Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections
Abstract
:1. Introduction
2. Results
2.1. Method Optimization and Characterization
2.1.1. Analytical Column and Ammonium Formate Concentration
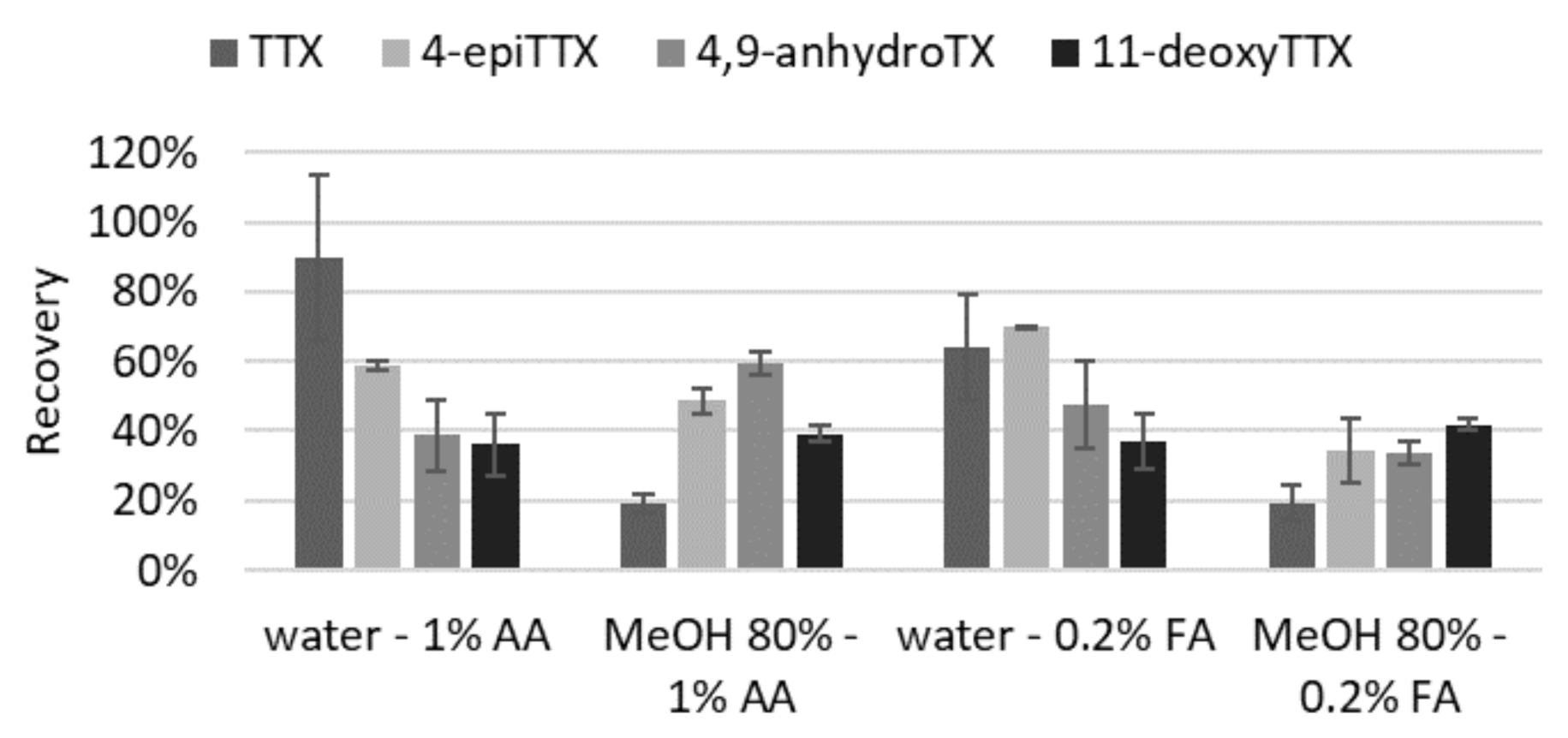
2.1.2. Extraction (Solvent and Volume) and Purification
2.1.3. Method Performance
2.2. Stability of TTXs in Acetic Acid Extracts and Oyster Tissues
2.3. TTXs in Bivalves Collected In Situ
2.4. TTXs in Marine Bacterial Strains
3. Discussion
- (1)
- (2)
- Are we overestimating the number of bacterial TTX-producing strains? Some of the bacteria mentioned in the review of Magarlamov et al. [24] might have been incorrectly reported as a TTX-producer, as for example a strain of V. alginolyticus that was classified as a false-positive due to the presence of a low molecular weight compound with the same molecular weight as TTX [49]. Similarly, we were not able to detect any TTXs in the V. alginolyticus strain ATCC 17749 although considered as a producer of 4,9-anhydroTTX [31] and TTX [32]. We did use culture conditions identical to those of the latter group, however, chromatographic evidence may need to be carefully reviewed before concluding on the toxin production of this strain. This is why highly specific and reliable methods are recommended to ascertain the presence of TTXs. We assumed our method was adapted for our screening purpose, both in terms of recovery and sensitivity, although showing a high signal enhancement due to the bacterial matrix.
- (3)
- Are we using the appropriate culture conditions to elicit TTX production? Indeed, Chau et al. [23] stated that bacteria could require specific inducers to promote TTX production. As discussed by Melnikova et al. [50], the medium content including phosphate concentration, temperature, growth phase and duration of cultivation may modulate the TTX content of bacteria. Turner et al. [48] confirmed that lower temperature (i.e., 22 °C compared to 23.5 and 41 °C) were required to detect TTX in P. luteola and V. alginolyticus isolated from worms. The production and accumulation of TTX-like compounds (revealed by confocal laser scanning microscopy using anti-TTX-antibodies) in Bacillus sp. 1839 was restricted to spores and sporulation would ultimately result in the presence of TTX in the ribbon worm Cephalothrix