The Synergism of PGN, LTA and LPS in Inducing Transcriptome Changes, Inflammatory Responses and a Decrease in Lactation as Well as the Associated Epigenetic Mechanisms in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Effects of LPS Stimulation and Co-Stimulation with PGN, LTA and LPS (PLL) on Transcriptome
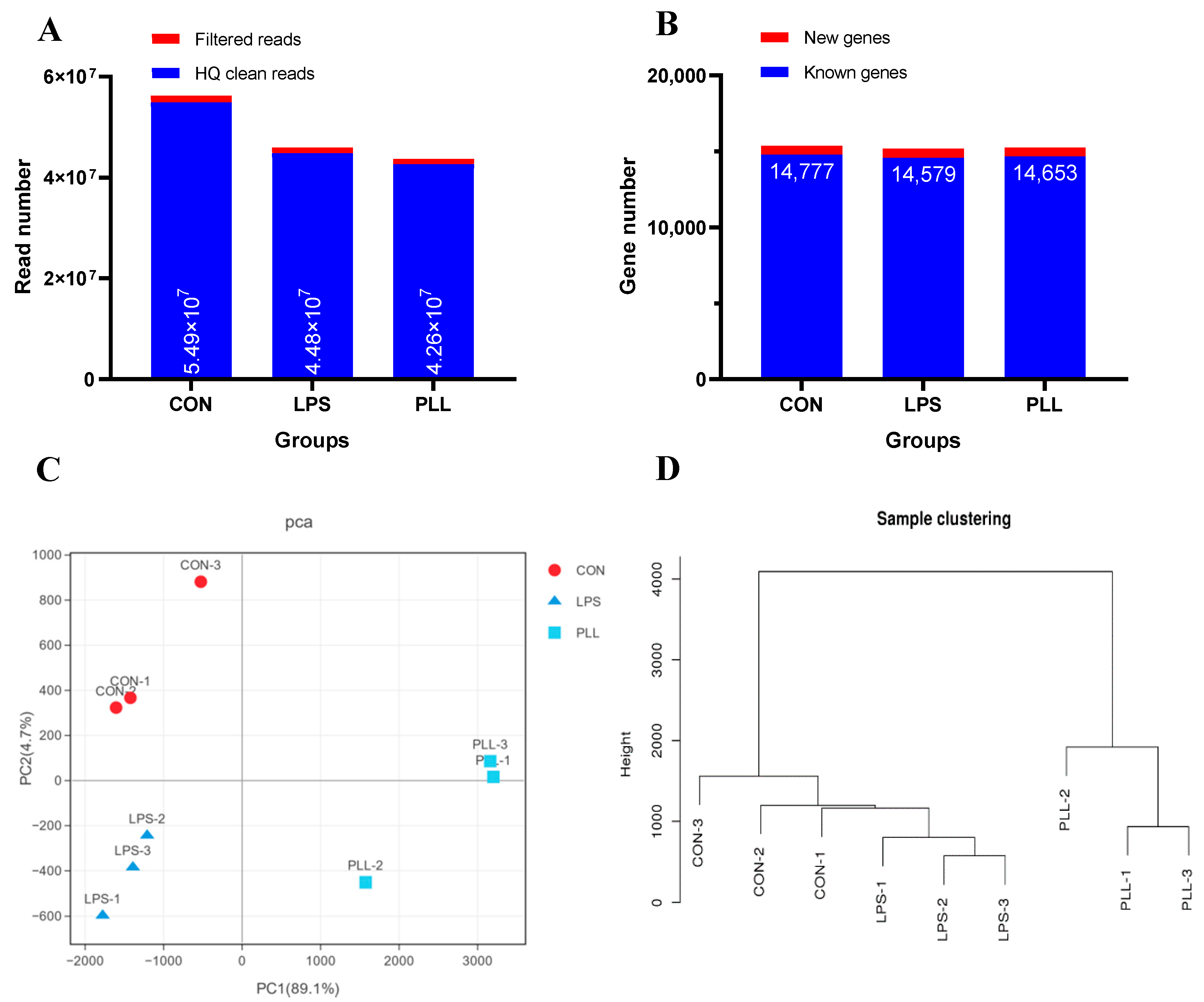
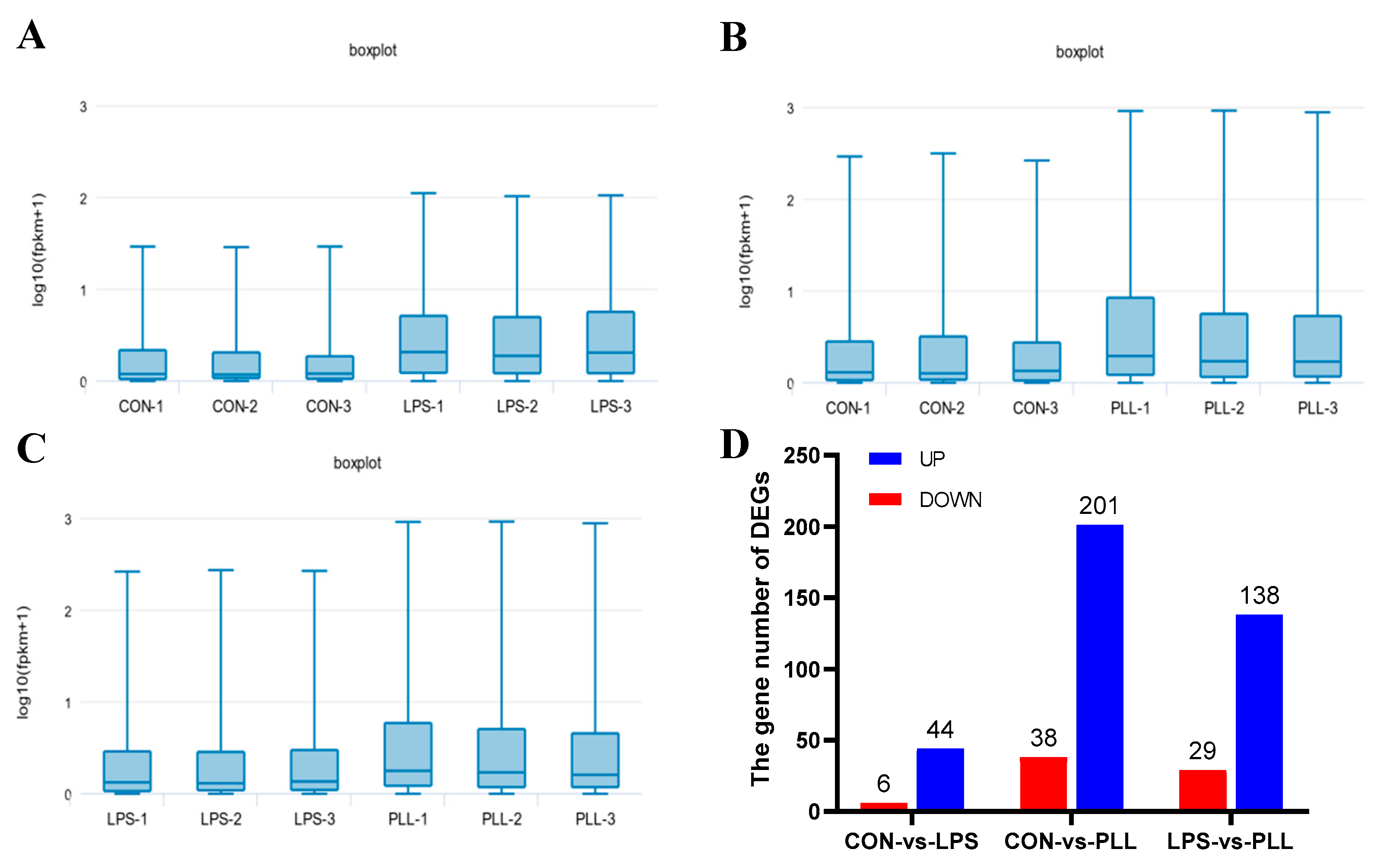
2.1.1. Overview of RNA Sequencing (RNA-Seq) Data
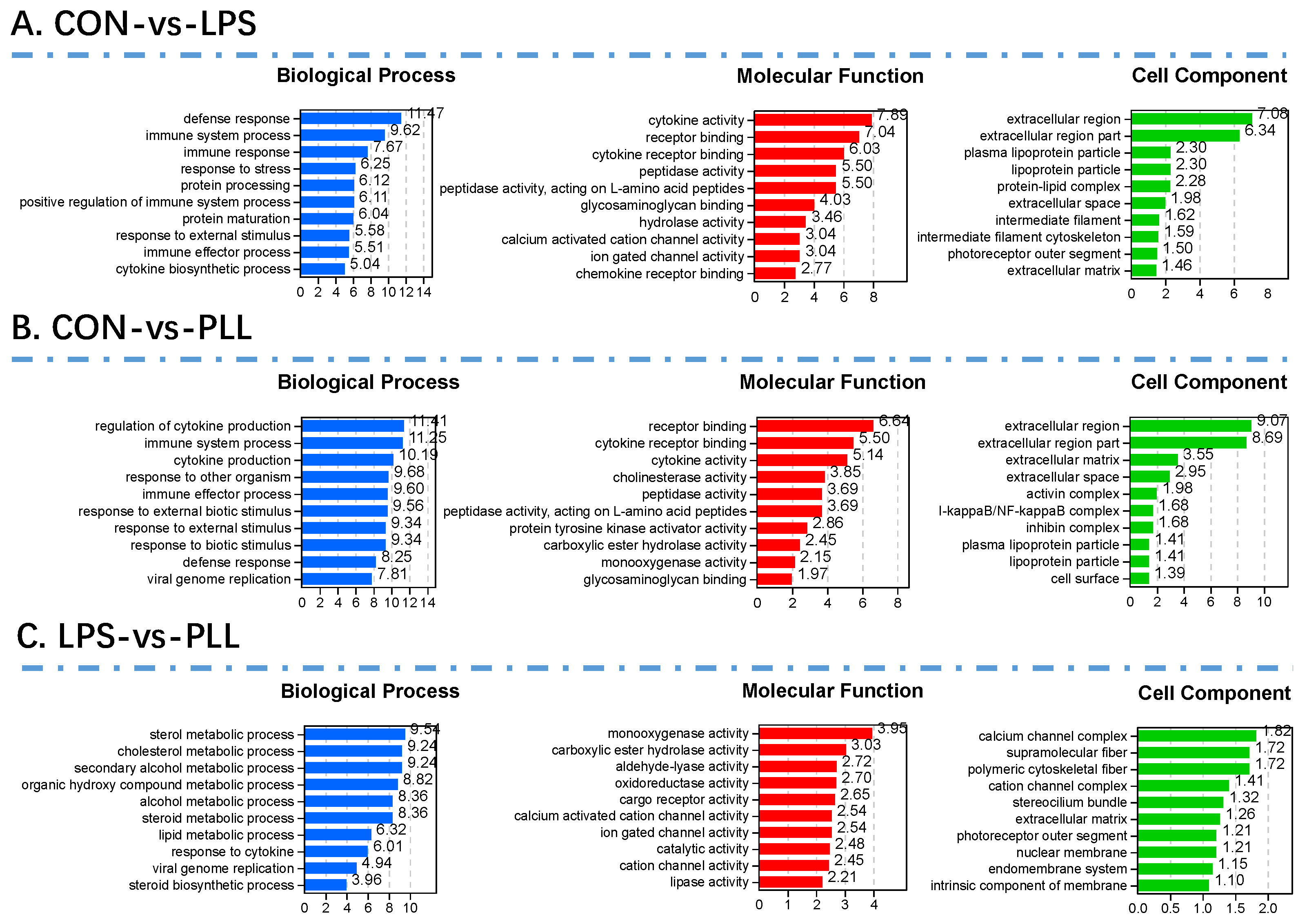
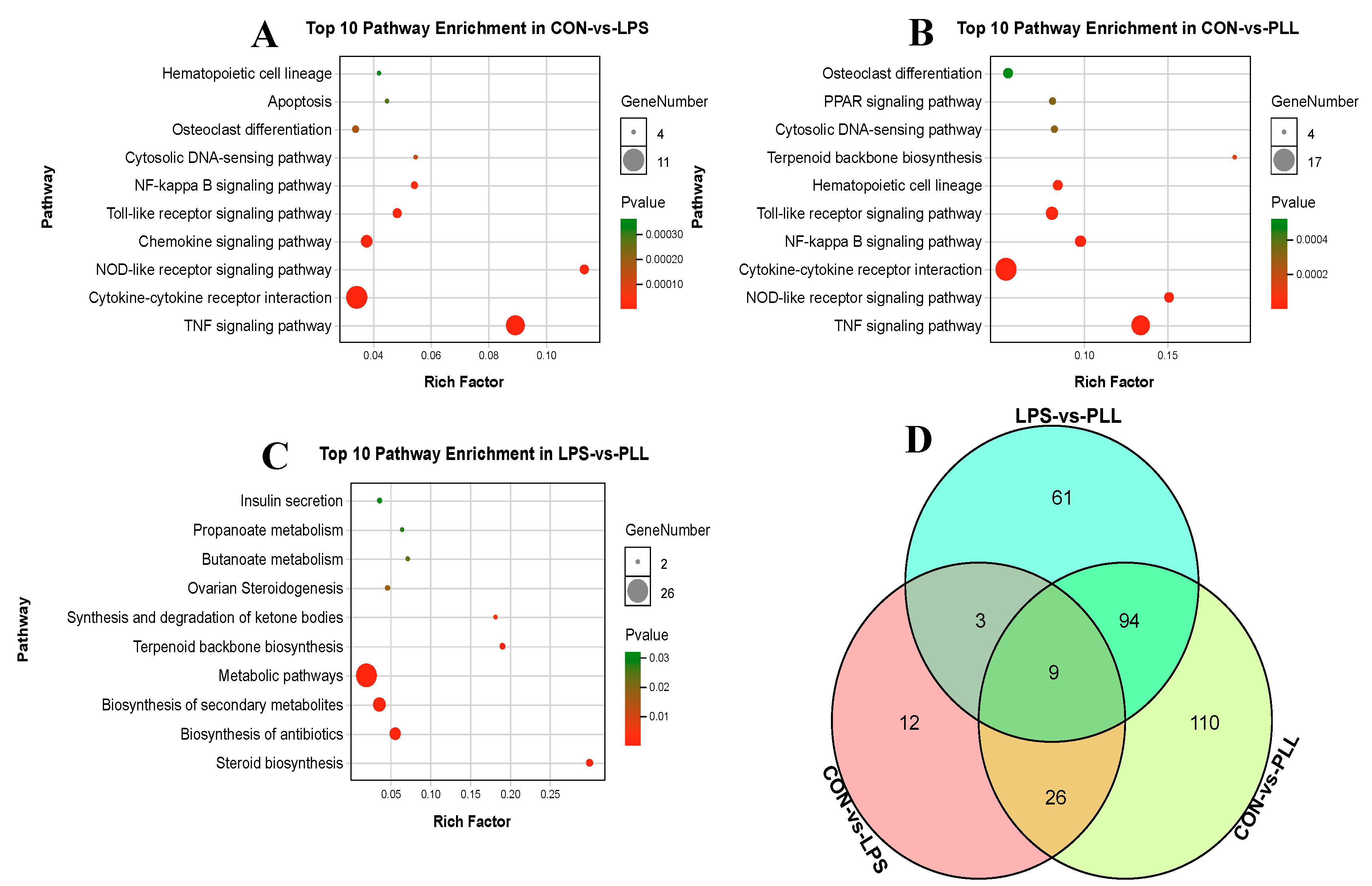
2.1.2. Gene Ontology (GO) Function Annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses of DEGs
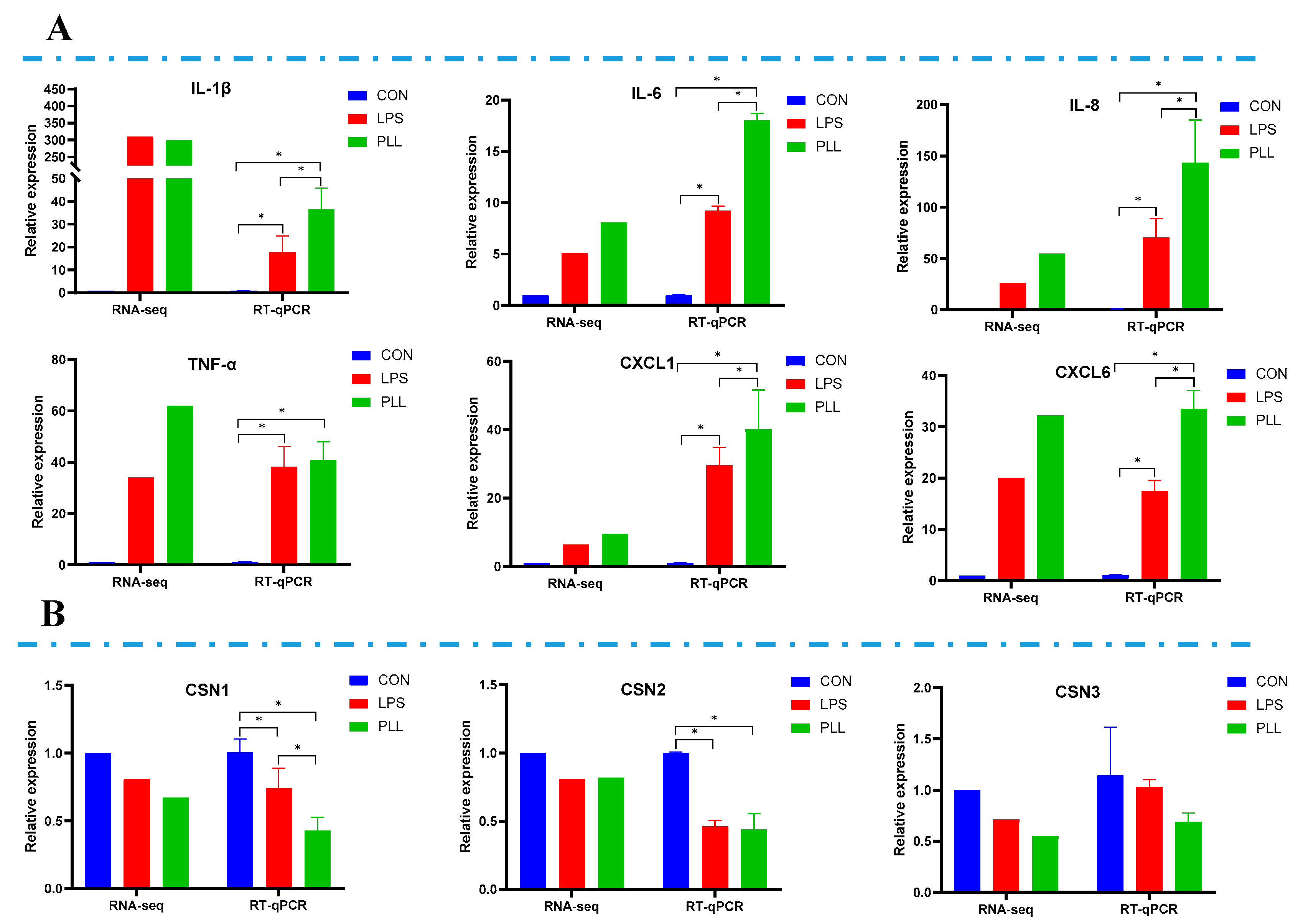
2.2. Validation of RNA-Seq Data by Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analyses
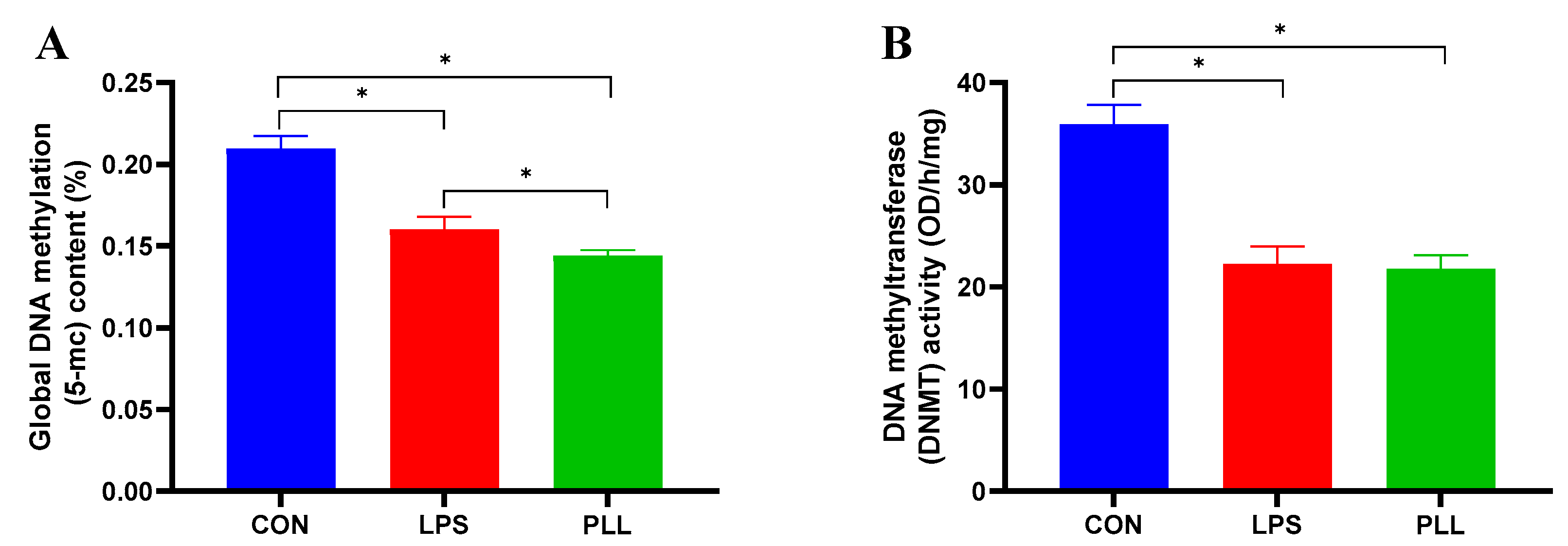
2.3. LPS and PLL Reduced Global DNA Methylation Levels by Suppressing DNMT Activity
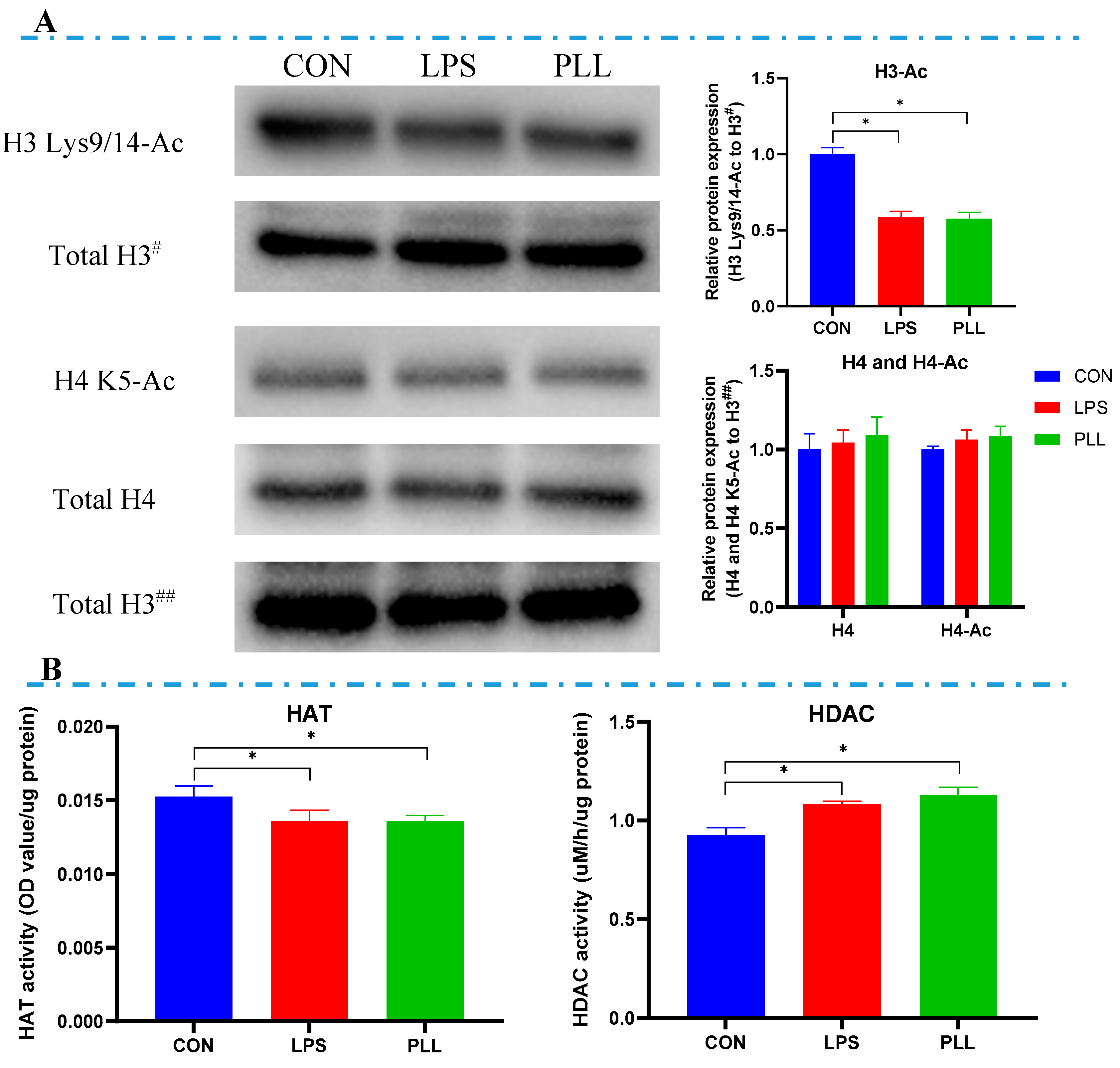
2.4. LPS and PLL Reduced Histone H3 Acetylation Levels by Suppressing HAT Activity and Promoting HDAC Activity
2.5. LPS and PLL Promoted Inflammation but Reduced Lactation
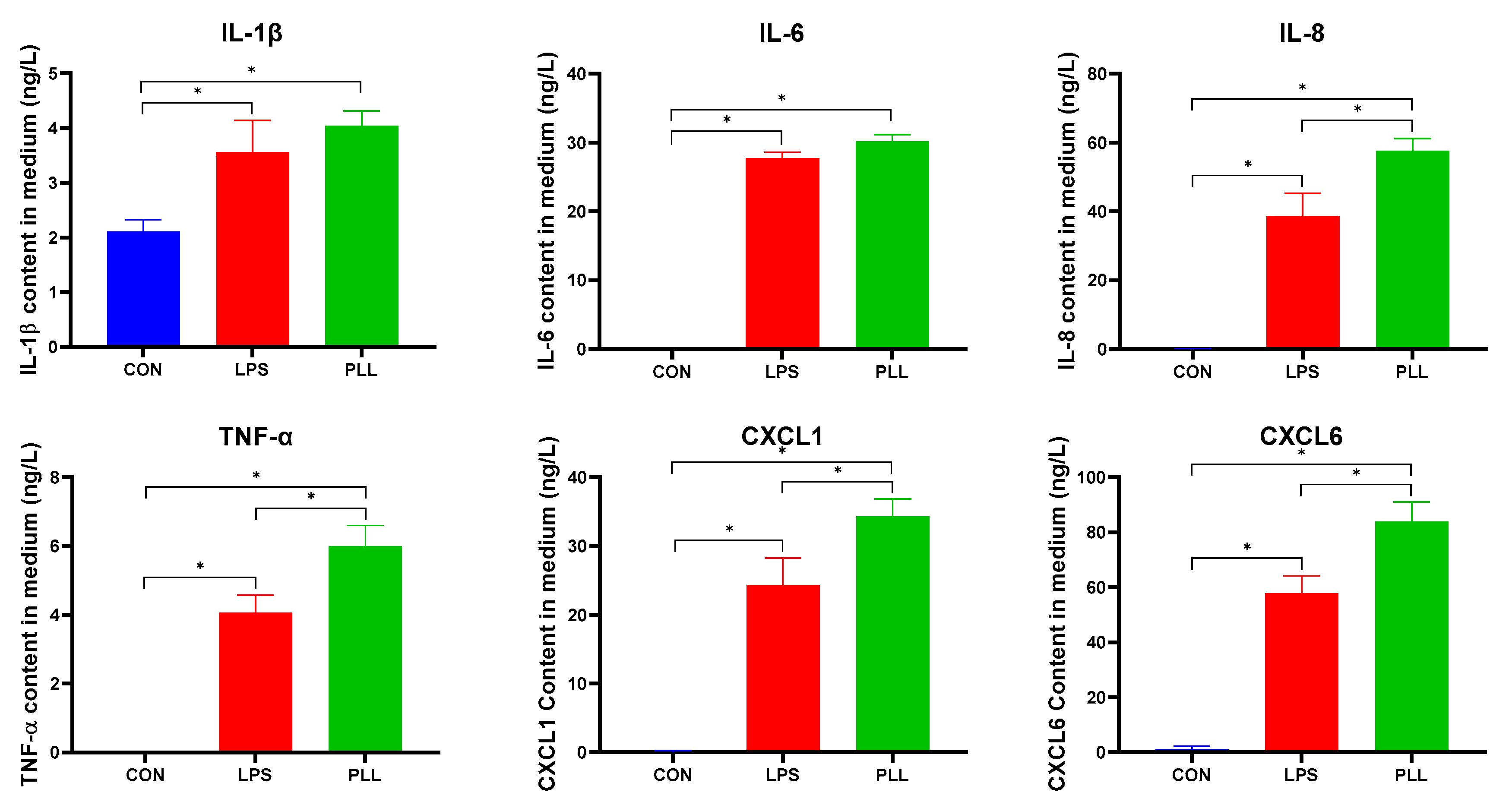
2.5.1. LPS and PLL Increased Inflammatory Factor Release
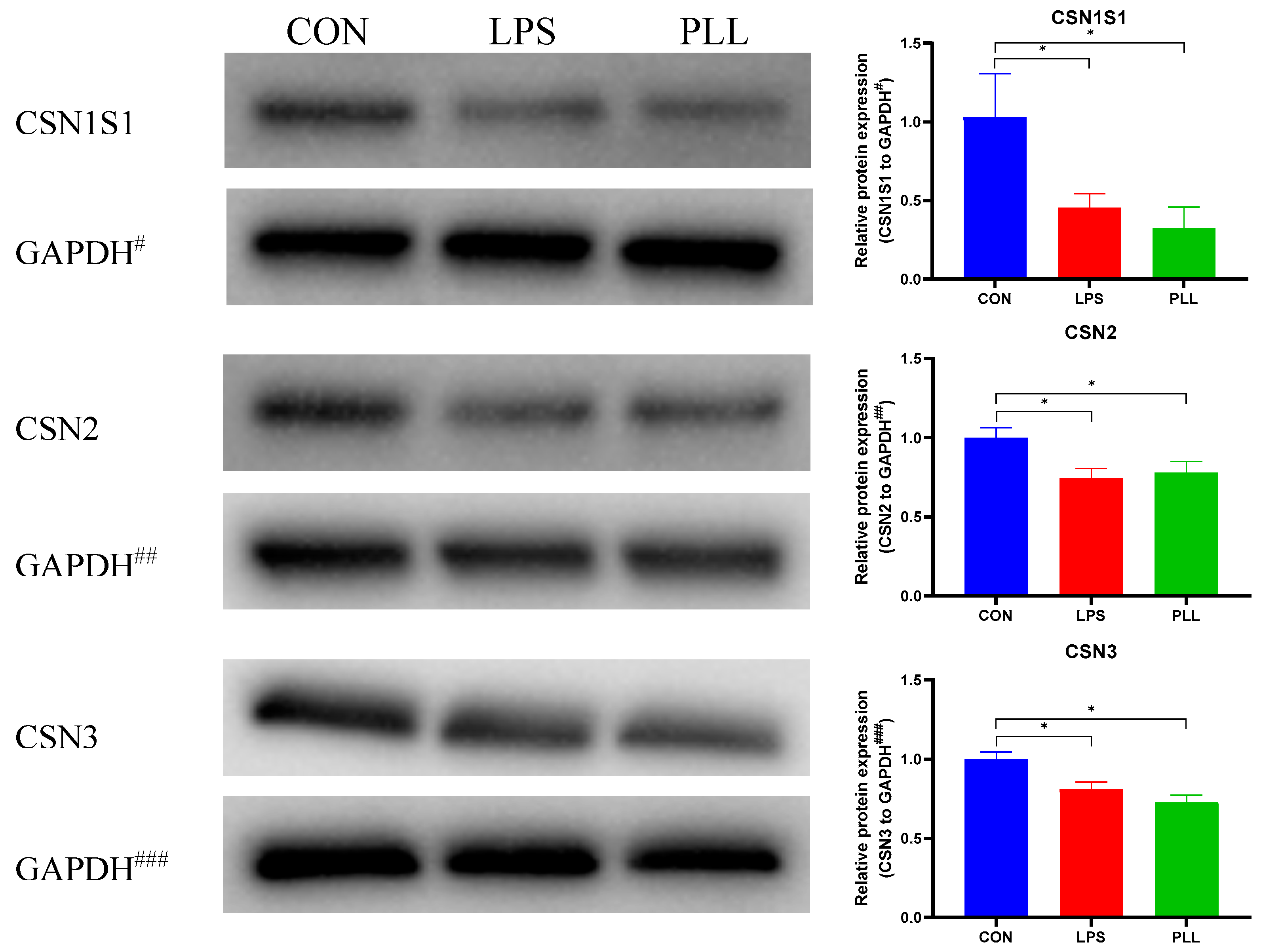
2.5.2. LPS and PLL Decreased Casein Synthesis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Treatments
5.2. RNA-Seq and RT-qPCR Validation
5.3. Western Blot and Enzyme-Linked Immunosorbent Assay (ELISA)
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, K.; Guenther, J.; Talbot, R.; Petzl, W.; Zerbe, H.; Schuberth, H.-J.; Seyfert, H.-M.; Glass, E.J. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genom. 2013, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Mount, J.A.; Karrow, N.A.; Caswell, J.L.; Boermans, H.J.; Leslie, K.E. Assessment of bovine mammary chemokine gene expression in response to lipopolysaccharide, lipotechoic acid plus peptidoglycan, and CpG oligodeoxynucleotide 2135. Can. J. Vet. Res. 2009, 73, 49–57. [Google Scholar]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Szyda, J.; Mielczarek, M.; Fraszczak, M.; Minozzi, G.; Williams, J.L.; Wojdak-Maksymiec, K. The genetic background of clinical mastitis in Holstein-Friesian cattle. Animal 2019, 13, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Bailey, T.L. Understanding the Basics of Mastitis; Virginia Cooperative Extension Publication, Virginia State University: Petersburg, VA, USA, 2009; pp. 1–7. [Google Scholar]
