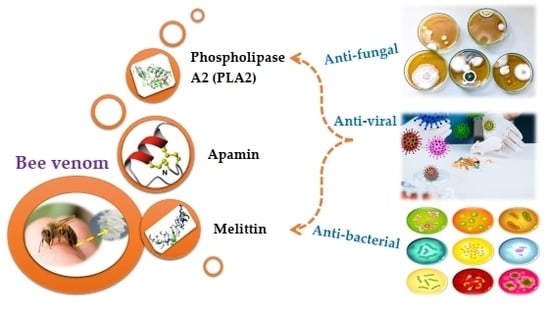
Antimicrobial Properties of Apis mellifera’s Bee Venom
Abstract
1. Introduction
2. Antimicrobial Properties of Bee Venom and Mode of Action for the Venom and its Derived Compounds
2.1. Antibacterial
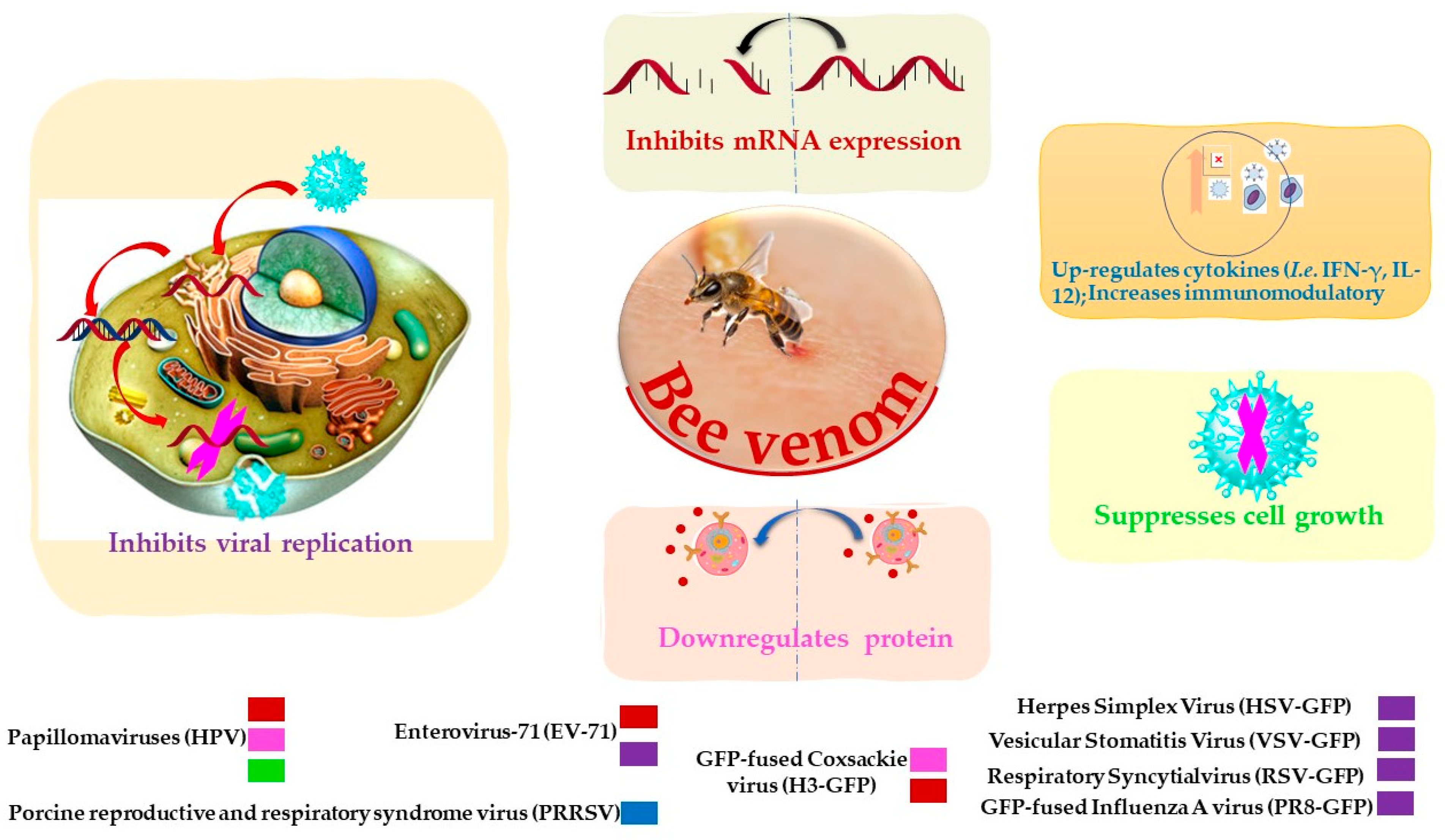
2.2. Anti-Viral
2.3. Anti-fungal
3. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Rehman, K.; Fiayyaz, F.; Khurshid, M.; Sabir, S. Antibiotics and Antimicrobial Resistance: Temporal and Global Trends in the Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 2; ISBN 9780128188828. [Google Scholar]
- Memariani, H.; Memariani, M.; Shahidi-Dadras, M.; Nasiri, S.; Akhavan, M.M.; Moravvej, H. Melittin: From honeybees to superbugs. Appl. Microbiol. Biotechnol. 2019, 103, 3265–3276. [Google Scholar] [CrossRef]
- Shin, S.; Ye, M.; Choi, S.; Park, K. The effects of melittin and apamin on airborne Fungi-Induced chemical mediator and extracellular matrix production from nasal polyp fibroblasts. Toxicon 2017, 9, 384. [Google Scholar] [CrossRef]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A. Antimicrobial activity of apitoxin, melittin and phospholipase A 2 of honey bee (Apis mellifera) venom against oral pathogens. An. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial activity and antibiotic-enhancing effects of honeybee venom against methicillin-resistant Staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef]
- Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial activity of bee venom and melittin against borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, T.; Rima, M.; Karam, M.; Fajloun, J.M.S. Antimicrobials from venomous animals: An oeriew. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- De Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake venom cathelicidins as natural antimicrobial peptides. Front. Pharmacol. 2019, 10, 1415–1427. [Google Scholar] [CrossRef]
- Das Neves, R.C.; Mortari, M.R.; Schwartz, E.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimicrobial and antibiofilm effects of peptides from venom of social wasp and scorpion on multidrug-resistant Acinetobacter baumannii. Toxins 2019, 11, 216. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Flávia, A.; Pereira, M.; Albano, M.; Cristina, F.; Alves, B.; Fernanda, B.; Teles, M.; Furlanetto, A.; Mores, V.L. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar]
- Wang, L.; Zhao, X.; Zhu, C.; Zhao, Y.; Liu, S.; Xia, X.; Liu, X.; Zhang, H.; Xu, Y.; Hang, B.; et al. The antimicrobial peptide MPX kills Actinobacillus pleuropneumoniae and reduces its pathogenicity in mice. Vet. Microbiol. 2020, 243, 108634. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557. [Google Scholar] [CrossRef]
- Pak, S.C. An introduction to the Toxins special issue on “Bee and wasp venoms: Biological characteristics and therapeutic application”. Toxins 2016, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y. Bee Venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Janik, J.E.; Wania-Galicia, L.; Kalauokalani, D. Bee stings—A remedy for postherpetic neuralgia? A case report. Reg. Anesth. Pain Med. 2007, 32, 533–535. [Google Scholar] [CrossRef]
- Dong, J.; Ying, B.; Huang, S.; Ma, S.; Long, P.; Tu, X.; Yang, W.; Wu, Z.; Chen, W.; Miao, X. High-Performance liquid chromatography combined with intrinsic fluorescence detection to analyse melittin in individual honeybee (Apis mellifera) venom sac. J. Chromatogr. B 2015, 1002, 139–143. [Google Scholar] [CrossRef]
- Huh, J.; Kang, J.W.; Nam, D.; Baek, Y.; Choi, D. Melittin suppresses VEGFA-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J. Nat. Prod. 2012, 75, 1922–1929. [Google Scholar] [CrossRef]
- Buku, A.; Reibman, J.; Pistelli, A.; Blandina, P.; Gazis, D. Mast cell degranulating (MCD) peptide analogs with reduced ring structure. J. Protein Chem. 1992, 11, 275–280. [Google Scholar] [CrossRef]