simula [51]. This Bacillus sp. strain appears to continuously produce TTX over time, as confirmed by the presence of TTX in spore-enriched cultures by LC-MS/MS, which makes this specific strain unique according to the authors [50]. Indeed, some studies revealed the complete loss of toxin production when bacteria were cultivated on artificial media (see references in Magarlamov et al. [24]). Finally, the possibility that unculturable microorganisms play a role in TTX biosynthesis cannot be ignored [23].
- (4)
- Should we focus on other source(s) of TTXs? It was suggested that some dinoflagellates, namely Alexandrium tamarense [52] and Prorocentrum cordatum (formerly P. minimum [10]) could be other sources of TTX, as they were concomitantly present at significant concentrations at the time when TTX-containing bivalves were harvested. Again, this hypothesis of a link between either P. cordatum or A. tamarense was tested but not confirmed or even refuted [30,35,39,47]. The search for the origin of TTX in the Greek mussels led to the discovery of two C9-based TTX-like compounds in cultivated strains of P. minimum thanks to Precursor Ion scan analyses [29]. The authors suggested that the origin was “associated with bacteria”. Unfortunately, we could not detect any C9-based TTX in our bacteria. Recently, picocyanobacteria (especially the genera Synechococcus, Cyanobium and Prochlorococcus) were proposed as a new hypothetic source of TTX, after they were detected in 70–90% of the core bacterial communities of TTX-bearing P. australis in New Zealand, but not in the sympatric and non-TTX containing clam Austrovenus stutchburyi [53]. The hypothesis of an algal source of TTX is also potentially confounded with the increasing knowledge on bacterial components of the phycosphere [54,55,56,57], which may result in microalgal transport of bacterial toxins into bivalves. In addition, as bivalves are not efficient at filtering free-living bacteria directly from seawater as a food source due to their small size [58], it is reasonable to assume that if bacteria are the biological source they may enter the bivalve digestive system as “back-packers”, i.e., as part of the phycosphere or from protists [59] or aggregates [60] or via another vector such as toxic flatworm larvae as recently proven by Okabe et al. [61].