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakula, Z.; Wlazlo, L.; Woch, P. Prevalence of Prototheca spp. on dairy farms in Poland- a cross-country study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Hertl, J.A.; Groehn, Y.T.; Leach, J.D.G.; Bar, D.; Bennett, G.J.; Gonzalez, R.N.; Rauch, B.J.; Welcome, F.L.; Tauer, L.W.; Schukken, Y.H. Effects of clinical mastitis caused by gram-positive and gram-negative bacteria and other organisms on the probability of conception in New York State Holstein dairy cows. J. Dairy Sci. 2010, 93, 1551–1560. [Google Scholar] [CrossRef]
- Hertl, J.A.; Schukken, Y.H.; Bar, D.; Bennett, G.J.; Gonzalez, R.N.; Rauch, B.J.; Welcome, F.L.; Tauer, L.W.; Groehn, Y.T. The effect of recurrent episodes of clinical mastitis caused by gram-positive and gram-negative bacteria and other organisms on mortality and culling in Holstein dairy cows. J. Dairy Sci. 2011, 94, 4863–4877. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Coliform mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef]
- Hoekstra, J.; Rutten, V.; Lam, T.; Van Kessel, K.P.M.; Spaninks, M.P.; Stegeman, J.A.; Benedictus, L.; Koop, G. Activation of a Bovine Mammary Epithelial Cell Line by Ruminant-Associated Staphylococcus aureus is Lineage Dependent. Microorganisms 2019, 7, 688. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Guenther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.J.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Li, C.; Wang, G.; Xing, G. Gene microarray integrated with iTRAQ-based proteomics for the discovery of NLRP3 in LPS-induced inflammatory response of bovine mammary epithelial cells. J. Dairy Res. 2019, 86, 416–424. [Google Scholar] [CrossRef]
- Xu, T.; Deng, R.; Li, X.; Zhang, Y.; Gao, M.-Q. RNA-seq analysis of different inflammatory reactions induced by lipopolysaccharide and lipoteichoic acid in bovine mammary epithelial cells. Microb. Pathog. 2019, 130, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, K.; Pires, J.A.A.; Faulconnier, Y.; Chambon, C.; Germon, P.; Boby, C.; Leroux, C. Mammary Gland Transcriptome and Proteome Modifications by Nutrient Restriction in Early Lactation Holstein Cows Challenged with Intra-Mammary Lipopolysaccharide. Int. J. Mol. Sci. 2019, 20, 1156. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Zhou, Z.; Graugnard, D.E.; Rodriguez-Zas, S.L.; Palladino, A.R.; Cardoso, F.C.; Trevisi, E.; Loor, J.J. Acute mammary and liver transcriptome responses after an intramammary Escherichia coli lipopolysaccharide challenge in postpartal dairy cows. Physiol. Rep. 2015, 3, e12388. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granie, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 1–22. [Google Scholar] [CrossRef]
