Clostridium perfringens Epsilon-Toxin Impairs the Barrier Function in MDCK Cell Monolayers in a Ca2+-Dependent Manner
Abstract
1. Introduction
2. Results
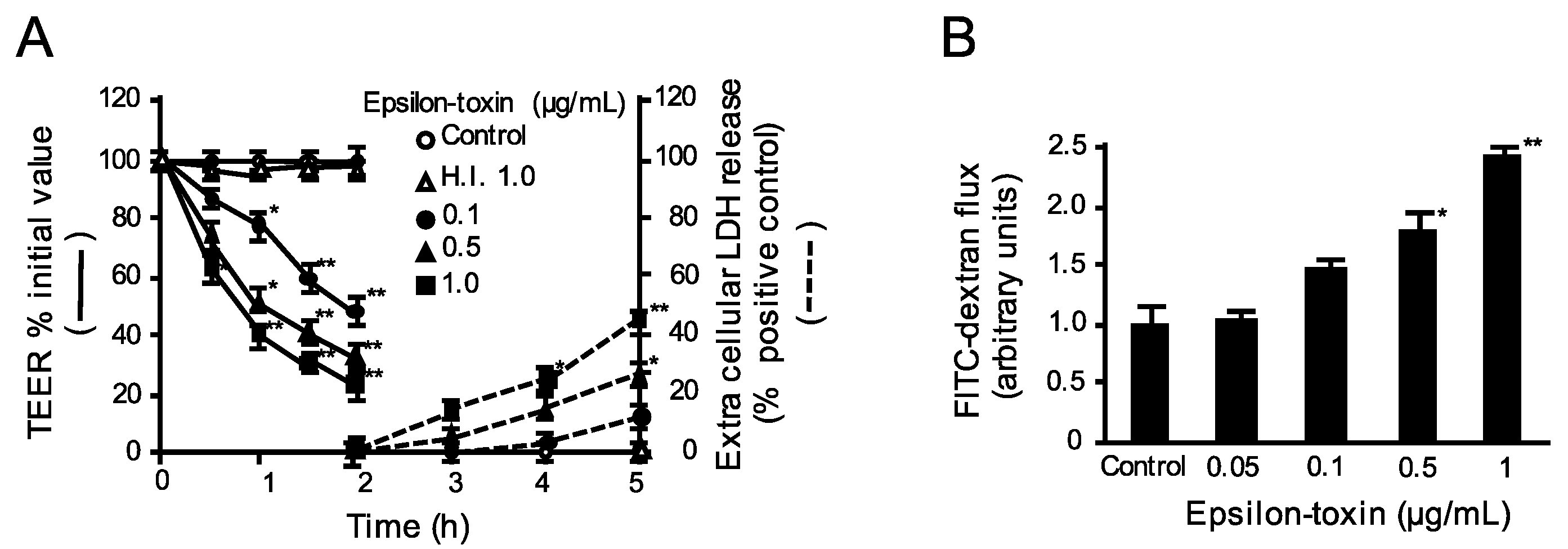
2.1. Epsilon-Toxin Induces Barrier Dysfunction in MDCK Cells
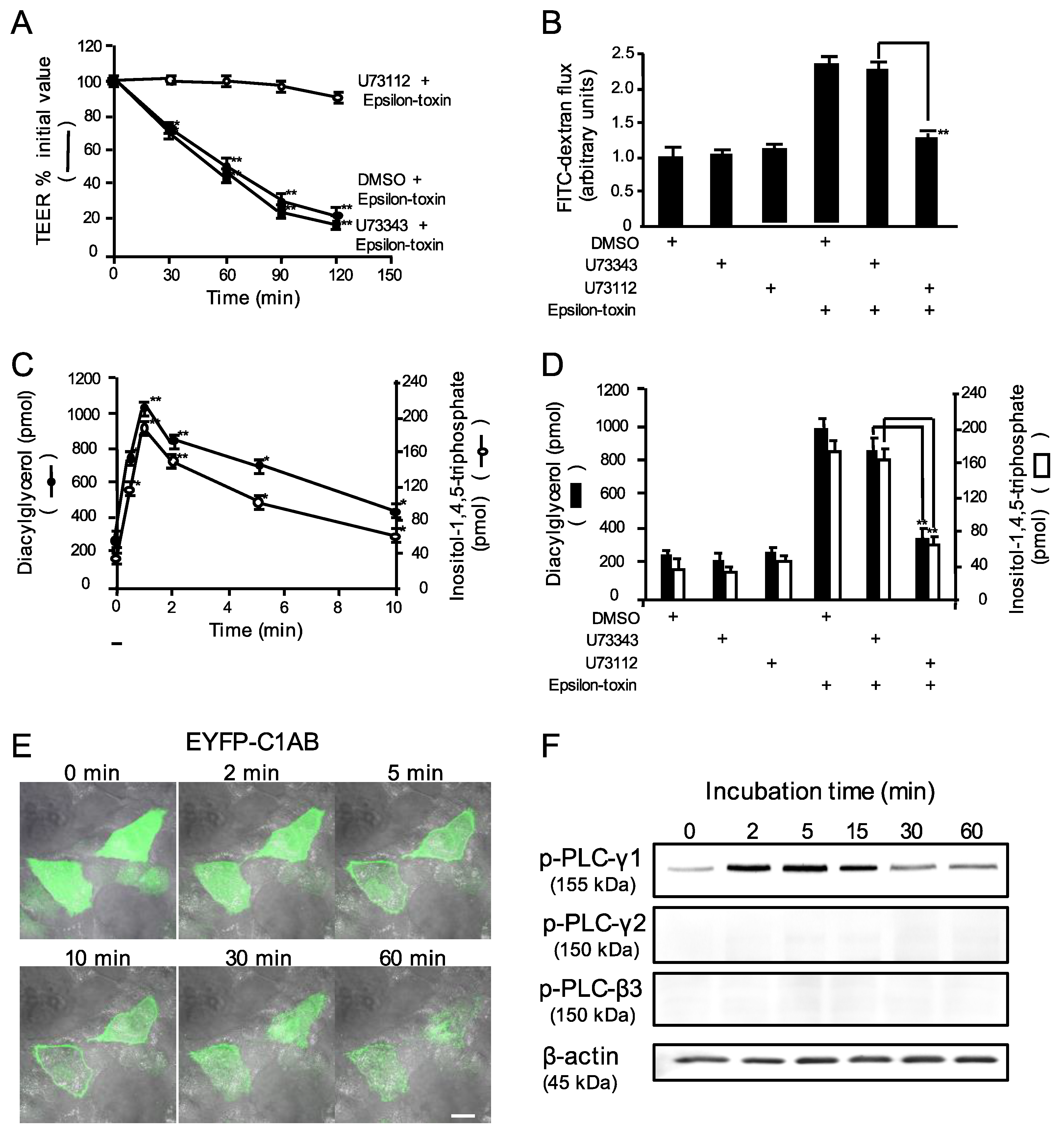
2.2. Epsilon-Toxin Activates Phospholipase Cγ-1 in MDCK Cells
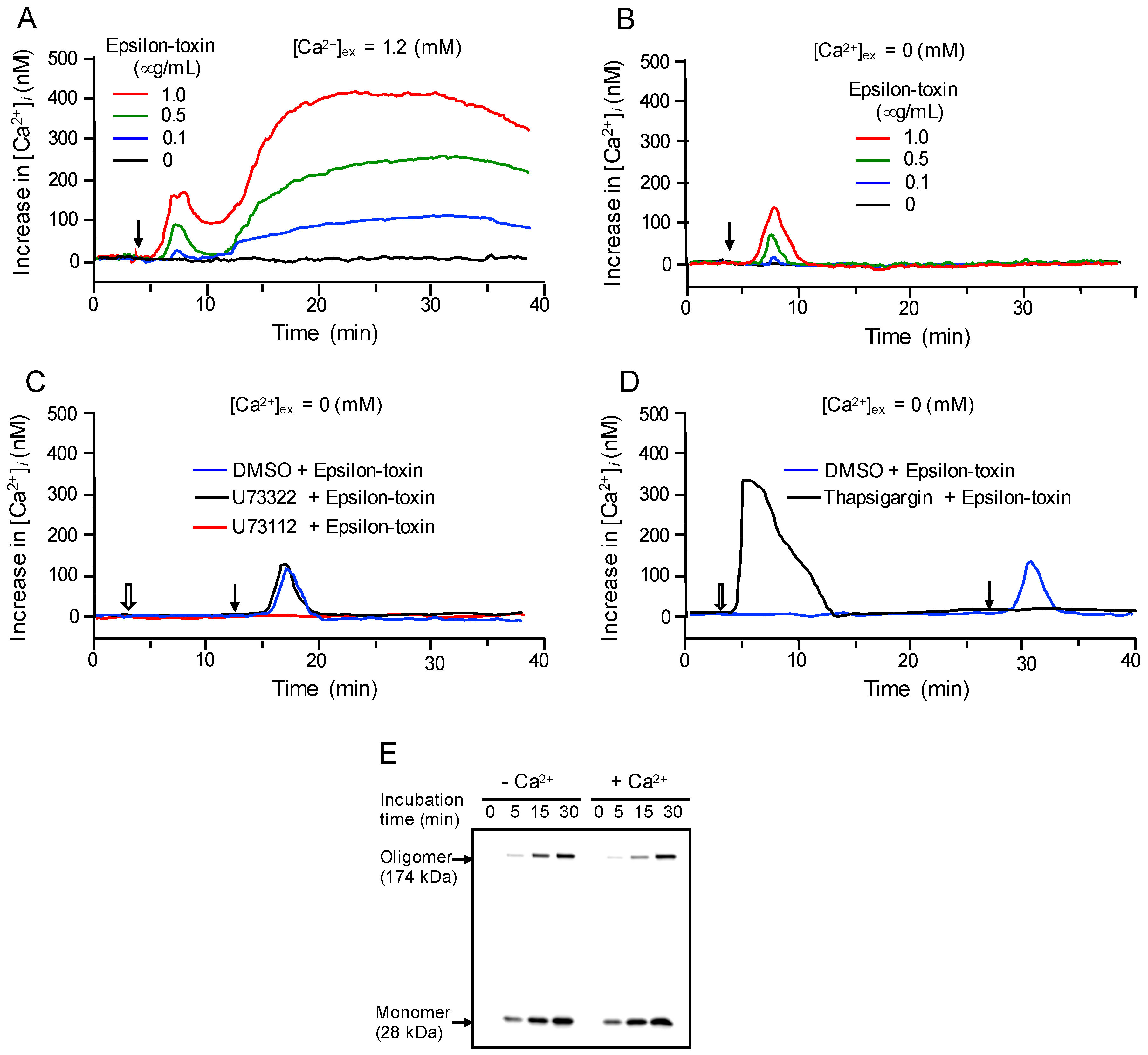
2.3. Epsilon-Toxin Induces Ca2+ Influx in MDCK Cells
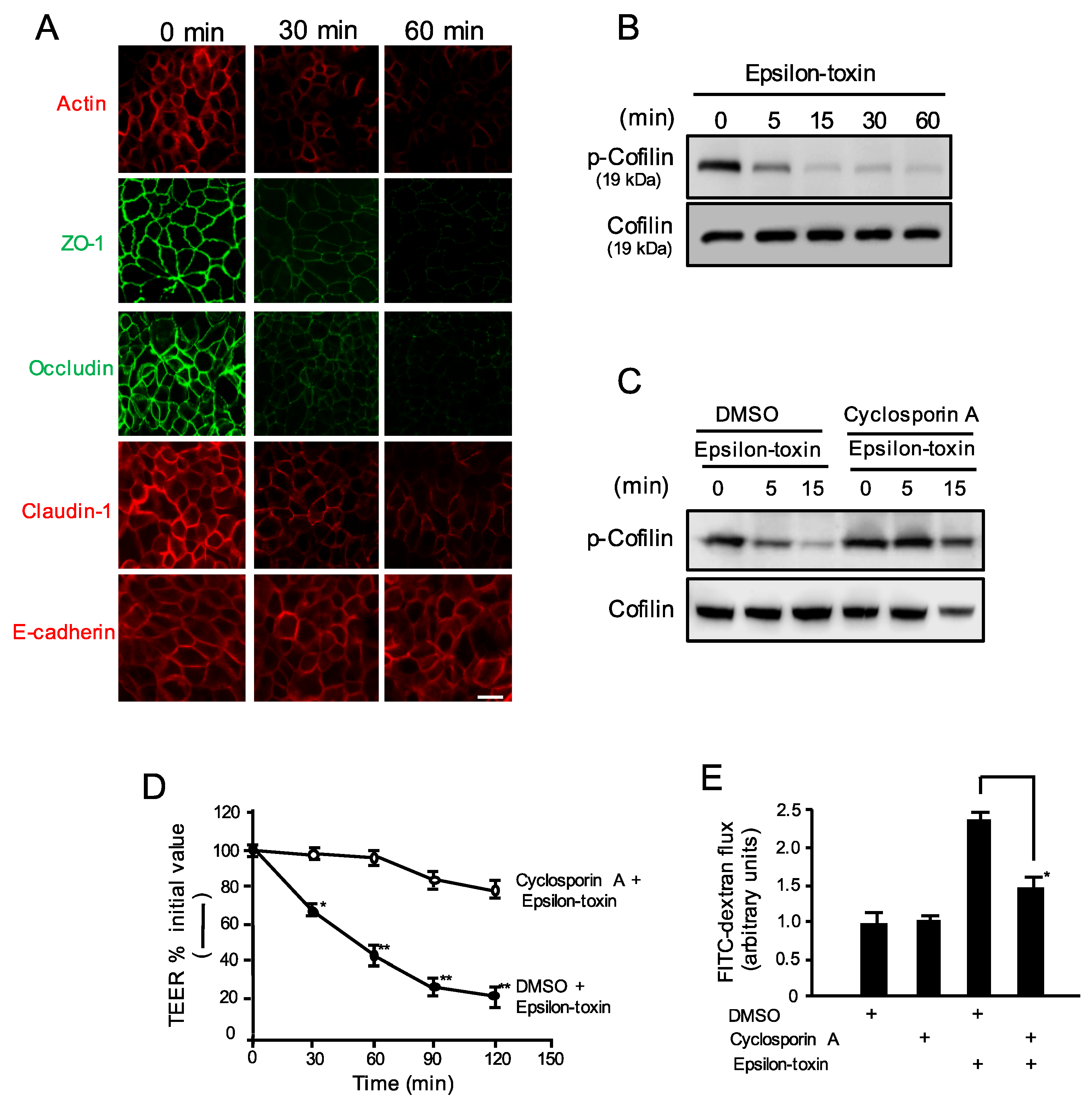
2.4. Epsilon-Toxin Changes the Organization of Junctional Proteins
2.5. Epsilon-Toxin Induces Cofilin Dephosphorylation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Cell Culture
5.3. Transepithelial Electrical Resistance (TEER) Measurements
5.4. Permeability of MDCK Monolayer
5.5. Leakage of Lactate Dehydrogenase (LDH) Activity
5.6. Determination of Diacylglycerol
5.7. Determination of Inositol 1,4,5-Trisphophate
5.8. Western Immunoblotting
5.9. Confocal Imaging of Diacylglycerol
5.10. Immunofluorescence Technique
5.11. Determination of Intracellular Ca2+ Concentrations
5.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Songer, J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996, 9, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Nagahama, M.; Ochi, S. Major toxins of Clostridium perfringens. J. Toxicol. Toxin Rev. 1997, 16, 195–214. [Google Scholar] [CrossRef]
- Popoff, M.R.; Bouvet, P. Clostridial toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef] [PubMed]
- Bokori-Brown, M.; Savva, C.G.; Fernandes da Costa, S.P.; Naylor, C.E.; Basak, A.K.; Titball, R.W. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J. 2011, 278, 4589–4601. [Google Scholar] [CrossRef]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE. 2013, 8, e76359. [Google Scholar] [CrossRef]