- Mourelle, D.; Brigatte, P.; Bringanti, L.D.B.; De Souza, B.M.; Arcuri, H.A.; Gomes, P.C.; Baptista-Saidemberg, N.B.; Ruggiero Neto, J.; Palma, M.S. Hyperalgesic and edematogenic effects of Secapin-2, a peptide isolated from Africanized honeybee (Apis mellifera) venom. Peptides 2014, 59, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, X.X.; Sheng, Y.X.; Zhang, J.L.; Yu, D.Q. A novel peptide from Apis mellifera and solid-phase synthesis of its analogue. Chin. Chem. Lett. 2012, 23, 1161–1164. [Google Scholar] [CrossRef]
- Gauldie, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. The structures of some peptides from bee venom. Eur. J. Biochem. 1978, 83, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Shkenderov, S.; Koburova, K. Adolapin—A newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon 1982, 20, 317–321. [Google Scholar] [CrossRef]
- Drici, M.D.; Diochot, S.; Terrenoire, C.; Romey, G.; Lazdunski, M. The bee venom peptide tertiapin underlines the role of I(KACh) in acetylcholine-induced atrioventricular blocks. Br. J. Pharmacol. 2000, 131, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Greunke, K.; Genov, N.; Betzel, C. 3-D Model of the bee venom acid phosphatase: Insights into allergenicity. Biochem. Biophys. Res. Commun. 2009, 378, 711–715. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H.; Szczęsna, T. HPLC study of chemical composition of honeybee (Apis mellifera L.) venom. J. Apic. Sci. 2004, 48, 103–109. [Google Scholar]
- Shipolini, R.A.; Callewaert, G.L.; Cottrell, R.C.; Doonan, S.; Vernon, C.A.; Banks, B.E. Phospholipase A from bee venom. Eur. J. Biochem. 1971, 20, 459–468. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Bockisch, B.; Braren, I.; Cifuentes, L.; McIntyre, M.; Rühl, D.; Ring, J.; Bredehorst, R.; Ollert, M.W.; et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J. Immunol. 2010, 184, 5403–5413. [Google Scholar]
- Kettner, A.; Hughes, G.J.; Frutiger, S.; Astori, M.; Roggero, M.; Spertini, F.; Corradin, G. Api m 6: A new bee venom allergen. J. Allergy Clin. Immunol. 2001, 107, 914–920. [Google Scholar] [CrossRef]
- Georgieva, D.; Greunke, K.; Betzel, C. Three-dimensional model of the honeybee venom allergen Api m 7: Structural and functional insights. Mol. Biosyst. 2010, 6, 1056–1060. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Michel, Y.; Mcintyre, M.; Cifuentes, L.; Braren, I.; Grunwald, T.; Darsow, U.; Ring, J.; Bredehorst, R.; et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Eur. J. Allergy Clin. Immunol. 2011, 66, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Bantleon, F.I.; McIntyre, M.; Ollert, M.; Spillner, E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin. Exp. Allergy 2012, 42, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Peiren, N.; de Graaf, D.C.; Vanrobaeys, F.; Danneels, E.L.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon 2008, 52, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Pickets, J.A.; Williams, I.H.; Martin, A.P. (Z)-11-Eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). J. Chem. Ecol. 1982, 8, 163–175. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on freund’s complete adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; João, M.; Queiroz, R.P.; Ricardo, C. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal Filipa. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Varanda, E.A.; Tavares, D.C. Radioprotection: Mechanisms and radioprotective agents including honeybee venom. J. Venom. Anim. Toxins 1998, 4, 5–21. [Google Scholar] [CrossRef]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Baek, H.J.; Park, K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J. Med. Plants Res. 2010, 4, 459–464. [Google Scholar]