- Guenther, J.; Petzl, W.; Zerbe, H.; Schuberth, H.-J.; Koczan, D.; Goetze, L.; Seyfert, H.-M. Lipopolysaccharide priming enhances expression of effectors of immune defence while decreasing expression of pro-inflammatory cytokines in mammary epithelia cells from cows. BMC Genom. 2012, 13, 17. [Google Scholar] [CrossRef]
- Im, J.; Lee, T.; Jeon, J.H.; Baik, J.E.; Kim, K.W.; Kang, S.S.; Yun, C.H.; Kim, H.; Han, S.H. Gene expression profiling of bovine mammary gland epithelial cells stimulated with lipoteichoic acid plus peptidoglycan from Staphylococcus aureus. Int. Immunopharmacol. 2014, 21, 231–240. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Sun, Y.; Dong, X.; Wang, Z.; Chen, J.; Dong, G. PGN and LTA from Staphylococcus aureus Induced Inflammation and Decreased Lactation through Regulating DNA Methylation and Histone H3 Acetylation in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 238. [Google Scholar] [CrossRef]
- Yi, S.-J.; Lee, H.; Lee, J.; Lee, K.; Kim, J.; Kim, Y.; Park, J.-I.; Kim, K. Bone Remodeling: Histone Modifications as Fate Determinants of Bone Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, P.Y.; Hsiao, C.C.; Lin, M.C. Epigenetics: A Potential Mechanism Involved in the Pathogenesis of Various Adverse Consequences of Obstructive Sleep Apnea. Int. J. Mol. Sci. 2019, 20, 2937. [Google Scholar] [CrossRef]
- Sui, Y.H.; Wu, W.M.; Wang, Z.; Wang, J.X.; Wang, Z.H.; Wu, R.L. A case-control design for testing and estimating epigenetic effects on complex diseases. Brief. Bioinform. 2014, 15, 319–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pearce, K.; Cai, D.C.; Roberts, A.C.; Glanzman, D.L. Role of protein synthesis and DNA methylation in the consolidation and maintenance of long-term memory in Aplysia. eLife 2017, 6, e18299. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Hu, X.; Gao, Y.; Wang, Z.; Li, J.; Wong, J. Activated MEK/ERK Pathway Drives Widespread and Coordinated Overexpression of UHRF1 and DNMT1 in Cancer cells. Sci. Rep. 2019, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Hutnick, L.K.; Golshani, P.; Namihira, M.; Xue, Z.G.; Matynia, A.; Yang, X.W.; Silva, A.J.; Schweizer, F.E.; Fan, G.P. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum. Mol. Genet. 2009, 18, 2875–2888. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hou, C.; Wisler, J.; Singh, K.; Wu, C.; Xie, Z.G.; Lu, Q.J.; Zhou, Z.G. Elevated histone H3 acetylation is associated with genes involved in T lymphocyte activation and glutamate decarboxylase antibody production in patients with type 1 diabetes. J. Diabetes Investig. 2019, 10, 51–61. [Google Scholar] [CrossRef]
- Han, Z.; Yu, H.; Zhao, Z.; Hunter, D.; Luo, X.; Duan, J.; Tian, L. AtHD2D Gene Plays a Role in Plant Growth, Development, and Response to Abiotic Stresses in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 310. [Google Scholar] [CrossRef]
- Mottamal, M.; Zheng, S.L.; Huang, T.L.; Wang, G.D. Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, C.; Li, W.J.; Wu, R.Y.; Guo, Y.; Cheng, D.; Yang, Y.Q.; Androulakis, I.P.; Kong, A.N. Pharmacokinetics and Pharmacodynamics of the Triterpenoid Ursolic Acid in Regulating the Antioxidant, Anti-inflammatory, and Epigenetic Gene Responses in Rat Leukocytes. Mol. Pharm. 2017, 14, 3709–3717. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, J.; Yoon, S.Y.; Joo, J.C.; Park, S.J.; Park, Y.J. Promotion of Cell Death in Cisplatin-Resistant Ovarian Cancer Cells through KDM1B-DCLRE1B Modulation. Int. J. Mol. Sci. 2019, 20, 2443. [Google Scholar] [CrossRef]
- Cai, L.; Zhan, M.; Li, Q.; Li, D.; Xu, Q. DNA methyltransferase DNMT1 inhibits lipopolysaccharide-induced inflammatory response in human dental pulp cells involving the methylation changes of IL-6 and TRAF6. Mol. Med. Rep. 2020, 21, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Garcia-Gomez, A.; Morante-Palacios, O.; Ciudad, L.; Ozkaramehmet, S.; Van Dijck, E.; Rodriguez-Ubreva, J.; Vaquero, A.; Ballestar, E. SIRT1/2 orchestrate acquisition of DNA methylation and loss of histone H3 activating marks to prevent premature activation of inflammatory genes in macrophages. Nucleic Acids Res. 2020, 48, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Pierre, W.C.; Legault, L.-M.; Londono, I.; McGraw, S.; Lodygensky, G.A. Alteration of the brain methylation landscape following postnatal inflammatory injury in rat pups. FASEB J. 2020, 34, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, F.T.; Kerr, D.E. Genome-wide methylation analysis reveals differentially methylated loci that are associated with an age-dependent increase in bovine fibroblast response to LPS. BMC Genom. 