- Rumah, K.R.; Ma, Y.; Linden, J.R.; Oo, M.L.; Anrather, J.; Schaeren-Wiemers, N.; Alonso, M.A.; Fischetti, V.A.; McClain, M.S.; Vartanian, T. The myelin and lymphocyte protein MAL is required for binding and activity of Clostridium perfringens epsilon-toxin. PLoS Pathog. 2015, 11, e1004896. [Google Scholar] [CrossRef]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C.; Gnanapavan, S.; Lewis, N.; Popoff, M.R.; Raciborska, D.; Nicholas, R.; et al. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. 2018, 25, 653–660. [Google Scholar] [CrossRef]
- Gardner, D.E. Pathology of Clostridium welchii type D enterotoxaemia. II Structural and ultrastructural alterations in the tissues of lambs and mice. J. Comp. Pathol. 1973, 83, 509–524. [Google Scholar] [CrossRef]
- Adamson, R.H.; Ly, J.C.; Fernandez-Miyakawa, M.; Ochi, S.; Sakurai, J.; Uzal, F.; Curry, F.E. Clostridium perfringens epsilon-toxin increases permeability of single perfused microvessels of rat mesentery. Infect. Immun. 2005, 73, 4879–4887. [Google Scholar] [CrossRef][Green Version]
- Nagahama, M.; Sakurai, J. Distribution of labeled Clostridium perfringens epsilon-toxin in mice. Toxicon 1991, 29, 211–217. [Google Scholar] [CrossRef]
- Tamai, E.; Ishida, T.; Miyata, S.; Matsushita, O.; Suda, H.; Kobayashi, S.; Sonobe, H.; Okabe, A. Accumulation of Clostridium perfringens epsilon-toxin in the mouse kidney and its possible biological significance. Infect. Immun. 2003, 71, 5371–5375. [Google Scholar] [CrossRef] [PubMed]
- Soler-Jover, A.; Blasi, J.; Gomez de Aranda, I.; Navarro, P.; Gibert, M.; Popoff, M.R.; Martín-Satué, M. Effect of epsilon toxin-GFP on MDCK cells and renal tubules in vivo. J. Histochem. Cytochem. 2004, 52, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Soler-Jover, A.; Dorca, J.; Popoff, M.R.; Gibert, M.; Saura, J.; Tusell, J.M.; Serratosa, J.; Blasi, J.; Martín-Satué, M. Distribution of Clostridium perfringens epsilon toxin in the brains of acutely intoxicated mice and its effect upon glial cells. Toxicon 2007, 50, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [CrossRef]