- McGhee, S.; Visovksy, C.; Zambroski, C.; Finnegan, A. Lyme disease: Recognition and management for emergency nurses. Emerg. Nurse 2018, 28. [Google Scholar] [CrossRef]
- Arteaga, V.; Lamas, A.; Regal, P.; Vázquez, B.; Manuel, J.; Cepeda, A.; Manuel, C. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pthogenesis 2019, 137, 103771–103776. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Lee, J.; Park, S.; Hyun, P.; Park, J.; Suh, G. Immunoprophylactic effects of administering honeybee (Apis melifera ) venom spray against salmonella gallinarum in broiler chicks. J. Vet. Sci. 2013, 75, 1287–1295. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model membrane and cell studies of antimicrobial activity of melittin analogues. Curr. Top. Med. Chem. 2016, 16, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; D’Ávila Vargas, G.; de Oliveira Hübner, S.; Fischer, G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef]
- Lubke, L.L.; Garon, C.F. The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the lyme disease spirochete. Clin. Infect. Dis. 1997, 25, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Navarre, W.W.; Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef]
- Lüderitz, O.; Freudenberg, M.A.; Galanos, C.; Lehmann, V.; Rietschel, E.T.; Shaw, D.H. Lipopolysaccharides of gram-negative bacteria. Curr. Top. Membr. Transp. 1982, 17, 79–151. [Google Scholar]
- Jamasbi, E.; Batinovic, S.; Sharples, R.A.; Sani, M.A.; Robins-Browne, R.M.; Wade, J.D.; Separovic, F.; Hossain, M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 2014, 46, 2759–2766. [Google Scholar] [CrossRef]
- Park, D.; Jung, J.W.; Lee, M.O.; Lee, S.Y.; Kim, B.; Jin, H.J.; Kim, J.; Ahn, Y.J.; Lee, K.W.; Song, Y.S.; et al. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 2014, 53, 185–193. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi, M.; Ali, V.; Hossein, H.; Jean, A.; Sabatier, M. Action mechanism of melittin—derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Dosler, S.; Gerceker, A.A. In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 2012, 24, 137–143. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’Amato, G.; Silvestri, C.; Del Prete, M.S.; Łukasiak, J.; Scalise, G. Comparative activities of cecropin A, melittin, and cecropin A–melittin peptide CA (1–7) M (2–9) NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 2003, 24, 1315–1318. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi-vala, M.; Pashaie, F.; Bevalian, P.; Hashemi, A.; Bagheri, K.P. Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of acinetobacter baumannii and pseudomonas aeruginosa. Microb. Drug Resist. 2018, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-T.; Hwang, J.-Y.; Sung, M.-S.; Je, S.-Y.; Bae, D.-R.; Han, S.-M.; Lee, S.-H. The minimum inhibitory concentration (MIC) of bee venom against bacteria isolated from pigs and chickens. Korean J. Vet. Serv 2006, 29, 19–26. [Google Scholar]
- Fernández, N.J.; Porrini, M.P.; Podaza, E.A.; Damiani, N.; Gende, L.B.; Eguaras, M.J. A scientific note on the first report of honeybee venom inhibiting Paenibacillus larvae growth. Apidologie 2014, 45, 719–721. [Google Scholar] [CrossRef]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee venom (Apis mellifera) an effective potential alternative to gentamicin for specific bacteria strains: Bee venom an effective potential for bacteria. J. Pharmacopunct. 2016, 19, 225–230. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First characterization of the venom from Apis mellifera syriaca, a honeybee from the middle east region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef]
- Vila-Farres, X.; Garcia de la Maria, C.; López-Rojas, R.; Pachón, J.; Giralt, E.; Vila, J. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2012, 18, 383–387. [Google Scholar] [CrossRef]
