Therapeutic Efficacy of onabotulinumtoxinA Delivered Using Various Approaches in Sensory Bladder Disorder
Abstract
1. Introduction
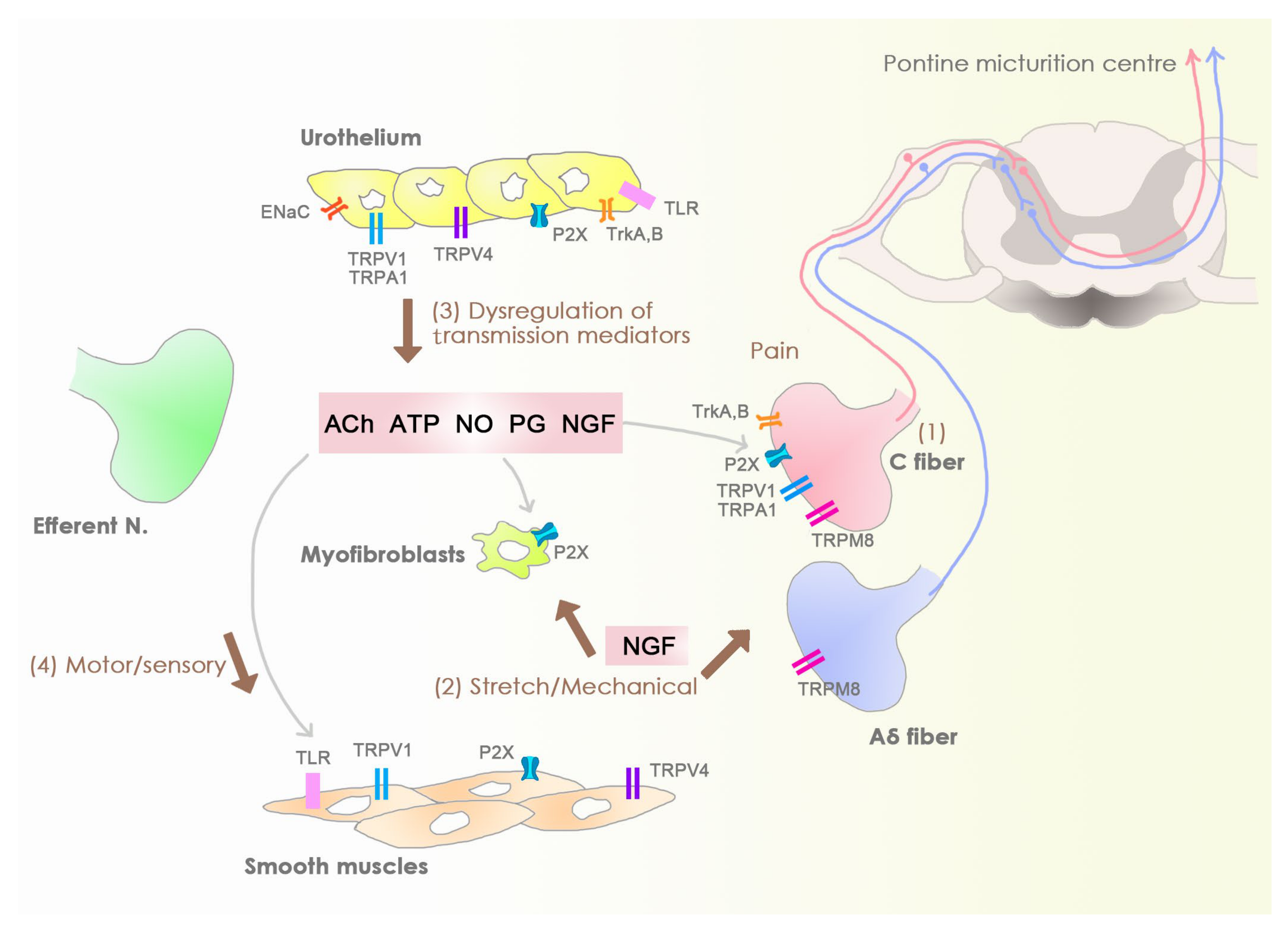
2. Sensory Bladder Disorders
3. Mechanism of Action of onaBoNTA
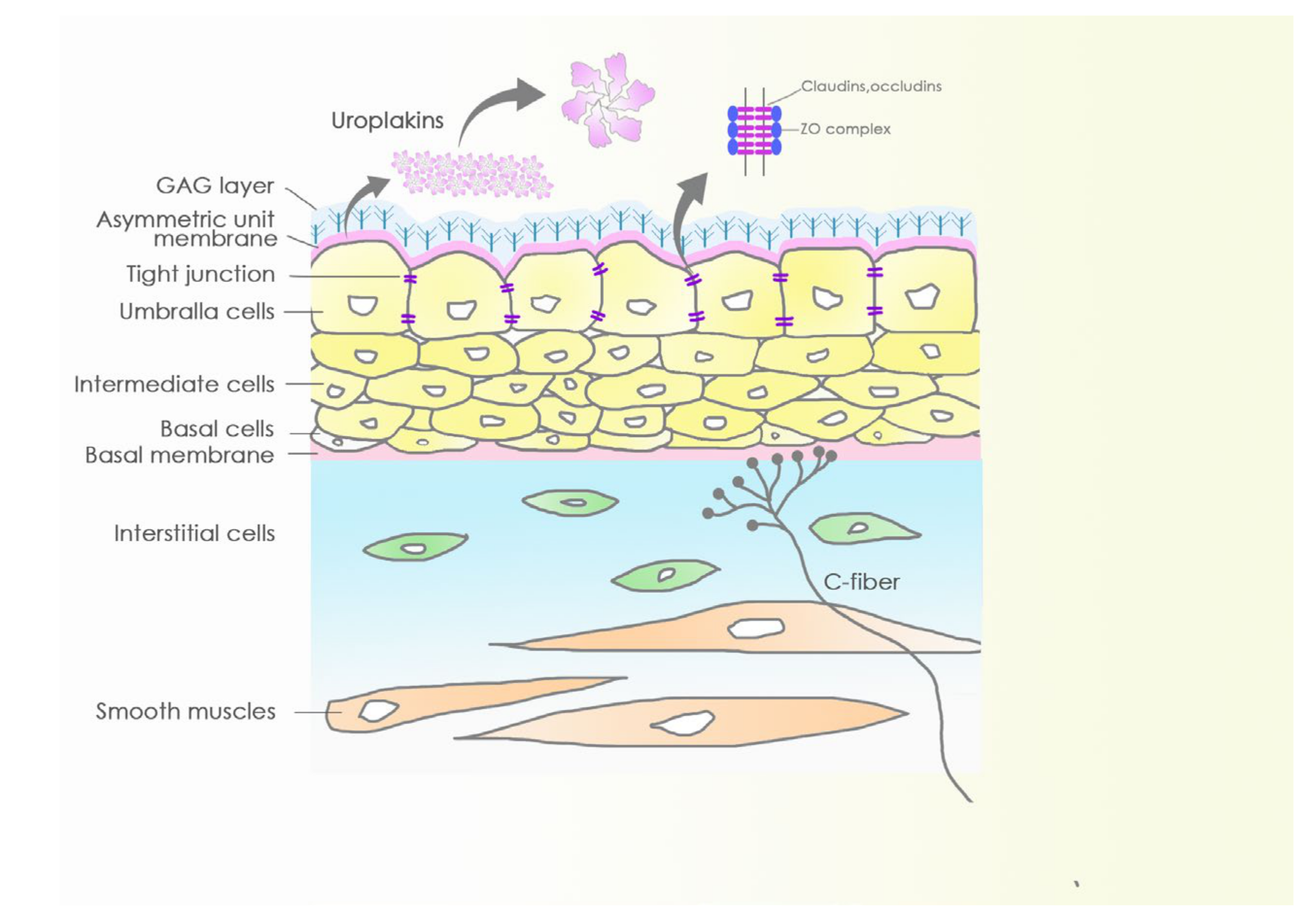
4. Barriers and Sensory Web of the Bladder Mucosa and Submucosa
5. Intravesical Delivery of onaBoNTA
5.1. Passive Diffusion
5.2. Disrupt Barrier
5.2.1. Protamine Sulfate
5.2.2. Dimethyl Sulphoxide
5.3. Increase Permebility
5.3.1. Electromotive Drug Administration
5.3.2. Low-Energy Shock Wave
5.4. Carrier Transportation
5.4.1. Liposome Formulation of onaBoNTA
5.4.2. Intravesical Thermosensitive Hydrogel
5.4.3. Hyaluronan-Phosphatidylethanolamine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dykstra, D.D.; Sidi, A.A.; Scott, A.B.; Pagel, J.M.; Goldish, G.D. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J. Urol. 1988, 139, 919–922. [Google Scholar] [CrossRef]
- Jhang, J.F.; Kuo, H.C. Botulinum Toxin A and Lower Urinary Tract Dysfunction: Pathophysiology and Mechanisms of Action. Toxins 2016, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Liao, C.H.; Kuo, H.C. Current and potential urological applications of botulinum toxin A. Nat. Rev. Urol. 2015, 12, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Caldwell, A.; Brierley, S.M. Mechanisms Underlying Overactive Bladder and Interstitial Cystitis/Painful Bladder Syndrome. Front. Neurosci. 2018, 12, 931. [Google Scholar] [CrossRef]
- Steers, W.D. Pathophysiology of overactive bladder and urge urinary incontinence. Rev. Urol. 2002, 4 (Suppl. S4), S7–S18. [Google Scholar]
- Chuang, Y.C.; Liu, S.P.; Lee, K.S.; Liao, L.; Wang, J.; Yoo, T.K.; Chu, R.; Sumarsono, B. Prevalence of overactive bladder in China, Taiwan and South Korea: Results from a cross-sectional, population-based study. Low. Urin. Tract Symptoms 2019, 11, 48–55. [Google Scholar] [CrossRef]
- Reynolds, W.S.; Fowke, J.; Dmochowski, R. The Burden of Overactive Bladder on US Public Health. Curr. Bladder Dysfunct. Rep. 2016, 11, 8–13. [Google Scholar] [CrossRef]
- Berry, S.H.; Elliott, M.N.; Suttorp, M.; Bogart, L.M.; Stoto, M.A.; Eggers, P.; Nyberg, L.; Clemens, J.Q. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 2011, 186, 540–544. [Google Scholar] [CrossRef]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef]