- Parker, M.W.; van der Goot, F.G.; Buckley, J.T. Aerolysin: The ins and outs of a model channel-forming toxin. Mol. Microbiol. 1996, 19, 205–212. [Google Scholar] [CrossRef]
- Nagahama, M.; Ochi, S.; Sakurai, J. Assembly of Clostridium perfringens epsilon-toxin on MDCK cell membrane. J. Nat. Toxins 1998, 7, 291–302. [Google Scholar]
- Nagahama, M.; Hara, H.; Fernandez-Miyakawa, M.; Itohayashi, Y.; Sakurai, J. Oligomerization of Clostridium perfringens epsilon-toxin is dependent upon membrane fluidity in liposomes. Biochemistry 2006, 45, 296–302. [Google Scholar] [CrossRef]
- Takagishi, T.; Oda, M.; Takehara, M.; Kobayashi, K.; Nagahama, M. Oligomer formation of Clostridium perfringens epsilon-toxin is induced by activation of neutral sphingomyelinase. Biochim. Biophys. Acta 2016, 1858, 2681–2688. [Google Scholar] [CrossRef]
- Chassin, C.; Bens, M.; de Barry, J.; Courjaret, R.; Bossu, J.L.; Cluzeaud, F.; Ben Mkaddem, S.; Gibert, M.; Poulain, B.; Popoff, M.R.; et al. Pore-forming epsilon toxin causes membrane permeabilization and rapid ATP depletion-mediated cell death in renal collecting duct cells. Am. J. Physiol. Renal Physiol. 2007, 293, F927–F937. [Google Scholar] [CrossRef]
- Nagahama, M.; Itohayashi, Y.; Hara, H.; Higashihara, M.; Fukatani, Y.; Takagishi, T.; Oda, M.; Kobayashi, K.; Nakagawa, I.; Sakurai, J. Cellular vacuolation induced by Clostridium perfringens epsilon-toxin. FEBS J. 2011, 278, 3395–3407. [Google Scholar] [CrossRef]
- Ivie, S.E.; Fennessey, C.M.; Sheng, J.; Rubin, D.H.; McClain, M.S. Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens epsilon-toxin. PLoS ONE 2011, 6, e17787. [Google Scholar] [CrossRef] [PubMed]