- Pashaei, F.; Bevalian, P.; Akbari, R.; Bagheri, K.P. Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb. Pathog. 2019, 127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Nagashima, Y.; Shiomi, K. Interaction of grammistins with lipids and their antibacterial activity. Fish. Sci. 2001, 67, 928–933. [Google Scholar] [CrossRef]
- Karyne, R.; Lechuga, G.C.; Souza, A.L.A.; das Silva Carvalho, J.P.R.; Bôas, M.H.S.V.; De Simone, S.G. Pan-drug resistant acinetobacter baumannii, but not other strains, are resistant to the bee venom peptide mellitin. Antibiotics 2020, 9, 178. [Google Scholar]
- Jamasbi, E.; Lucky, S.S.; Li, W.; Akhter, M.; Gopalakrishnakone, P.; Separovic, F. Effect of dimerized melittin on gastric cancer cells and antibacterial activity. Amino Acids 2018, 50, 1101–1110. [Google Scholar] [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Zhang, L.; Zhang, Y.; Cao, B. Cecropin A—melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014, 451, 650–655. [Google Scholar] [CrossRef]
- Saugar, J.M.; Alarcón, T.; López-Hernández, S.; López-Brea, M.; Andreu, D.; Rivas, L. Activities of polymyxin B and cecropin A-melittin peptide CA (1–8) M (1–18) against a multiresistant strain of acinetobacter baumannii. Antimicrob. Agents Chemother. 2002, 46, 875–878. [Google Scholar] [CrossRef]
- Saravanan, R.; Bhunia, A.; Bhattacharjya, S. Micelle-bound structures and dynamics of the hinge deleted analog of melittin and its diastereomer: Implications in cell selective lysis by d-amino acid containing antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 128–139. [Google Scholar] [CrossRef]
- Sun, X.; Chen, S.; Li, S.; Yan, H.; Fan, Y.; Mi, H. Deletion of two C-terminal Gln residues of 12–26-residue fragment of melittin improves its antimicrobial activity. Peptides 2005, 26, 369–375. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Soboksa, N.E.; Gari, S.R.; Hailu, A.B.; Alemu, B.M. Association between microbial water quality, sanitation and hygiene practices and childhood diarrhea in Kersa and Omo Nada districts of Jimma Zone, Ethiopia. PLoS ONE 2020, 15, e0229303. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Prim. 2017, 3, 17086–17101. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K. Water-Associated Infectious Diseases; Springer Nature Singapore Pte Ltd.: Singapore, 2019; ISBN 9789811391972. [Google Scholar]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Lee, B.-H.; Nikapitiya, C.; Kim, J.-H.; Kim, T.-H.; Lee, H.-C.; Kim, C.G.; Lee, J.-S.; Kim, C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Chaturvedi, P.; Chun, S.; Lee, Y.; Ahn, W. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and�E7 expression on cervical cancer cell line. Oncol. Rep. 2015, 33, 1675–1682. [Google Scholar] [CrossRef]
- Lee, J.-A.; Kim, Y.-M.; Hyun, P.-M.; Jeon, J.-W.; Park, J.-K.; Suh, G.-H.; Jung, B.-G.; Lee, B.-J. Honeybee (Apis mellifera) venom reinforces viral clearance during the early stage of infection with porcine reproductive and respiratory syndrome virus through the up-regulation of Th1-specific immune responses. Toxins 2015, 7, 1837–1853. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.-C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.-C.; Lazdunski, M.; Doglio, A. Secreted phospholipases A 2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K. Broad-spectrum antiviral agents: Secreted phospholipase A 2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017, 7, 15931. [Google Scholar] [CrossRef]
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Baghian, A.; Kousoulas, K.G. Role of the Na+, K+ pump in herpes simplex type 1-induced cell fusion: Melittin causes specific reversion of syncytial mutants with the syn1 mutation to Syn+ (wild-type) phenotype. Virology 1993, 196, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef]