- Coelho, A.; Cruz, F.; Cruz, C.D.; Avelino, A. Effect of onabotulinumtoxinA on intramural parasympathetic ganglia: An experimental study in the guinea pig bladder. J. Urol. 2012, 187, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Somogyi, G.T.; Salas, N.A.; Kiss, S.; Boone, T.B.; Smith, C.P. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology 2005, 66, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Cruz, F.; Cruz, C.D.; Avelino, A. Spread of onabotulinumtoxinA after bladder injection. Experimental study using the distribution of cleaved SNAP-25 as the marker of the toxin action. Eur. Urol. 2012, 61, 1178–1184. [Google Scholar] [CrossRef]
- Haynes, M.D.; Martin, T.A.; Jenkins, S.A.; Kynaston, H.G.; Matthews, P.N.; Jiang, W.G. Tight junctions and bladder cancer (review). Int. J. Mol. Med. 2005, 16, 3–9. [Google Scholar] [CrossRef]
- Tzan, C.J.; Berg, J.; Lewis, S.A. Effect of protamine sulfate on the permeability properties of the mammalian urinary bladder. J. Membr. Biol. 1993, 133, 227–242. [Google Scholar] [CrossRef]
- Stein, P.C.; Pham, H.; Ito, T.; Parsons, C.L. Bladder injury model induced in rats by exposure to protamine sulfate followed by bacterial endotoxin. J. Urol. 1996, 155, 1133–1138. [Google Scholar] [CrossRef]
- Stemler, K.M.; Crock, L.W.; Lai, H.H.; Mills, J.C.; Gereau, R.W.t.; Mysorekar, I.U. Protamine sulfate induced bladder injury protects from distention induced bladder pain. J. Urol. 2013, 189, 343–351. [Google Scholar] [CrossRef][Green Version]
- Stewart, B.H.; Branson, A.C.; Hewitt, C.B.; Kiser, W.S.; Straffon, R.A. The treatment of patients with interstitial cystitis, with special reference to intravesical DMSO. J. Urol. 1972, 107, 377–380. [Google Scholar] [CrossRef]
- Grover, S.; Srivastava, A.; Lee, R.; Tewari, A.K.; Te, A.E. Role of inflammation in bladder function and interstitial cystitis. Adv. Urol. 2011, 3, 19–33. [Google Scholar] [CrossRef]
- Shiga, K.I.; Hirano, K.; Nishimura, J.; Niiro, N.; Naito, S.; Kanaide, H. Dimethyl sulphoxide relaxes rabbit detrusor muscle by decreasing the Ca2+ sensitivity of the contractile apparatus. Br. J. Pharmacol. 