The Pharmacological Mechanism of Diabetes Mellitus-Associated Overactive Bladder and Its Treatment with Botulinum Toxin A
Abstract
1. Introduction
2. Urodynamic Finding in Patients with DM-Associated OAB
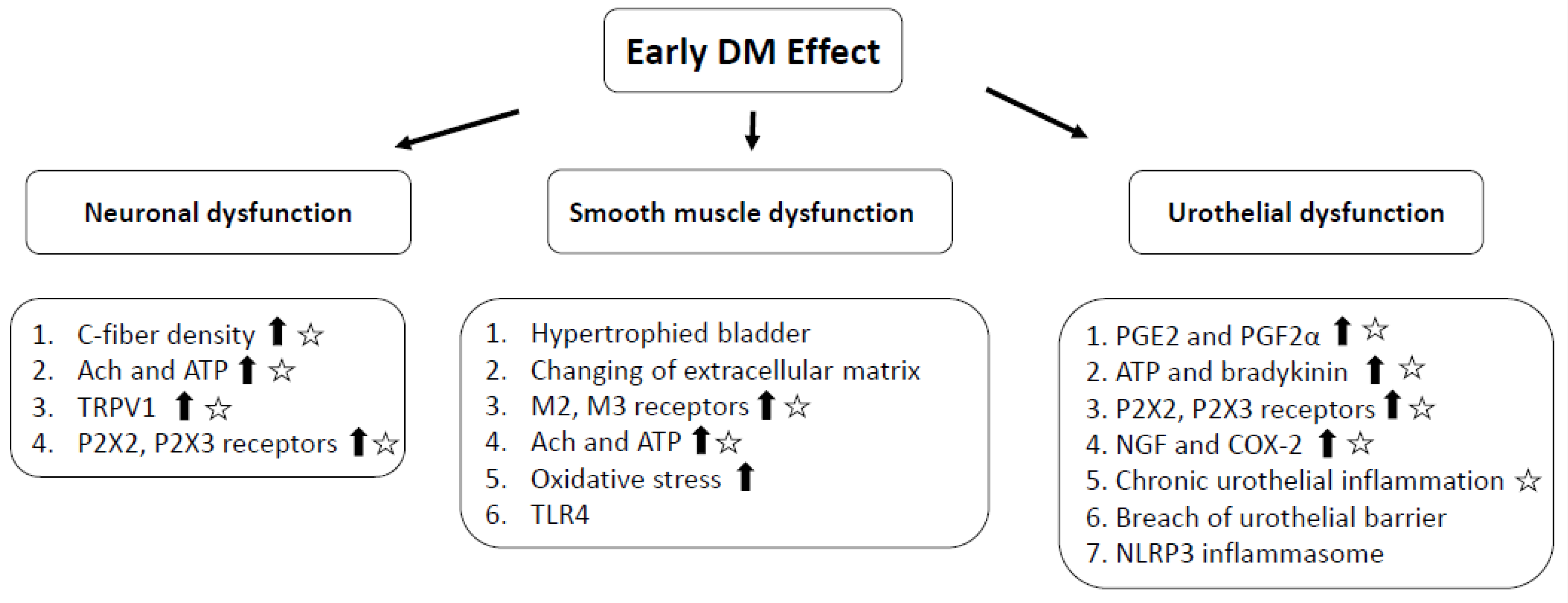
3. Pathophysiology of DM-Associated OAB
4. Diabetes and Bladder Inflammation
5. Inhibition of Chronic Inflammation and Hypersensitivity by Intravesical Botulinum Toxin A Injection
6. Clinical Outcomes of Intravesical Botulinum Toxin A Injection for Patients with DM-Associated OAB
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, D.; Zhao, M.; Huang, L.; Wang, K. Overactive bladder symptom severity, bother, help-seeking behavior, and quality of life in patients with type 2 diabetes: A path analysis. Health Qual. Life Outcomes 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Vittinghoff, E.; Kanaya, A.M.; Miles, T.P.; Resnick, H.E.; Kritchevsky, S.B.; Simonsick, E.M.; Brown, J.S. Urinary incontinence in elderly women: Findings from the Health, Aging, and Body Composition Study. Obstet. Gynecol. 2004, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Hu, S.W.; Chen, Y.C.; Lin, T.L.; Lin, L.Y. Prevalence and correlations of anal incontinence and constipation in Taiwanese women. Neurourol. Urodyn. 2003, 22, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.F.; Huang, M.H.; Wang, C.C.; Kuo, H.C. Higher glycosylated hemoglobin levels increase the risk of overactive bladder syndrome in patients with type 2 diabetes mellitus. Int. J. Urol. 2012, 19, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chancellor, M.B.; Lin, J.M.; Hsieh, J.H.; Yu, H.J. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int. 2010, 105, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Janning, S.W.; Haas, G.P.; Wilson, K.L.; Smith, D.M.; Reckard, G.; Quan, S.-P.; Bukofzer, S. Comparative persistence and adherence to overactive bladder medications in patients with and without diabetes. Int. J. Clin. Pract. 2012, 66, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Moller, C.F.; Olesen, K.P. Diabetic cystopathy. IV: Micturition cystourethrography compared with urodynamic investigation. Dan. Med. Bull. 1976, 23, 291–294. [Google Scholar]
- Karoli, R.; Bhat, S.; Fatima, J.; Priya, S. A study of bladder dysfunction in women with type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2014, 18, 552–557. [Google Scholar] [CrossRef]
- Majima, T.; Matsukawa, Y.; Funahashi, Y.; Takai, S.; Kato, M.; Yamamoto, T.; Gotoh, M. Urodynamic analysis of the impact of diabetes mellitus on bladder function. Int. J. Urol. 2019, 26, 618–622. [Google Scholar] [CrossRef]
- Bansal, R.; Agarwal, M.M.; Modi, M.; Mandal, A.K.; Singh, S.K. Urodynamic profile of diabetic patients with lower urinary tract symptoms: Association of diabetic cystopathy with autonomic and peripheral neuropathy. Urology 2011, 77, 699–705. [Google Scholar] [CrossRef]