- Kamal, S.A. In vitro study on the effect of bee venom on some cell lines and lumpy skin disease virus. J. Agric. Sci. Technol. A 2016, 6, 124–135. [Google Scholar]
- Fujii, G.; Horvath, S.; Woodward, S.; Eiserling, F.; Eisenberg, D. A molecular model for membrane fusion based on solution studies of an amphiphilic peptide from HIV gp41. Protein Sci. 1992, 1, 1454–1464. [Google Scholar] [CrossRef]
- Yasin, B.; Pang, M.; Turner, J.S.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I.; Wagar, E.A. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B. Antifungal activity of bee venom and sweet bee venom against clinically isolated candida albicans. J. Pharmacopunct. 2016, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.R.; Kim, J.J.; Park, G.S.; Oh, S.M.; Han, C.S.; Lee, M.Y. The antifungal activity of bee venom against dermatophytes. J. Appl. Biol. Chem. 2012, 55, 7–11. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol. Lett. 2014, 355, 36–42. [Google Scholar] [CrossRef]
- Park, C.; Lee, D.G. Melittin induces apoptotic features in Candida albicans. Biochem. Biophys. Res. Commun. 2010, 394, 170–172. [Google Scholar] [CrossRef]
- Ali, E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014, 53, 441–451. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Influence of the hydrophobic amino acids in the N- and C-terminal regions of pleurocidin on antifungal activity. J. Microbiol. Biotechnol. 2010, 20, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gupta, A.; Karne, S.P.; Kamble, S.; Shinde, B. Anti-inflammatory activity of sting protein from Apis mellifera. Int. J. Life Sci. Sci. Res. 2017, 3, 914–919. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.R.; Kim, K.H.; An, H.J.; Chang, Y.C.; Han, S.M.; Park, Y.Y.; Pak, S.C.; Park, K.K. Effects of bee venom against Propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015, 35, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Pak, S.C.; Nicholls, Y.M.; Macfarlane, N. Evaluation of anti-acne property of purified bee venom serum in humans. J. Cosmet. Dermatol. 2016, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, L.; Ewan, P.; Huber, P.A.J.; Clark, A.; Nasser, S.; Krishna, M.T. The impact of national guidelines on venom immunotherapy practice in the United Kingdom. Clin. Exp. Allergy 2016, 46, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Safaeinejad, Z.; Nabiuni, M.; Nazari, Z. Potentiation of a novel palladium (II) complex lethality with bee venom on the human T-cell acute lymphoblastic leukemia cell line (MOLT-4). J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Alizadehnohi, M.; Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Irian, S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J. Venom Res. 2012, 3, 22–27. [Google Scholar]
- Trindade, R.A.; Kiyohara, P.K.; De Araujo, P.S.; da Costa Bueno, M.H. PLGA microspheres containing bee venom proteins for preventive immunotherapy. Int. J. Pharm. 2012, 423, 124–133. [Google Scholar] [CrossRef]
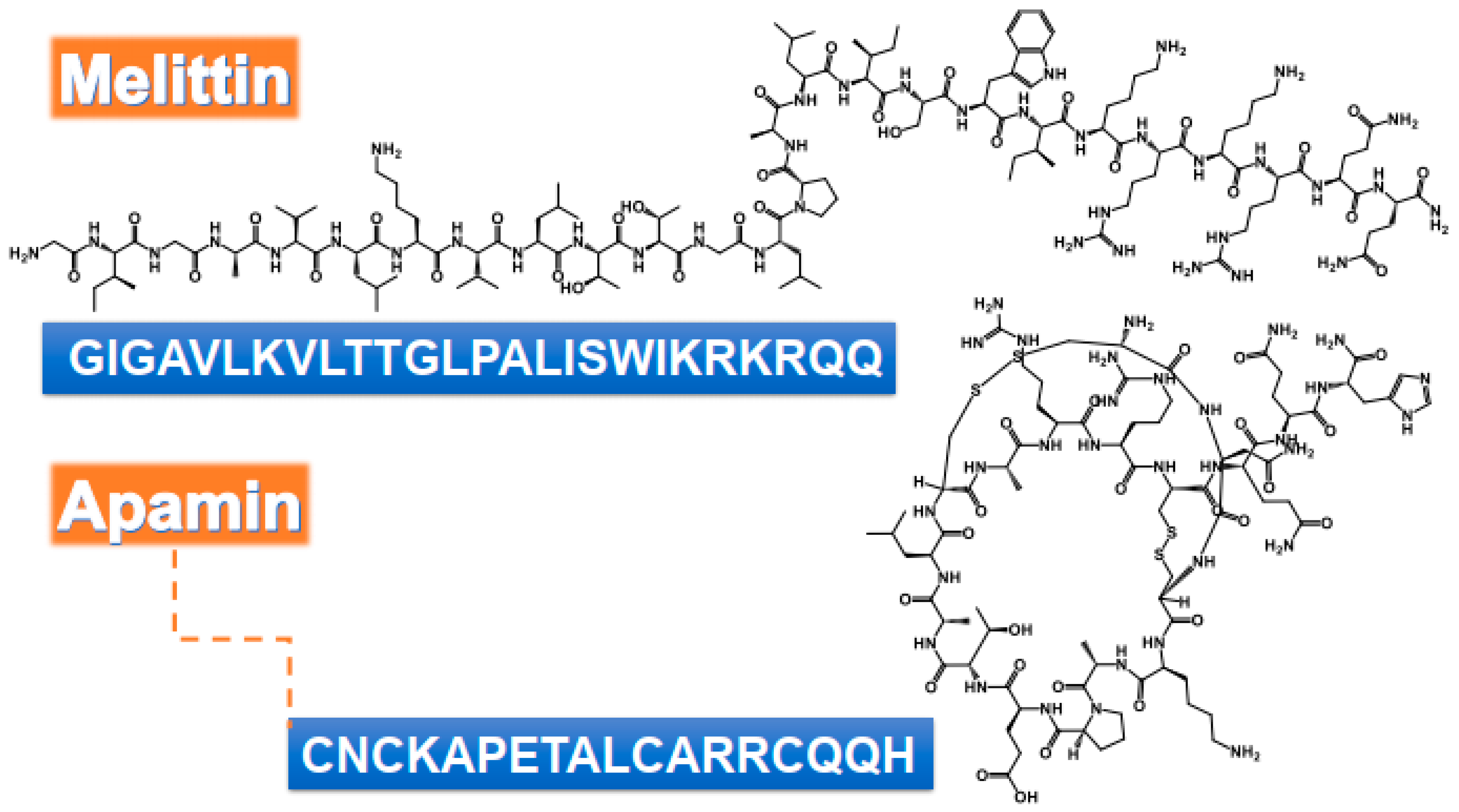
| Bee Venom/Isolated Compounds | Organism | Method | Dose/Mode of Action | Reference |
|---|---|---|---|---|
| Bee venom | S. aureus | Disc diffusion | MIC 8 µg/mL | [57] |
| MBC 16 µg/mL | ||||
| S. aureus Gp | Disc-diffusion | At 100 µg/mL give inhibition zone 23.2 mm after 24 h | [10] | |
| MRSA CCARM 3366 | Broth microdilution | MIC 0.085 μg/mL | [5]. | |
| MBC 0.106 μg/ mL | ||||
| S. aureus CCARM 3708 | Broth microdilution | MIC 0.11 μg/mL | [5]. | |
| MBC 0.14 μg/mL | ||||
| MR S. aureus ATCC 33591 | Broth microdilution | MIC90% 7.2 μg/mL | [11] | |
| MBC90% 28.7 μg/mL | ||||
| PC: Cephalothin | ||||
| MIC90% 2 μg/mL | ||||
| MBC90% 2 μg/mL | ||||
| S. aureus enterotoxin ATCC 23235 | Broth microdilution | MIC 0.7 μg/mL | [11] | |
| PC: Cephalothin and Oxacillin | ||||
| MIC < 0.5 μg /mL | ||||
| S. hyicus | Disc diffusion | MIC 128 µg/mL | [57] | |