2017, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Sun, Y.; Dong, X.; Wang, Z.; Zhang, Z.; Xiao, Y.; Dong, G. Bacterial Lipopolysaccharide Induced Alterations of Genome-Wide DNA Methylation and Promoter Methylation of Lactation-Related Genes in Bovine Mammary Epithelial Cells. Toxins 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Li, D.; Feng, Z.; Xu, Q. MyD88 hypermethylation mediated by DNMT1 is associated with LTA-induced inflammatory response in human odontoblast-like cells. Cell Tissue Res. 2019, 376, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Shuto, T.; Furuta, T.; Oba, M.; Xu, H.; Li, J.-D.; Cheung, J.; Gruenert, D.C.; Uehara, A.; Suico, M.A.; Okiyoneda, T.; et al. Promoter hypomethylation of Toll-like receptor-2 gene is associated with increased proinflammatory response toward bacterial peptidoglycan in cystic fibrosis bronchial epithelial cells. FASEB J. 2006, 20, 782–784. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.; Sun, Y.; Dong, X.; Wang, Z.; Zhang, Z.; Xiao, Y.; Dong, G. Bacterial endotoxin decreased histone H3 acetylation of bovine mammary epithelial cells and the adverse effect was suppressed by sodium butyrate. BMC Vet. Res. 2019, 15, 267. [Google Scholar] [CrossRef]
- Ling, B.B.; Alcorn, J. LPS-induced inflammation downregulates mammary gland glucose, fatty acid, and L-carnitine transporter expression at different lactation stages. Res. Vet. Sci. 2010, 89, 200–202. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, G.; Ao, C.; Zhang, S.; Qiu, M.; Wang, X.; Wu, Y.; Erdene, K.; Jin, L.; Lei, C.; et al. Feeding a high-concentrate corn straw diet increased the release of endotoxin in the rumen and pro-inflammatory cytokines in the mammary gland of dairy cows. BMC Vet. Res. 2014, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Kiku, Y.; Nagasawa, Y.; Tanabe, F.; Sugawara, K.; Watanabe, A.; Hata, E.; Ozawa, T.; Nakajima, K.-i.; Arai, T.; Hayashi, T. The cell wall component lipoteichoic acid of Staphylococcus aureus induces chemokine gene expression in bovine mammary epithelial cells. J. Vet. Med. Sci. 2016, 78, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Kratochvilova, L.; Kharkevich, K.; Slama, P. TNF-alpha and IL-10 are Produced by Leukocytes during the Experimental Inflammatory Response of Bovine Mammary Gland Induced by Peptidoglycan; Mendel University: Brno, Czech Republic, 2018; pp. 376–379. [Google Scholar]