- Lee, W.C.; Wu, H.P.; Tai, T.Y.; Yu, H.J.; Chiang, P.H. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J. Urol. 2009, 181, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gali, A.; Mucciardi, G.; Buttice, S.; Subba, E.; D’Amico, C.; Lembo, F.; Magno, C. Correlation between advanced glycation end-products, lower urinary tract symptoms and bladder dysfunctions in patients with type 2 diabetes mellitus. Low. Urin. Tract. Symptoms 2017, 9, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Tai, H.C.; Yu, H.J. Urodynamic findings in female diabetic patients with and without overactive bladder symptoms. Neurourol. Urodyn. 2010, 29, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Chancellor, M.B.; Andersson, K.E.; Christ, G.J. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005, 95, 733–738. [Google Scholar] [CrossRef]
- Tong, Y.C.; Chin, W.T.; Cheng, J.T. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci. Lett. 1999, 277, 173–176. [Google Scholar] [CrossRef]
- Tong, Y.C.; Cheng, J.T. Alteration of M(3) subtype muscarinic receptors in the diabetic rat urinary bladder. Pharmacology 2002, 64, 148–151. [Google Scholar] [CrossRef]
- Klee, N.S.; Moreland, R.S.; Kendig, D.M. Detrusor contractility to parasympathetic mediators is differentially altered in the compensated and decompensated states of diabetic bladder dysfunction. Am. J. Physiol. Renal. Physiol. 2019, 317, F388–F398. [Google Scholar] [CrossRef]
- Gray, M.A.; Wang, C.C.; Sacks, M.S.; Yoshimura, N.; Chancellor, M.B.; Nagatomi, J. Time-dependent alterations of select genes in streptozotocin-induced diabetic rat bladder. Urology 2008, 71, 1214–1219. [Google Scholar] [CrossRef]
- Wang, C.C.; Nagatomi, J.; Toosi, K.K.; Yoshimura, N.; Hsieh, J.H.; Chancellor, M.B.; Chancellor, M.B.; Sacks, M.S. Diabetes-induced alternations in biomechanical properties of urinary bladder wall in rats. Urology 2009, 73, 911–915. [Google Scholar] [CrossRef]
- Tanik, N.; Tanik, S.; Albayrak, S.; Zengin, K.; Inan, L.E.; Caglayan, E.K.; Celikbilek, A.; Kirboga, K.; Gurdal, M. Association Between Overactive Bladder and Polyneuropathy in Diabetic Patients. Int. Neurourol. J. 2016, 20, 232–239. [Google Scholar] [CrossRef]
- Lee, W.C.; Wu, H.C.; Huang, K.H.; Wu, H.P.; Yu, H.J.; Wu, C.C. Hyposensitivity of C-fiber afferents at the distal extremities as an indicator of early stages diabetic bladder dysfunction in type 2 diabetic women. PLoS ONE 2014, 9, e86463. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Ruiz, G.W.; Daneshgari, F.; Liu, G.; Apodaca, G.; Birder, L.A. Impact of diabetes mellitus on bladder uroepithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R84–R93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Kuo, H.C. Urothelial Dysfunction and Chronic Inflammation in Diabetic Patients with Overactive Bladder. Low. Urin. Tract. Symptoms 2017, 9, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Landman, G.W.; Kleefstra, N.; Groenier, K.H.; Bakker, S.J.; Groeneveld, G.H.; Bilo, H.J.; Van Hateren, K.J. Inflammation biomarkers and mortality prediction in patients with type 2 diabetes (ZODIAC-27). Atherosclerosis 2016, 250, 46–51. [Google Scholar] [CrossRef]
- Ristikj-Stomnaroska, D.; Risteska-Nejashmikj, V.; Papazova, M. Role of Inflammation in the Pathogenesis of Diabetic Peripheral Neuropathy. Open Access Maced. J. Med. Sci. 2019, 7, 2267–2270. [Google Scholar] [CrossRef]