2007, 151, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.P.; Parker, A.S.; Crook, J.E.; Rogers, A.; Metz-Kudashick, D.; Thiel, D.D. Botulinum a toxin/dimethyl sulfoxide bladder instillations for women with refractory idiopathic detrusor overactivity: A phase 1/2 study. Mayo Clin. Proc. 2009, 84, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Gurpinar, T.; Truong, L.D.; Wong, H.Y.; Griffith, D.P. Electromotive drug administration to the urinary bladder: An animal model and preliminary results. J. Urol. 1996, 156, 1496–1501. [Google Scholar] [CrossRef]
- Slater, S.E.; Patel, P.; Viney, R.; Foster, M.; Porfiri, E.; James, N.D.; Montgomery, B.; Bryan, R.T. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Ann. R. Coll. Surg. Engl. 2014, 96, 415–419. [Google Scholar] [CrossRef][Green Version]
- Di Stasi, S.M.; Giannantoni, A.; Stephen, R.L.; Capelli, G.; Navarra, P.; Massoud, R.; Vespasiani, G. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: A prospective randomized study. J. Urol. 2003, 170, 777–782. [Google Scholar] [CrossRef]
- Di Stasi, S.M.; Valenti, M.; Verri, C.; Liberati, E.; Giurioli, A.; Leprini, G.; Masedu, F.; Ricci, A.R.; Micali, F.; Vespasiani, G. Electromotive instillation of mitomycin immediately before transurethral resection for patients with primary urothelial non-muscle invasive bladder cancer: A randomised controlled trial. Lancet. Oncol. 2011, 12, 871–879. [Google Scholar] [CrossRef]
- Di Stasi, S.M.; Giannantoni, A.; Massoud, R.; Dolci, S.; Navarra, P.; Vespasiani, G.; Stephen, R.L. Electromotive versus passive diffusion of mitomycin C into human bladder wall: Concentration-depth profiles studies. Cancer Res. 1999, 59, 4912–4918. [Google Scholar]
- CADTH Rapid Response Reports. The Use of the Electromotive Drug Administration System in Patients with Overactive Bladder: A Review of the Clinical Effectiveness, Safety, and Cost-Effectiveness; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2014. [Google Scholar]
- Kajbafzadeh, A.M.; Ahmadi, H.; Montaser-Kouhsari, L.; Sharifi-Rad, L.; Nejat, F.; Bazargan-Hejazi, S. Intravesical electromotive botulinum toxin type A administration--part II: Clinical application. Urology 2011, 77, 439–445. [Google Scholar] [CrossRef]
- Ladi-Seyedian, S.S.; Sharifi-Rad, L.; Kajbafzadeh, A.M. Intravesical Electromotive Botulinum Toxin Type “A” Administration for Management of Urinary Incontinence Secondary to Neuropathic Detrusor Overactivity in Children: Long-term Follow-up. Urology 2018, 114, 167–174. [Google Scholar] [CrossRef]