| MBC 128 µg/mL | ||||
| S. chromogenes | Disc diffusion | MIC 128 µg/mL | [57] | |
| MBC 128 µg/mL | ||||
| S. salivarius | Broth microdilution | MIC 20 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 0.9 µg/mL | ||||
| S. sanguinis | Broth microdilution | MIC 30 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 3.7 µg/mL | ||||
| S. sobrinus | Broth microdilution | MIC 40 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 0.9 µg/mL | ||||
| S. mitis | Broth microdilution | MIC 40 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 3.7 µg/mL | ||||
| S. mutans | Broth microdilution | MIC 20 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 0.9 µg/mL | ||||
| Klebsiella pneumonia | Broth microdilution | MIC 30 µg/mL for 24 h | [16] | |
| Bacillus subtilis | Broth microdilution | MIC 30 µg/mL for 24 h | [16] | |
| Paenibacillus larvae | Resazurin method | MIC 3.12 μg/mL | [58] | |
| MBC 4.16 μg/mL | ||||
| PC: Oxytetracycline | ||||
| MIC 0.63 μg/mL | ||||
| MBC 0.83 μg/mL | ||||
| E. faecalis | Broth microdilution | MIC 20 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 3.7 µg/mL | ||||
| L. casei | Broth microdilution | MIC 20 µg/mL | [4] | |
| PC: Chlorhexidine digluconate | ||||
| MIC 0.9 µg/mL | ||||
| Salmonella typhimurium | Disc-diffusion | Inhibition zone was 15.88 mm at 45 μg | [59] | |
| PC: Gentamicin | ||||
| Inhibition zone was 19 mm at 10 μg/mL | ||||
| E. coli | Disc-diffusion | At 45 μg inhibits 32.46 mm | [59] | |
| PC: Gentamicin | ||||
| At 10 μg/mL inhibits 20 mm | ||||
| P. aeruginosa | NR | The antibacterial activity was 38% at 50 µg/mL | [60] | |
| Borrelial spirochetes | Direct counting method | MIC 200 µg/mL | [6] | |
| PC: Doxycycline, cefoperazone, and daptomycin | ||||
| MIC 10 µg/mL | ||||
| Melittin | S. salivarius | Broth microdilution | MIC 10 µg/mL | [4] |
| E. faecalis | Broth microdilution | MIC 6 µg/mL | [4] | |
| L. casei | Broth microdilution | MIC 4 µg/mL | [4] | |
| S. sanguinis | Broth microdilution | MIC 10 µg/mL | [4] | |
| S. sobrinus | Broth microdilution | MIC 10 µg/mL | [4] | |
| S. mitis | Broth microdilution | MIC 10 µg/mL | [4] | |
| S. mutans | Broth microdilution | MIC 40 µg/mL | [4] | |
| K. pneumonia | Broth microdilution | MIC 8 µg/mL throughout 24 h | [16] | |
| B. subtilis | Broth microdilution | MIC 6 µg/mL for 24 h | [16] | |
| Susceptible colistin- A. baumannii | Broth microdilution | MIC 4 mg/L after 24 h | [61] | |
| Acinetobacter spp. | Disc diffusion | Cell lysis | [62] | |
| Membranolytic effect | ||||
| MIC 0.5 µg/mL | ||||
| Colistin-resistant A. baumannii | Broth microdilution | MIC 2 mg/L after 24 h | [61] | |
| Listeria monocytogenes F4244 | Agar well diffusion | MIC 0.315 µg/mL | [53] | |
| MBC 3.263 µg/mL | ||||
| MR S. aureus ATCC 33591 | Broth microdilution | MIC90% 6.7 μg/mL | [11] | |
| MBC90% 26 μg/mL | ||||
| PC: Cephalothin | ||||
| MIC90% 2 μg/mL | ||||
| MBC90% 2 μg/mL | ||||
| S. aureus enterotoxin ATCC 23235 | Broth microdilution | MIC 3.6 μg/mL | [11] | |
| PC: Cephalothin and Oxacillin | ||||
| MIC <0.5 μg /mL | ||||
| S. aureus | Microtiter broth dilution | MIC 6.25 μg/mL | [63] | |
| B. spirochetes | Direct counting method | MIC 200 µg/mL | [6] | |
| PC: Doxycycline, cefoperazone, and daptomycin | ||||
| MIC 10 µg/mL | ||||
| A. baumannii ATCC 19606 | Broth microdilution | MIC 17µg/mL | [64] | |
| PC: Polymyxin | ||||
| MIC 0.25 µg/mL | ||||
| Imipenem: | ||||
| MIC ≤ 0.125 0.25 µg/mL | ||||
| A. baumannii 31852 (S) | Broth microdilution | MIC 20 µg/mL | [64] | |
| PC: Polymyxin | ||||
| MIC 0.25 µg/mL | ||||
| Imipenem: | ||||
| MIC 0.25 µg/mL | ||||
| A. baumannii 33677 (XDR) | Broth microdilution | MIC 31 µg/mL | [64] | |
| PC: Polymyxin | ||||
| MIC 0.25 µg/mL | ||||
| Imipenem: | ||||
| MIC 16 µg/mL | ||||
| A. baumannii 96734 (XDR) | Broth microdilution | MIC 45.5 µg/mL | [64] | |