- Yan, P.; Xu, Y.; Zhang, Z.; Gao, C.; Zhu, J.; Li, H.; Wan, Q. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Cytokine 2019, 113, 356–364. [Google Scholar] [CrossRef]
- Yeniel, A.O.; Ergenoglu, A.M.; Meseri, R.; Kismali, E.; Ari, A.; Kavukcu, G.; Aydin, H.H.; Ak, H.; Atay, S.; Itil, I.M. Is overactive bladder microvasculature disease a component of systemic atheroscleorosis? Neurourol. Urodyn. 2018, 37, 1372–1379. [Google Scholar] [CrossRef]
- Inouye, B.M.; Hughes, F.M., Jr.; Jin, H.; Lutolf, R.; Potnis, K.C.; Routh, J.C.; Rouse, D.C.; Foo, W.-C.; Purves, J.T. Diabetic bladder dysfunction is associated with bladder inflammation triggered through hyperglycemia, not polyuria. Res. Rep. Urol. 2018, 10, 219–225. [Google Scholar] [CrossRef]
- Xiao, N.; Wang, Z.; Huang, Y.; Daneshgari, F.; Liu, G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J. Urol. 2013, 189, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M., Jr.; Hirshman, N.A.; Inouye, B.M.; Jin, H.; Stanton, E.W.; Yun, C.E.; Davis, L.G.; Routh, J.C.; Purves, J.T. NLRP3 promotes diabetic bladder dysfunction and changes in symptom-specific bladder innervation. Diabetes 2019, 68, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M., Jr.; Sexton, S.J.; Ledig, P.D.; Yun, C.E.; Jin, H.; Purves, J.T. Bladder decompensation and reduction in nerve density in a rat model of chronic bladder outlet obstruction are attenuated with the NLRP3 inhibitor glyburide. Am. J. Physiol. Renal. Physiol. 2019, 316, F113–F120. [Google Scholar] [CrossRef] [PubMed]
- Szasz, T.; Wenceslau, C.F.; Burgess, B.; Nunes, K.P.; Webb, R.C. Toll-like receptor 4 activation contributes to diabetic bladder dysfunction in a murine model of type 1 diabetes. Diabetes 2016, 65, 3754–3764. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Kuo, H.C. Botulinum toxin injection for lower urinary tract dysfunction. Int. J. Urol. 2013, 20, 40–55. [Google Scholar] [CrossRef]
- Kuo, H.C. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004, 63, 868–872. [Google Scholar] [CrossRef]
- Jhang, J.F.; Kuo, H.C. Botulinum toxin A and lower urinary tract dysfunction: Pathophysiology and mechanisms of action. Toxins 2016, 8, 120. [Google Scholar] [CrossRef]
- Akaike, N.; Shin, M.-C.; Wakita, M.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kato, K.; Kaji, R.; Kozaki, S. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J. Physiol. 2013, 591, 1031–1043. [Google Scholar] [CrossRef]
- Papagiannopoulou, D.; Vardouli, L.; Dimitriadis, F.; Apostolidis, A. Retrograde transport of radiolabelled botulinum neurotoxin type A to the CNS after intradetrusor injection in rats. BJU Int. 2016, 117, 697–704. [Google Scholar] [CrossRef]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef]