4. Materials and Methods
4.1. Samples
4.1.1. Sampling of Bivalves
4.1.2. Bacteria from Culture Collections
4.2. Chemicals and Reagents
4.3. Method Optimization
4.3.1. Solvent and Volume of Extraction
4.3.2. Purification
4.3.3. Analytical Column and Mobile Phases
4.4. Optimized Extraction and Analysis
4.4.1. Extraction of TTXs from Shellfish and Bacteria
4.4.2. HILIC-MS/MS Analysis of TTXs
4.5. Characterization of the HILIC-MS/MS Method
4.5.1. Limit of Detection and Limit of Quantification
4.5.2. Recoveries and Matrix Effects
4.6. Stability of TTXs in Acetic Acid Extracts and Oyster Tissues
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lago, J.; Rodríguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2020, 36, 645–667. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and Accumulation in Aquatic Organisms, and Cases of Human Intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Giráldez, J.; Rodrigues, S.; Leão, J.; Pinto, E.; Soliño, L.; Gago-Martínez, A. High Levels of Tetrodotoxin (TTX) in Trumpet Shell Charonia lampas from the Portuguese Coast. Toxins 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef]
- Katikou, P. Public Health Risks Associated with Tetrodotoxin and Its Analogues in European Waters: Recent Advances after the EFSA Scientific Opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef] [Green Version]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First Toxicity Report of Tetrodotoxin and 5,6,11-TrideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Turner, A.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS Potentially Linked to the Presence of the Dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.; Loreal, H.; Toyofuku, H.; Hess, P.; Iddya, K.; Ababouch, L. Assessment and Management of Biotoxin Risks in Bivalve Molluscs; FAO Fisheries and Aquaculture Technical Paper No. 551; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; 337p, Available online: http://www.fao.org/3/i2356e/i2356e.pdf (accessed on 27 August 2021).
- McFarren, E.F. Report on Collaborative Studies of the Bioassay for Paralytic Shellfish Poison. J. AOAC Int. 1959, 42, 263–271. [Google Scholar] [CrossRef]
- Onoue, Y.; Noguchi, T.; Maruyama, J.; Hashimoto, K.; Seto, H. Properties of two toxins newly isolated from oysters. J. Agric. Food Chem. 1983, 31, 420–423. [Google Scholar] [CrossRef]
- Sommer, H.; Whedon, W.F.; Kofoid, C.A.; Stohler, R. Relation of paralytic shellfish poison to certain plankton organisms of the genus Gonyaulax. Arch. Pathol. 1937, 24, 537–559. [Google Scholar]
- Sommer, H.; Meyer, K.F. Paralytic shellfish poisoning. Arch. Pathol. 1937, 24, 560–598. [Google Scholar]
- Botana, L.M.; Hess, P.; Munday, R.; Nathalie, A.; DeGrasse, S.L.; Feeley, M.; Suzuki, T.; van den Berg, M.; Fattori, V.; Gamarro, E.G.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with Bivalve Molluscs. Trends Food Sci. Technol. 2017, 59, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off. J. 1986, L358, 1–29. [Google Scholar]