- Ivie, S.E.; McClain, M.S. Identification of amino acids important for binding of Clostridium perfringens epsilon-toxin to host cells and to HAVCR1. Biochemistry 2012, 51, 7588–7595. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Minami, J.; Tamai, E.; Matsushita, O.; Shimamoto, S.; Okabe, A. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of MDCK cells and rat synaptosomes. J. Biol. Chem. 2002, 277, 39463–39468. [Google Scholar] [CrossRef] [PubMed]
- Fennessey, C.M.; Sheng, J.; Rubin, D.H.; McClain, M.S. Oligomerization of Clostridium perfringens epsilon toxin is dependent upon caveolins 1 and 2. PLoS ONE 2012, 7, e46866. [Google Scholar] [CrossRef]
- Cole, A.R.; Gibert, M.; Popoff, M.R.; Moss, D.S.; Titball, R.W.; Basak, A.K. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat. Struct. Mol. Biol. 2004, 11, 797–798. [Google Scholar] [CrossRef]
- Savva, C.G.; Clark, A.R.; Naylor, C.E.; Popoff, M.R.; Moss, D.S.; Basak, A.K.; Titball, R.W.; Bokori-Brown, M. The pore structure of Clostridium perfringens epsilon toxin. Nat. Commun. 2019, 10, 2641. [Google Scholar] [CrossRef]
- Chansard, M.; Liang, J.; Iwahana, E.; Baker, T.; Whittaker, J.; Fukuhara, C. Role of calcium in the gating of isoproterenol-induced arylalkylamine N-acetyltransferase gene expression in the mouse pineal gland. J. Pineal Res. 2006, 41, 85–94. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Anderova, M.; Chvatal, A. Differential calcium signalling in neuronal-glial networks. Front. Biosci. 2009, 14, 2004–2016. [Google Scholar] [CrossRef]
- Meldolesi, J. Calcium signaling: Oscillation, activation, expression. Nature 1998, 392, 865–866. [Google Scholar] [CrossRef]