- Smith, C.P.; Gangitano, D.; Munoz, A.; Salas, N.A.; Boone, T.B.; Aoki, K.R.; Francis, J.; Somogyi, G.T. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 2008, 52, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Ha, U.S.; Park, E.Y.; Kim, J.C. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 2011, 78, 721. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Baukloh, H.; Priefert, J.; Knispel, H.H.; Lawrence, G.W.; Miller, K.; Neuhaus, J. Botulinum toxin A detrusor injections reduce postsynaptic muscular M2, M3, P2X2, and P2X3 receptors in children and adolescents who have neurogenic detrusor overactivity: A single-blind study. Urology 2013, 81, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Top, T.; Sekerci, C.A.; Isbilen-Basok, B.; Tanidir, Y.; Tinay, I.; Isman, F.K.; Akbal, C.; Şimşek, F.; Tarcan, T. The effect of intradetrusor botulinum neurotoxin type A on urinary NGF, TGF BETA-1, TIMP-2 levels in children with neurogenic detrusor overactivity due to myelodysplasia. Neurourol. Urodyn. 2017, 36, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Liao, C.H.; Kuo, H.C. Diabetes mellitus does not affect the efficacy and safety of intravesical onabotulinumtoxina injection in patients with refractory detrusor overactivity. Neurourol. Urodyn. 2014, 33, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Daneshgari, F.; Liu, G.; Birder, L.; Hanna-Mitchell, A.T.; Chacko, S. Diabetic bladder dysfunction: Current translational knowledge. J. Urol. 2009, 182 (Suppl. 6), S18–S26. [Google Scholar] [CrossRef]
- Chancellor, M.B. The overactive bladder progression to underactive bladder hypothesis. Int. Urol. Nephrol. 2014, 46 (Suppl. 1), S23–S27. [Google Scholar] [CrossRef]
- Wang, C.C.; Lee, C.L.; Kuo, H.C. Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility. Toxins 2016, 8, 82. [Google Scholar] [CrossRef]
- Kuo, H.C.; Liao, C.H.; Chung, S.D. Adverse events of intravesical botulinum toxin a injections for idiopathic detrusor overactivity: Risk factors and influence on treatment outcome. Eur. Urol. 2010, 58, 919–926. [Google Scholar] [CrossRef]
| Author [reference] | Patients (n) | Mean Age (years) | DM Duration (years) | DO | DHIC | DU | Normal | SUI |
|---|---|---|---|---|---|---|---|---|
| Majima [9] | 57M | 65.8 | 10 | 5 (9%) | 18 (32%) | 22 (39%) | 12 (23%) | NA |
| Karoli [8] | 44F | 54.8 | 11.6 | 10 (23%) | NA | 5 (11%) | 9 (16%) | 22 (48%) |
| Bansal [10] | 52M | 61.3 | 11 | 20 (39%) | NA | 41 (79%) | NA | NA |
| Gali [12] | 21M + 19F | 64.5 | 10.9 | 7 (18%) | 24 (60%) | 4 (10%) | 5 (13%) | NA |
| Lee [11] | 86F | 66.9 | 11.4 | 12 (14%) | NA | 30 (35%) | 33 (38%) | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Jiang, Y.-H.; Kuo, H.-C. The Pharmacological Mechanism of Diabetes Mellitus-Associated Overactive Bladder and Its Treatment with Botulinum Toxin A. Toxins 2020, 12, 186. https://doi.org/10.3390/toxins12030186
Wang C-C, Jiang Y-H, Kuo H-C. The Pharmacological Mechanism of Diabetes Mellitus-Associated Overactive Bladder and Its Treatment with Botulinum Toxin A. Toxins. 2020; 12(3):186. https://doi.org/10.3390/toxins12030186
Chicago/Turabian StyleWang, Chung-Cheng, Yung-Hong Jiang, and Hann-Chorng Kuo. 2020. "The Pharmacological Mechanism of Diabetes Mellitus-Associated Overactive Bladder and Its Treatment with Botulinum Toxin A" Toxins 12, no. 3: 186. https://doi.org/10.3390/toxins12030186
APA StyleWang, C.-C., Jiang, Y.-H., & Kuo, H.-C. (2020). The Pharmacological Mechanism of Diabetes Mellitus-Associated Overactive Bladder and Its Treatment with Botulinum Toxin A. Toxins, 12(3), 186. https://doi.org/10.3390/toxins12030186