- Kharkevich, K.; Kratochvilova, L.; Slama, P. Transforming Growth Factor Beta 1 Production during Inflammatory Response of Mammary Gland Induced by Peptidoglycan; Mendel University: Brno, Czech Republic, 2018; pp. 366–369. [Google Scholar]
- Zhang, W.Y.; Li, X.Z.; Xu, T.; Ma, M.R.; Zhang, Y.; Gao, M.Q. Inflammatory responses of stromal fibroblasts to inflammatory epithelial cells are involved in the pathogenesis of bovine mastitis. Exp. Cell Res. 2016, 349, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Jansson, P.A.; Perfilyev, A.; Volkov, P.; Pedersen, M.; Svensson, M.K.; Poulsen, P.; Ribel-Madsen, R.; Pedersen, N.L.; Almgren, P.; et al. Altered DNA Methylation and Differential Expression of Genes Influencing Metabolism and Inflammation in Adipose Tissue From Subjects With Type 2 Diabetes. Diabetes 2014, 63, 2962–2976. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Moreno-Indias, I.; Sanchez-Alcoholado, L.; Ramos-Molina, B.; Alcaide-Torres, J.; Morcillo, S.; Ocana-Wilhelmi, L.; Tinahones, F.; Queipo-Ortuno, M.I.; Cardona, F. Altered Adipose Tissue DNA Methylation Status in Metabolic Syndrome: Relationships Between Global DNA Methylation and Specific Methylation at Adipogenic, Lipid Metabolism and Inflammatory Candidate Genes and Metabolic Variables. J. Clin. Med. 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Zhao, T.; Yang, X.Z.; Sun, B.Y.; Li, Y.; Duan, J.C.; Sun, Z.W. PM2.5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Sci. Total Environ. 2019, 650, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Jiang, Q.; Wang, J.; Wang, X.; Yang, C.; Sun, Y.; Zhang, Y.; Wang, C.; Gao, Y.; Wei, X.; et al. Genome-wide methylation and transcriptome of blood neutrophils reveal the roles of DNA methylation in affecting transcription of protein-coding genes and miRNAs in E. coli-infected mastitis cows. BMC Genom. 2020, 21, 102. [Google Scholar] [CrossRef]
- Song, M.Y.; He, Y.H.; Zhou, H.K.; Zhang, Y.; Li, X.Z.; Yu, Y. Combined analysis of DNA methylome and transcriptome reveal novel candidate genes with susceptibility to bovine Staphylococcus aureus subclinical mastitis. Sci. Rep. 2016, 6, 29390. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Hon, G.C.; Hawkins, R.D.; Kheradpour, P.; Stark, A.; Harp, L.F.; Ye, Z.; Lee, L.K.; Stuart, R.K.; Ching, C.W.; et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 2009, 459, 108–112. [Google Scholar] [CrossRef]