| PC: Polymyxin | ||||
| MIC 0.25 µg/mL | ||||
| Imipenem: | ||||
| MIC 16 µg/mL | ||||
| Synthetic Melittin and Its Analogues | ||||
| Synthetic melittin | P. aeruginosa ATCC 47085 | Luria broth | MIC 12.1 µM | [65] |
| E. coli ATCC 29222 | Luria broth | MIC 13.2 µM | [65] | |
| E. coli DH5 | NR | MIC 3.9 µM | [66] | |
| PC: Tetracycline | ||||
| MIC 1.2 µM | ||||
| K. pneumoniae ATCC 13883 | Luria broth | MIC 14.9 µM | [65] | |
| A. baumannii ATCC 19606 | Luria broth | MIC 8.3 µM | [65] | |
| B. subtilis | NR | MIC 2 µM | [66] | |
| PC: Tetracycline | ||||
| MIC 0.2 µM | ||||
| S. aureus | NR | MIC 3.6 µM | [66] | |
| PC: Tetracycline | ||||
| MIC 4 µM | ||||
| Melittin Hybrid | ||||
| Cecropin A–melittin (CAM) | E. coli | Microtiter broth dilution | MIC 3.7 µg /mL | [67] |
| CAM-W | E. coli | Microtiter broth dilution | MIC 0.3 µg/mL | [67] |
| Cecropin A-melittin CA(1–8)M(1–18) | A. baumannii | Mueller-Hinton broth | MIC 2 µM | [68] |
| PC: Polymyxin B | ||||
| MIC 1 µM | ||||
| Mutant melittin I17K | L. monocytogenes F4244 | Agar well diffusion | MIC 0.814 µg/mL | [53] |
| MBC 7.412 µg/mL | ||||
| Mutant melittin G1I | L. monocytogenes F4244 | Agar well diffusion | MIC 0.494 µg/mL | [53] |
| MBC 5.366 µg/mL | ||||
| MM-1 | B. subtilis | NR | MIC 2.4 µM | [66] |
| PC: Tetracycline | ||||
| MIC 0.2 µM | ||||
| MM-2 | B. subtilis | NR | MIC 1.8 µM | [66] |
| PC: Tetracycline | ||||
| MIC 0.2 µM | ||||
| Mel-H | E. coli | Microtiter broth dilution | MIC 11.25 µM | [69] |
| P. aeruginosa ATCC27853 | Microtiter broth dilution | MIC 11.25 µM | [69] | |
| S. aureus ATCC25923 | Microtiter broth dilution | MIC 5.6 µM | [69] | |
| Mel(12–24) | B. subtilis | Broth microdilution | MIC 0.65 µg/mL | [70] |
| PC: Melittin | ||||
| MIC 0.18 µg/mL | ||||
| S. aureus | Broth microdilution | MIC 1.3 µg/mL | [70] | |
| PC: melittin | ||||
| MIC 0.72 µg/mL | ||||
| Phospholipase A2 | S. aureus Gp | Disc-diffusion | Hydrolysis of phospholipids | [10] |
| At 100 µg/mL inhibits 13.33 mm after 24 h | ||||
| L. casei | Broth microdilution | MIC 400 µg/mL | [4] | |
| Bee Venom/Isolated Compounds | Organism | Method | Dose/Mode of Action | Reference |
|---|---|---|---|---|
| Bee venom | Papillomaviruses (HPV16 E6) | Reverse transcription assay | Inhibits mRNA expression. | [77] |
| Suppresses cell growth. | ||||
| Downregulates protein. | ||||
| At 10 µg/mL inhibits 0.35 ± 0.06-fold after 24 h. | ||||
| Papillomaviruses (HPV16 E7) | Reverse transcription assay | Inhibits mRNA expression. | [77] | |
| Suppresses cell growth. | ||||
| Downregulates protein. | ||||
| At 10 µg/mL inhibits 0.44 ± 0.07-fold after 24 h. | ||||
| PRRSV | Enzyme-linked immunosorbent assay | Increases immunomodulatory against the virus. | [78] | |
| Significant up-regulate Th1 cytokines (IFN-γ and IL-12) and several types of immune cells. | ||||
| Vesicular stomatitis virus (VSV) | Plaque assay | Inhibits virus replication | [76] | |
| EC50 0.5 ± 0.06 μg/mL | ||||
| HSV | Plaque assay | Inhibits virus replication | [76] | |
| EC50 1.52 ± 0.11 μg/mL | ||||
| Coxsackie virus (H3) | Plaque assay | Inhibits mRNA expression | [76] | |
| Inhibits virus replication | ||||
| EC50 0.5 ± 0.04 μg/mL | ||||
| RSV | Plaque assay | Inhibits virus replication | [76] | |
| EC50 1.17 ± 0.09 μg/mL | ||||
| PR8 | Plaque assay | Inhibits virus replication. | [76] | |
| EC50 1.81 ± 0.08 μg/mL | ||||
| EV-71 | Plaque assay | Inhibits mRNA expression. | [76] | |
| Inhibits virus replication | ||||
| EC50 0.49 ± 0.02 μg/mL | ||||
| Lumpy skin disease virus (LSDV) | Agar gel precipitation test | At the dose 0.5 μg/mL | [85] | |
| Melittin | Immunodeficiency virus (HIV) | Lysis and fusion assays | Lytic and fusogenic | [86] |
| Herpes simplex (HSV-1) | Plaque assay Virus penetration assay | Inhibits cell fusion. | [83] | |