- Hess, P.; Grune, B.; Anderson, D.B.; Aune, T.; Botana, L.M.; Caricato, P.; van Egmond, H.P.; Halder, M.; Hall, S.; Lawrence, J.F.; et al. Three Rs approaches in marine biotoxin testing—The report and recommendations of a joint ECVAM/DG SANCO workshop (ECVAM workshop 55). ATLA Altern. Lab. Anim. 2006, 34, 193–224. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; De Astudillo, L.R.; Biré, R.; Burdaspal, P.A.; Ceredi, A.; Davis, B.; Dias, E.; Eaglesham, G.; et al. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, A.D.; Boundy, M.J.; Rapkova, M.D. Development and Single-Laboratory Validation of a Liquid Chromatography Tandem Mass Spectrometry Method for Quantitation of Tetrodotoxin in Mussels and Oysters. J. AOAC Int. 2017, 100, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-Producing Bacteria: Detection, Distribution and Migration of the Toxin in Aquatic Systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, N.; Sugimoto, K.; Kudo, Y.; Onodera, K.-I.; Cho, Y.; Konoki, K.; Nishikawa, T.; Yotsu-Yamashita, M. Spiro Bicyclic Guanidino Compounds from Pufferfish: Possible Biosynthetic Intermediates of Tetrodotoxin in Marine Environments. Chem. Eur. J. 2018, 24, 7250–7258. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Pinto, E.P.; Oliveira, P.; Pedro, S.; Costa, P.R. Evaluation of the Occurrence of Tetrodotoxin in Bivalve Mollusks from the Portuguese Coast. J. Mar. Sci. Eng. 2019, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.; Stramenga, A.; et al. Tetrodotoxins (TTXs) and Vibrio alginolyticus in Mussels from Central Adriatic Sea (Italy): Are They Closely Related? Mar. Drugs 2021, 19, 304. [Google Scholar] [CrossRef]
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, O.; Gago-Martínez, A. Preliminary Results on the Evaluation of the Occurrence of Tetrodotoxin Associated to Marine Vibrio spp. in Bivalves from the Galician Rias (Northwest of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, srep40880. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of Tetrodotoxin Shellfish Poisoning (TSP) Toxins and Causative Factors in Bivalve Molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef] [Green Version]
- Simidu, U.; Noguchi, T.; Hwang, D.F.; Shida, Y.; Hashimoto, K. Marine bacteria which produce tetrodotoxin. Appl. Environ. Microbiol. 1987, 53, 1714–1715. [Google Scholar] [CrossRef] [Green Version]
- Pratheepa, V.; Alex, A.; Silva, M.; Vasconcelos, V. Bacterial diversity and tetrodotoxin analysis in the viscera of the gastropods from Portuguese coast. Toxicon 2016, 119, 186–193. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Marine Biotoxins. Determination of Tetrodotoxin by HILIC-MS/MS. Available online: https://www.aesan.gob.es/en/CRLMB/web/home.html (accessed on 17 September 2021).
- Blanco, L.; Lago, J.; González, V.; Paz, B.; Rambla-Alegre, M.; Cabado, A.G. Occurrence of Tetrodotoxin in Bivalves and Gastropods from Harvesting Areas and Other Natural Spaces in Spain. Toxins 2019, 11, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control. 2021, 120, 107510. [Google Scholar] [CrossRef]
- Biessy, L.; Smith, K.F.; Boundy, M.J.; Webb, S.C.; Hawes, I.; Wood, S.A. Distribution of Tetrodotoxin in the New Zealand Clam, Paphies australis, Established Using Immunohistochemistry and Liquid Chromatography-Tandem Quadrupole Mass Spectrometry. Toxins 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanji-Rapkova, M.; Turner, A.; Baker-Austin, C.; Huggett, J.; Ritchie, J. Distribution of Tetrodotoxin in Pacific Oysters (Crassostrea gigas). Mar. Drugs 2021, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Patria, F.P.; Pekar, H.; Zuberovic-Muratovic, A. Multi-Toxin Quantitative Analysis of Paralytic Shellfish Toxins and Tetrodotoxins in Bivalve Mollusks with Ultra-Performance Hydrophilic Interaction LC-MS/MS—An In-House Validation Study. Toxins 2020, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M.; Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food Chem. 2018, 269, 166–172. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Z.; Wang, Y.; Jiang, T.; Wang, J.; Sun, X.; Guo, Y. Immunoaffinity Chromatography Purification and Ultrahigh Performance Liquid Chromatography Tandem Mass Spectrometry Determination of Tetrodotoxin in Marine Organisms. J. Agric. Food Chem. 2015, 63, 3129–3134. [Google Scholar] [CrossRef]
- Boundy, M.J.; Biessy, L.; Roughan, B.; Nicolas, J.; Harwood, D.T. Survey of Tetrodotoxin in New Zealand Bivalve Molluscan Shellfish over a 16-Month Period. Toxins 2020, 12, 512. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First Detection of Tetrodotoxin in the Bivalve Paphies australis by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry with and without Precolumn Reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Biessy, L.; Smith, K.F.; Harwood, D.T.; Boundy, M.J.; Hawes, I.; Wood, S.A.; Harwood, T. Spatial variability and depuration of tetrodotoxin in the bivalve Paphies australis from New Zealand. Toxicon X 2019, 2, 100008. [Google Scholar] [CrossRef]
- Numano, S.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Temporal Variation of the Profile and Concentrations of Paralytic Shellfish Toxins and Tetrodotoxin in the Scallop, Patinopecten yessoensis, Cultured in a Bay of East Japan. Mar. Drugs 2019, 17, 653. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New Invasive Nemertean Species (Cephalothrix Simula) in England with High Levels of Tetrodotoxin and a Microbiome Linked to Toxin Metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.; Hedström, M.; Seth, H.; McEvoy, E.G.; Jacobsson, E.; Göransson, U.; Andersson, H.S.; Sundberg, P. The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus—Just a False Positive? Mar. Drugs 2016, 14, 63. [Google Scholar] [CrossRef] [Green Version]
- Melnikova, D.I.; Vlasenko, A.E.; Magarlamov, T.Y. Stable Tetrodotoxin Production by Bacillus sp. Strain 1839. Mar. Drugs 2019, 17, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magarlamov, T.Y.; Beleneva, I.A.; Chernyshev, A.V.; Kuhlevsky, A.D. Tetrodotoxin-producing Bacillus sp. from the ribbon worm (Nemertea) Cephalothrix simula (Iwata, 1952). Toxicon 2014, 85, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef]
- Biessy, L.; Pearman, J.K.; Smith, K.F.; Hawes, I.; Wood, S.A. Seasonal and Spatial Variations in Bacterial Communities from Tetrodotoxin-Bearing and Non-tetrodotoxin-Bearing Clams. Front. Microbiol. 2020, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Martin, J.L.; Giewat, M.W.; Rooney-Varga, J.N. Microbial community diversity in the phycosphere of natural populations of the toxic alga, Alexandrium fundyense. Environ. Microbiol. 2007, 9, 3108–3121. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.; Amin, S.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Feng, J.; Sardar, A.; Hu, Z.; Wang, H. Tropicibacter alexandrii sp. nov., a novel marine bacterium isolated from the phycosphere of a dinoflagellate, Alexandrium minutum. Antonie van Leeuwenhoek 2020, 113, 311–320. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Xie, Z.; Zhang, S.; Wu, Y.; Ge, Y.; Zhang, X. Haliea alexandrii sp. nov., isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1133–1138. [Google Scholar] [CrossRef]
