastasis ↑ Tumour progression ↑ | [64] | |
| Liver | 1 | ↓ | Invasiveness ↑ Metastasis ↑ | [65] |
| 3 | ↓ | Invasiveness ↑ Metastasis ↑ Colony formation ↑ | [66] | |
| 10 | ↑ | Angiogenesis ↑ Invasiveness ↑ | [67] | |
| Gastric | 1 | ↑ | Apoptosis ↑ Invasiveness ↑ Migration ↑ Colony formation ↑ | [68,69,70] |
| 4 | ↑ | Invasiveness ↑ Migration ↑ | [71,72] | |
| 4 | ↓ | Migration ↑ Proliferation ↑ Invasiveness ↑ | [73] | |
| 6 | ↑ | Migration ↑ Proliferation ↑ Invasiveness ↑ Colony formation ↑ | [74,75] | |
| 7 | ↑ | Migration ↑ Proliferation ↑ Invasiveness ↑ Colony formation ↑ EMT ↑ | [75,76] | |
| 9 | ↑ | Migration ↑ Proliferation ↑ Invasiveness ↑ | [75] | |
| 11 | ↓ | Migration ↑ Invasiveness ↑ | [77] | |
| Colorectal | 1 | ↑ | Growth ↑ Colony formation ↑ Migration ↑ Invasiveness ↑ | [78,79] |
| 2 | ↑ | Colony formation ↑ Proliferation ↑ | [80] | |
| 3 | ↓ | Proliferation ↑ Invasiveness ↑ EMT ↑ | [81] | |
| 7 | ↓ | EMT ↑ Colony formation ↑ Growth ↑ Invasiveness ↑ | [82,83] |
| Mycotoxin | Total Tolerable Daily Intake | References |
|---|---|---|
| Aflatoxins | Not established | - |
| Fumonisin B1 | 1 µg/kg | [138] |
| Zearalenone | 0.25 µg/kg | [139] |
| Deoxynivalenol | 1 µg/kg | [140] |
| Patulin | Not established | - |
| Mycotoxin | Cell Line | TEER Values | Targeted Claudin | References |
|---|---|---|---|---|
| Aflatoxin B1 | Caco-2 | ↓ | CLDN3 ↓ | [213] |
| Ochratoxin A | Caco-2, HT-29-DR | ↓ | CLDN3, CLDN4 ↓ | [213,221,222] |
| Patulin | Caco-2 | ↓ | CLDN1, CLDN3, CLDN4) ↓ * | [204,209,213] |
| T-2 toxin | Caco-2 | ↓ | CLDN3, CLDN4 ↓ | [213] |
| Fumonisin B1 | Caco-2 | ↓ | CLDN3, CLDN4 ↓ | [213] |
| Deoxynivalenol | Caco-2, T84, HT-29-DR | ↓ | CLDN4 ↓ | [188,212] |
| AFM1 + ZEA + OTA (combination) | Caco-2 | ↓ | CLDN3, CLDN4 | [215] |
| AFM1 + OTA | Caco-2 | ↓ | CLDN3, CLDN4 | [217] |
| AFM1 + AFB1 | Caco-2 | ↓ | CLDN1 | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozieł, M.J.; Ziaja, M.; Piastowska-Ciesielska, A.W. Intestinal Barrier, Claudins and Mycotoxins. Toxins 2021, 13, 758. https://doi.org/10.3390/toxins13110758
Kozieł MJ, Ziaja M, Piastowska-Ciesielska AW. Intestinal Barrier, Claudins and Mycotoxins. Toxins. 2021; 13(11):758. https://doi.org/10.3390/toxins13110758
Chicago/Turabian StyleKozieł, Marta Justyna, Maksymilian Ziaja, and Agnieszka Wanda Piastowska-Ciesielska. 2021. "Intestinal Barrier, Claudins and Mycotoxins" Toxins 13, no. 11: 758. https://doi.org/10.3390/toxins13110758
APA StyleKozieł, M. J., Ziaja, M., & Piastowska-Ciesielska, A. W. (2021). Intestinal Barrier, Claudins and Mycotoxins. Toxins, 13(11), 758. https://doi.org/10.3390/toxins13110758