- Uhlén, P.; Laestadius, A.; Jahnukainen, T.; Söderblom, T.; Bäckhed, F.; Celsi, G.; Brismar, H.; Normark, S.; Aperia, A.; Richter-Dahlfors, A. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 2000, 405, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, S.; Gäbler, K.; Below, S.; Müller, C.; Kohler, C.; Engelmann, S.; Hildebrandt, P.; Völker, U.; Hecker, M.; Hildebrandt, J.P. Effects of Staphylococcus aureus-hemolysin A on calcium signalling in immortalized human airway epithelial cells. Cell Calcium 2009, 45, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.K.; Vikström, E.; Magnusson, K.E.; Vécsey-Semjén, B.; Colque-Navarro, P.; Möllby, R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012, 80, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.L.; Smedley, J.G., 3rd; Singh, U.; Chakrabarti, G.; Van Itallie, C.M.; Anderson, J.M.; McClane, B.A. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell. Microbiol. 2007, 9, 2734–2755. [Google Scholar] [CrossRef]
- Takahashi, A.; Tanoue, N.; Nakano, M.; Hamamoto, A.; Okamoto, K.; Fujii, Y.; Harada, N.; Nakaya, Y. A pore-forming toxin produced by Aeromonas sobria activates Ca2+ dependent Cl- secretion. Microb. Pathog. 2005, 38, 173–180. [Google Scholar] [CrossRef]
- Ward, P.D.; Ouyang, H.; Thakker, D.R. Role of phospholipase C-beta in the modulation of epithelial tight junction permeability. J. Pharmacol. Exp. Ther. 2003, 304, 689–698. [Google Scholar] [CrossRef]
- Avaritt, B.R.; Swaan, P.W. Intracellular Ca2+ release mediates cationic but not anionic poly(amidoamine) (PAMAM) dendrimer-induced tight junction modulation. Pharm. Res. 2014, 31, 2429–2438. [Google Scholar] [CrossRef]
- Petit, L.; Gibert, M.; Gillet, D.; Laurent-Winter, C.; Boquet, P.; Popoff, M.R. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J. Bacteriol. 1997, 179, 6480–6487. [Google Scholar] [CrossRef]