- Barillé, L.; Prou, J.; Héral, M.; Bourgrier, S. No influence of food quality, but ration-dependent retention efficiencies in the Japanese oyster Crassostrea gigas. J. Exp. Mar. Biol. Ecol. 1993, 171, 91–106. [Google Scholar] [CrossRef]
- Dupuy, C.; Vaquer, A.; Lam-Höai, T.; Rougier, C.; Mazouni, N.; Lautier, J.; Collos, Y.; Le Gall, S. Feeding rate of the oyster Crassostrea gigas in a natural planktonic community of the Mediterranean Thau Lagoon. Mar. Ecol. Prog. Ser. 2000, 205, 171–184. [Google Scholar] [CrossRef]
- Kach, D.J.; Ward, J.E. The role of marine aggregates in the ingestion of picoplankton-size particles by suspension-feeding molluscs. Mar. Biol. 2008, 153, 797–805. [Google Scholar] [CrossRef]
- Okabe, T.; Saito, R.; Yamamoto, K.; Watanabe, R.; Kaneko, Y.; Yanaoka, M.; Furukoshi, S.; Yasukawa, S.; Ito, M.; Oyama, H.; et al. The role of toxic planocerid flatworm larvae on tetrodotoxin accumulation in marine bivalves. Aquat. Toxicol. 2021, 237, 105908. [Google Scholar] [CrossRef]
- Vaelli, P.M.; Theis, K.R.; Williams, J.E.; O’Connell, L.A.; Foster, J.A.; Eisthen, H.L. The skin microbiome facilitates adaptive tetrodotoxin production in poisonous newts. eLife 2020, 9, e53898. [Google Scholar] [CrossRef]
- Liu, J.; Wei, F.; Lu, Y.; Ma, T.; Zhao, J.; Gong, X.; Bao, B. Production level of tetrodotoxin in Aeromonas is associated with the copy number of a plasmid. Toxicon 2015, 101, 27–34. [Google Scholar] [CrossRef]
- Melnikova, D.I.; Magarlamov, T.Y. The Microbial Community of Tetrodotoxin-Bearing and Non-Tetrodotoxin-Bearing Ribbon Worms (Nemertea) from the Sea of Japan. Mar. Drugs 2020, 18, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnikova, D.; Nijland, R.; Magarlamov, T. The First Data on the Complete Genome of a Tetrodotoxin-Producing Bacterium. Toxins 2021, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, A.; Le Gac, M.; Labry, C.; Siano, R.; Quere, J.; Caradec, F.; Le Bec, C.; Nezan, E.; Doner, A.; Gouriou, J. The Bay of Brest (France), a new risky site for toxic Alexandrium minutum blooms and PSP shellfish contamination. Harmful Algae News 2015, 51, 4–5. [Google Scholar]
- Zobell, C.E. Studies on marine bacteria. I. The cultural requirements of heterotrophic aerobes. J. Mar. Res. 1941, 4, 42–75. [Google Scholar]
- Lamberty, A.; Schimmel, H.; Pauwels, J. The study of the stability of reference materials by isochronous measurements. Fresenius J. Anal. Chem. 1998, 360, 359–361. [Google Scholar] [CrossRef]
- Rey, V.; Botana, A.M.; Alvarez, M.; Antelo, A.; Botana, L.M. Liquid Chromatography with a Fluorimetric Detection Method for Analysis of Paralytic Shellfish Toxins and Tetrodotoxin Based on a Porous Graphitic Carbon Column. Toxins 2016, 8, 196. [Google Scholar] [CrossRef]
- Rodriguez, I.; Alfonso, A.; Gonzalez-Jartin, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Li, L.; Chen, S.; Yan, Z.; Gao, X.; Chang, J. Analysis and Evaluation of Tetrodotoxin in Coastal Aquatic Products of Zhejiang Province. J. Coast. Res. 2018, 380–385. [Google Scholar] [CrossRef]