- Deckert, J.; Struhl, K. Histone acetylation at promoters is differentially affected by specific activators and repressors. Mol. Cell. Biol. 2001, 21, 2726–2735. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, M.; Eisel, F.; Tchatalbachev, S.; Chakraborty, T.; Meinhardt, A.; Bhushan, S. Uropathogenic Escherichia coli Epigenetically Manipulate Host Cell Death Pathways. J. Infect. Dis. 2016, 213, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Solanki, B.; Singh, S.; Sahu, U.; Parvez, S.; Kar, S.; Ganguly, S. Persistent peripheral presence of Staphylococcus aureus promotes histone H3 hypoacetylation and decreases tyrosine hydroxylase protein level in rat brain tissues. Neuroreport 2019, 30, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Tagai, C.; Shiraishi, T.; Miyaji, K.; Iwamuro, S. Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against Gram-positive Staphylococcus aureus. Peptides 2013, 48, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Yoon, J.; Cho, H.S.; Lee, W.B.; Kim, J.; Song, Y.H.; Kim, S.N.; Yoon, J.H.; Kim-Ha, J.; Kim, Y.J. Downregulation of lipopolysaccharide response in drosophila by negative crosstalk between the AP1 and NF-kappa B signaling modules. Nat. Immunol. 2005, 6, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.T.; Robitaille, G.; Turner, J.D. Establishment of bovine mammary epithelial-cells (mac-t)-an invitro model for bovine lactation. Exp. Cell Res. 1991, 197, 191–199. [Google Scholar] [CrossRef]
| Gene Symbol | Gene Description | Fold Change * | |
|---|---|---|---|
| LPS | PLL | ||
| Cytokines and Chemokines | |||
| CCL20 | C-C motif chemokine 20 | 802.17 | 1266.00 |
| CCL5 | C-C motif chemokine 5 | 9.29 | 13.80 |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | 6.34 | 9.52 |
| CXCL6 | chemokine (C-X-C motif) ligand 6 | 20.07 | 32.26 |
| CXCL8 (IL-8) | interleukin-8 | 26.24 | 54.95 |
| IL1A (IL-1α) | interleukin-1 alpha | 3.73 | 5.76 |
| IL1B (IL-1β) | interleukin-1 beta | 310.00 | 300.00 |
| IL6 | interleukin-6 | 5.06 | 8.06 |
| NFKBIA | NF-kappa-B inhibitor alpha | 2.42 | 3.19 |
| TNF | tumor necrosis factor | 34.00 | 62.00 |
| CSF2 | granulocyte-macrophage colony-stimulating factor | – | 4.11 |
| Flt3lg | fms-related tyrosine kinase 3 ligand isoform X2 | – | 2.08 |
| TNFSF10 | tumor necrosis factor ligand superfamily member 10 | – | 2.20 |
| Another Immune Associated DEGs | |||