- Takagishi, T.; Oda, M.; Kabura, M.; Kurosawa, M.; Tominaga, K.; Urano, S.; Ueda, Y.; Kobayashi, K.; Kobayashi, T.; Sakurai, J.; et al. Clostridium perfringens alpha-toxin induces gm1a clustering and Trka phosphorylation in the host cell membrane. PLoS ONE 2015, 10, e0120497. [Google Scholar] [CrossRef]
- Wahl, M.I.; Nishibe, S.; Carpenter, G. Growth factor signaling pathways: Phosphoinositide metabolism and phosphorylation of phospholipase C. Cancer Cells 1989, 1, 101–107. [Google Scholar]
- Suzuki, T.; Seth, A.; Rao, R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J. Biol. Chem. 2008, 283, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Flick, J.; Levine, S.A.; Madara, J.L.; Sharp, G.W.; Donowitz, M. Regulation of tight junction resistance in T84 monolayers by elevation in intracellular Ca2+: A protein kinase C effect. J. Membr. Biol. 1996, 149, 71–79. [Google Scholar] [PubMed]
- Ye, J.; Tsukamoto, T.; Sun, A.; Nigam, S.K. A role for intracellular calcium in tight junction reassembly after ATP depletion-repletion. Am. J. Physiol. 1999, 277, F524–F532. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Han, J.; Bellila, A.; Isoda, H.; Tanaka, T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem. Biophys. Res. Commun. 2008, 377, 921–925. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamasaki, Y.; Sasaki-Yamaguchi, Y.; Ida-Koga, N.; Kamisuki, S.; Sugawara, F.; Nagumo, Y.; Usui, T. TRPA1-dependent reversible opening of tight junction by natural compounds with an α,β-unsaturated moiety and capsaicin. Sci. Rep. 2018, 8, 2251. [Google Scholar] [CrossRef]
- Zhao, J.W.; Gao, Z.L.; Ji, Q.Y.; Wang, H.; Zhang, H.Y.; Yang, Y.D.; Xing, F.J.; Meng, L.J.; Wang, Y. Regulation of cofilin activity by CaMKII and calcineurin. Am. J. Med. Sci. 2012, 344, 462–472. [Google Scholar] [CrossRef]