| Analog | Oyster | Bacteria | ||||
|---|---|---|---|---|---|---|
| Recovery (%) | Matrix Effect (%) | LOD/LOQ (µg/kg) | Recovery (%) | Matrix Effect (%) | LOD/LOQ (µg/kg) | |
| TTX | 100 ± 15 | 141 ± 25 | 3.8/5 (2019) 6.3/12.5 (2020) | 82 ± 5 | 355 ± 1 | 5/10 |
| 4-epiTTX | 89 ± 12 | 71 ± 20 | nd | 86 ± 1 | 123 ± 16 | nd |
| 4,9-anhydroTTX | 98 ± 12 | 57 ± 12 | nd | 73 ± 7 | 117 ±26 | nd |
| 11-deoxyTTX | 77 ± 15 | 58 ± 12 | nd | 77 ± 5 | 128 ± 23 | nd |
| Location | Year | Dates | Bivalve Species | Number of Samples |
|---|---|---|---|---|
| Antifer * (Bay of Seine) | 2018 | From 12 July to 9 October (bi-weekly) | Oyster (C. gigas) | 7 |
| Géfosse (Bay of Veys) | 2018 2019 | From 13 June to 24 October (bi-weekly) From 20 May to 26 September (bi-weekly) | Oyster (C. gigas) | 10 9 |
| Pointe du Puits (Rance estuary) | 2018 2019 | From 11 June to 6 August (weekly) From 3 June to 26 August (weekly) | Oyster (C. gigas) | 9 13 |
| Ville Ger (Rance estuary) | 2018 2019 | From 11 June to 6 August (weekly) From 3 June to 26 August (weekly) | Clam (R. philippinarum) | 9 13 |
| Kersanton ** (Bay of Brest) | 2019 | From 16 May to 30 August (weekly) | Oyster (C. gigas) | 14 |
| Bouzigues (Thau lagoon) | 2019 | From 27 May to 28 August (weekly) | Oyster (C. gigas) | 14 |
| Analytes | Q1 Mass (m/z) | Q3 Mass (m/z) | Transition Type | DP (V) | CE (eV) | CXP (V) | Ion Ratio (Quant/Qual) |
|---|---|---|---|---|---|---|---|
| TTX and 4-epiTTX | 320 | 302 | Quant | 86 | 35 | 48 | TTX = 1.9 4 epiTTX = 3.0 |
| 162 | Qual | 86 | 59 | 26 | |||
| 4,9-anhydroTTX | 302 | 162 | Quant | 121 | 47 | 26 | 1.3 |
| 256 | Qual | 121 | 37 | 44 | |||
| 11-deoxyTTX | 304 | 286 | Quant | 86 | 35 | 22 | 6.3 |
| 176 | Qual | 86 | 47 | 28 | |||
| norTTX | 290 | 272 | Qual | 121 | 35 | 22 | / |
| 162 | Qual | 121 | 35 | 22 | |||
| TrideoxyTTX | 272 | 254 | Qual | 86 | 47 | 28 | / |
| 162 | Qual | 86 | 47 | 28 | |||
| C9-based-TTX (265) | 265 | 179 | Qual | 86 | 59 | 26 | / |
| 162 | Qual | 86 | 59 | 26 | |||
| C9-based-TTX (308) | 308 | 180 | Qual | 86 | 59 | 26 | / |
| 162 | Qual | 86 | 59 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Réveillon, D.; Savar, V.; Schaefer, E.; Chevé, J.; Halm-Lemeille, M.-P.; Hervio-Heath, D.; Travers, M.-A.; Abadie, E.; Rolland, J.-L.; Hess, P. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins 2021, 13, 740. https://doi.org/10.3390/toxins13110740
Réveillon D, Savar V, Schaefer E, Chevé J, Halm-Lemeille M-P, Hervio-Heath D, Travers M-A, Abadie E, Rolland J-L, Hess P. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins. 2021; 13(11):740. https://doi.org/10.3390/toxins13110740
Chicago/Turabian StyleRéveillon, Damien, Véronique Savar, Estelle Schaefer, Julien Chevé, Marie-Pierre Halm-Lemeille, Dominique Hervio-Heath, Marie-Agnès Travers, Eric Abadie, Jean-Luc Rolland, and Philipp Hess. 2021. "Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections" Toxins 13, no. 11: 740. https://doi.org/10.3390/toxins13110740
APA StyleRéveillon, D., Savar, V., Schaefer, E., Chevé, J., Halm-Lemeille, M.-P., Hervio-Heath, D., Travers, M.-A., Abadie, E., Rolland, J.-L., & Hess, P. (2021). Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins, 13(11), 740. https://doi.org/10.3390/toxins13110740