| C1S | TPA: complement C1s subcomponent | 2.13 | - |
| C2 | complement C2 | 4.44 | 6.44 |
| CFB | complement factor B | 52.12 | 132.50 |
| ISG15 | ubiquitin-like protein ISG15 | 2.16 | 11.60 |
| MMP9 | matrix metalloproteinase-9 | 2.85 | 4.89 |
| MAP2K6 | dual specificity mitogen-activated protein kinase kinase 6 | – | 0.46 |
| MAP3K8 | mitogen-activated protein kinase kinase kinase 8 isoform X2 | – | 2.77 |
| CD14 | monocyte differentiation antigen CD14 isoform X2 | – | 5.77 |
| ASAP3 | arf-GAP with SH3 domain, ANK repeat and PH domain-containing protein 3 | – | 3.20 |
| TLR3 | toll-like receptor 3 isoform X1 | – | 2.53 |
| MEFV | pyrin isoform X1 | – | 10.67 |
| DHX58 | probable ATP-dependent RNA helicase DHX58 | – | 2.95 |
| DDX58 | probable ATP-dependent RNA helicase DDX58 | – | 2.10 |
| ZBP1 | TPA: Z-DNA binding protein 1-like | – | 3.47 |
| CD3G | T-cell surface glycoprotein CD3 gamma chain | – | 0.47 |
| TNFAIP3 | tumor necrosis factor alpha-induced protein 3 | – | 2.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sun, Y.; Dong, X.; Chen, J.; Wang, Z.; Chen, J.; Dong, G. The Synergism of PGN, LTA and LPS in Inducing Transcriptome Changes, Inflammatory Responses and a Decrease in Lactation as Well as the Associated Epigenetic Mechanisms in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 387. https://doi.org/10.3390/toxins12060387
Wu Y, Sun Y, Dong X, Chen J, Wang Z, Chen J, Dong G. The Synergism of PGN, LTA and LPS in Inducing Transcriptome Changes, Inflammatory Responses and a Decrease in Lactation as Well as the Associated Epigenetic Mechanisms in Bovine Mammary Epithelial Cells. Toxins. 2020; 12(6):387. https://doi.org/10.3390/toxins12060387
Chicago/Turabian StyleWu, Yongjiang, Yawang Sun, Xianwen Dong, Jingbo Chen, Zili Wang, Juncai Chen, and Guozhong Dong. 2020. "The Synergism of PGN, LTA and LPS in Inducing Transcriptome Changes, Inflammatory Responses and a Decrease in Lactation as Well as the Associated Epigenetic Mechanisms in Bovine Mammary Epithelial Cells" Toxins 12, no. 6: 387. https://doi.org/10.3390/toxins12060387
APA StyleWu, Y., Sun, Y., Dong, X., Chen, J., Wang, Z., Chen, J., & Dong, G. (2020). The Synergism of PGN, LTA and LPS in Inducing Transcriptome Changes, Inflammatory Responses and a Decrease in Lactation as Well as the Associated Epigenetic Mechanisms in Bovine Mammary Epithelial Cells. Toxins, 12(6), 387. https://doi.org/10.3390/toxins12060387