- Uzal, F.A.; Kelly, W.R.; Morris, W.E.; Assis, R.A. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J. Comp. Pathol. 2002, 126, 71–75. [Google Scholar] [CrossRef]
- Popoff, M.R. Epsilon toxin: A fascinating pore-forming toxin. FEBS J. 2011, 278, 4602–4615. [Google Scholar] [CrossRef]
- Lonchamp, E.; Dupont, J.L.; Wioland, L.; Courjaret, R.; Mbebi-Liegeois, C.; Jover, E.; Doussau, F.; Popoff, M.R.; Bossu, J.L.; de Barry, J.; et al. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS ONE 2010, 5, e13046. [Google Scholar] [CrossRef]
- Wioland, L.; Dupont, J.L.; Doussau, F.; Gaillard, S.; Heid, F.; Isope, P.; Pauillac, S.; Popoff, M.R.; Bossu, J.L.; Poulain, B. Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell. Microbiol. 2015, 17, 369–388. [Google Scholar] [CrossRef]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, N.; Seeger, W.; Dewein, E.; Bhakdi, S.; Roka, L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: Role of calcium. Am. J. Physiol. 1985, 248, C127–C134. [Google Scholar] [CrossRef]
- Cajnko, M.M.; Marušić, M.; Kisovec, M.; Rojko, N.; Benčina, M.; Caserman, S.; Anderluh, G. Listeriolysin O affects the permeability of Caco-2 monolayer in a pore-dependent and Ca2+-independent manner. PLoS ONE 2015, 10, e0130471. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Irvine, R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.S.; Putney, J.W., Jr. Pharmacology of store-operated calcium entry channels. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; Chapter 16. [Google Scholar]
- Moon, A.; Drubin, D.G. The ADF/cofilin proteins: Stimulus-responsive modulators of actin dynamics. Mol. Biol. Cell 1995, 6, 1423–1431. [Google Scholar] [CrossRef]
- Seike, S.; Takehara, M.; Takagishi, T.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Delta-toxin from Clostridium perfringens perturbs intestinal epithelial barrier function in Caco-2 cell monolayers. Biochim. Biophys. Acta 2018, 1860, 428–433. [Google Scholar] [CrossRef]