| Inhibits Na+, K+ pump activity. | ||||
| Inhibits virus adsorption and penetration to the cells. | ||||
| Immunodeficiency virus HIV-1 | Transient transfection Assays | Inhibits virus replication. | [84] | |
| Suppresses gene expression. | ||||
| Suppresses intracellular | ||||
| Protein and mRNA synthesis. | ||||
| Suppresses long terminal repeat (LTR) activity | ||||
| ID50 0.9–1.4 µM after 24 h. | ||||
| Arenavirus Junin (JV) | Plaque assay | Impedes the multiplication | [82] | |
| EC50 0.86 µM after 24 h. | ||||
| HSV-1 | Plaque assay | Impedes the multiplication | [82] | |
| EC50 1.35 µM after 24 h. | ||||
| Herpes simplex virus (HSV-2) | Plaque assay | Impedes the multiplication | [82] | |
| EC50 2.05 µM after 24 h. | ||||
| Herpes simplex virus 1 M (HSV-1 M) | Quantitative microplate assay | Viral inactivation at 100 µg/mL | [87] | |
| Herpes simplex virus 2 G (HSV-2 G) | Quantitative microplate assay | Viral inactivation at 100 µg/mL | [87] | |
| Phospholipase A2 (sPLA2) | Hepatitis C virus (HCV) | Plaque assay | IC50 117 ± 43 ng/mL after 24 h. | [81] |
| DENV | Plaque assay | IC50 183 ± 38 ng/mL after 24 h. | [81] | |
| JEV | Plaque assay | IC50 49 ± 13 ng/mL after 24 h. | [81] |
| Bee Venom/ Isolated Compounds | Organism | Method | Dose/Mode of Action | Reference |
|---|---|---|---|---|
| Bee venom | T. mentagrophytes | Broth dilution | At 0.63 ppm inhibits 92% After 1 h. | [89] |
| T. rubrum | Broth dilution | At 0.63 ppm inhibits 26% After 1 h. | [89] | |
| C. albicans | Disc diffusion | Prevents dimorphism MIC 40 µg/mL for 48 h. | [92] | |
| C. albicans | Broth microdilution | MIC 62.5–125 μg/mL for 24 h. | [88] | |
| Candida krusei | Broth microdilution | MIC 60 µg/mL throughout 48 h. | [16] | |
| A. alternate | NR | At 1 µg/mL inhibits 50% of interleukin (IL)-6 production. | [3] | |
| At 1 µg/mL inhibits 28.8% of interleukin (IL)-8 production. | [3] | |||
| Melittin | C. krusei | Broth microdilution | MIC 30 µg/mL for 48 h | [16] |
| C. albicans | NR | Disruptive the mitochondrial membrane. | [90] | |
| Apoptotic for 4 h | ||||
| Aspergillus flavus (KCTC 1375) | Microdilution method and MTT assay | MIC 1.25 µM | [93] | |
| PC: Amphotericin B: | ||||
| MIC 2.5 µM | ||||
| Fluconazole: MIC 10 µM | ||||
| Itraconazole: MIC 10 µM | ||||
| Malassezia furfur (KCTC 7744) | Microdilution method and MTT assay | MIC 1.25 µM | [93] | |
| PC: Amphotericin B: MIC 2.5 µM | ||||
| Fluconazole: MIC 5 µM | ||||
| Itraconazole: MIC 5 µM | ||||
| C. albicans (ATCC 90028) | Microdilution method and MTT assay | MIC 2.5 µM | [93] | |
| PC: Amphotericin B: MIC 5 µM | ||||
| Fluconazole: MIC 10 µM | ||||
| Itraconazole: MIC 10 µM | ||||
| Apamin | A. alternate | NR | At 1 µg/mL inhibits 42.6% of interleukin (IL)-6 production. | [3] |
| At 1 µg/mL inhibits 38.7% of interleukin (IL)-8 production. | [3] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Seedi, H.; Abd El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. https://doi.org/10.3390/toxins12070451
El-Seedi H, Abd El-Wahed A, Yosri N, Musharraf SG, Chen L, Moustafa M, Zou X, Al-Mousawi S, Guo Z, Khatib A, et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins. 2020; 12(7):451. https://doi.org/10.3390/toxins12070451
Chicago/Turabian StyleEl-Seedi, Hesham, Aida Abd El-Wahed, Nermeen Yosri, Syed Ghulam Musharraf, Lei Chen, Moustafa Moustafa, Xiaobo Zou, Saleh Al-Mousawi, Zhiming Guo, Alfi Khatib, and et al. 2020. "Antimicrobial Properties of Apis mellifera’s Bee Venom" Toxins 12, no. 7: 451. https://doi.org/10.3390/toxins12070451
APA StyleEl-Seedi, H., Abd El-Wahed, A., Yosri, N., Musharraf, S. G., Chen, L., Moustafa, M., Zou, X., Al-Mousawi, S., Guo, Z., Khatib, A., & Khalifa, S. (2020). Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins, 12(7), 451. https://doi.org/10.3390/toxins12070451