- Ochi, S.; Oda, M.; Nagahama, M.; Sakurai, J. Clostridium perfringens alpha-toxin-induced hemolysis of horse erythrocytes is dependent on Ca2+ uptake. Biochim. Biophys. Acta 2003, 1613, 79–86. [Google Scholar] [CrossRef]
- Ochi, S.; Oda, M.; Matsuda, H.; Ikari, S.; Sakurai, J. Clostridium perfringens alpha-toxin activates the sphingomyelin metabolism system in sheep erythrocytes. J. Biol. Chem. 2004, 279, 12181–12189. [Google Scholar] [CrossRef]
- Nagahama, M.; Umezaki, M.; Tashiro, R.; Oda, M.; Kobayashi, K.; Shibutani, M.; Takagishi, T.; Ishidoh, K.; Fukuda, M.; Sakurai, J. Intracellular trafficking of Clostridium perfringens iota-toxin b. Infect. Immun. 2012, 80, 3410–3416. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahama, M.; Seike, S.; Ochi, S.; Kobayashi, K.; Takehara, M. Clostridium perfringens Epsilon-Toxin Impairs the Barrier Function in MDCK Cell Monolayers in a Ca2+-Dependent Manner. Toxins 2020, 12, 286. https://doi.org/10.3390/toxins12050286
Nagahama M, Seike S, Ochi S, Kobayashi K, Takehara M. Clostridium perfringens Epsilon-Toxin Impairs the Barrier Function in MDCK Cell Monolayers in a Ca2+-Dependent Manner. Toxins. 2020; 12(5):286. https://doi.org/10.3390/toxins12050286
Chicago/Turabian StyleNagahama, Masahiro, Soshi Seike, Sadayuki Ochi, Keiko Kobayashi, and Masaya Takehara. 2020. "Clostridium perfringens Epsilon-Toxin Impairs the Barrier Function in MDCK Cell Monolayers in a Ca2+-Dependent Manner" Toxins 12, no. 5: 286. https://doi.org/10.3390/toxins12050286
APA StyleNagahama, M., Seike, S., Ochi, S., Kobayashi, K., & Takehara, M. (2020). Clostridium perfringens Epsilon-Toxin Impairs the Barrier Function in MDCK Cell Monolayers in a Ca2+-Dependent Manner. Toxins, 12(5), 286. https://doi.org/10.3390/toxins12050286