- Wang, H.J.; Cheng, J.H.; Chuang, Y.C. Potential applications of low-energy shock waves in functional urology. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2017, 24, 573–581. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Huang, T.L.; Tyagi, P.; Huang, C.C. Urodynamic and Immunohistochemical Evaluation of Intravesical Botulinum Toxin A Delivery Using Low Energy Shock Waves. J. Urol. 2016, 196, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Nageib, M.; El-Hefnawy, A.S.; Zahran, M.H.; El-Tabey, N.A.; Sheir, K.Z.; Shokeir, A.A. Delivery of intravesical botulinum toxin A using low-energy shockwaves in the treatment of overactive bladder: A preliminary clinical study. Arab J. Urol. 2019, 17, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-Y.; Chancellor, D.D.; Chancellor, M.B.; Chuang, Y.-C. Role of liposome in treatment of overactive bladder and interstitial cystitis. Urol. Sci. 2015, 26, 3–6. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lee, W.C.; Lee, W.C.; Chiang, P.H. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J. Urol. 2009, 182, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Tyagi, P.; Huang, C.C.; Yoshimura, N.; Wu, M.; Kaufman, J.; Chancellor, M.B. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J. Urol. 2009, 182, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Liu, H.T.; Chuang, Y.C.; Birder, L.A.; Chancellor, M.B. Pilot study of liposome-encapsulated onabotulinumtoxina for patients with overactive bladder: A single-center study. Eur. Urol. 2014, 65, 1117–1124. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Kaufmann, J.H.; Chancellor, D.D.; Chancellor, M.B.; Kuo, H.C. Bladder instillation of liposome encapsulated onabotulinumtoxina improves overactive bladder symptoms: A prospective, multicenter, double-blind, randomized trial. J. Urol. 2014, 192, 1743–1749. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Kuo, H.C. A Prospective, Multicenter, Double-Blind, Randomized Trial of Bladder Instillation of Liposome Formulation OnabotulinumtoxinA for Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2017, 198, 376–382. [Google Scholar] [CrossRef]
- Lee, W.C.; Su, C.H.; Tain, Y.L.; Tsai, C.N.; Yu, C.C.; Chuang, Y.C. Potential Orphan Drug Therapy of Intravesical Liposomal Onabotulinumtoxin-A for Ketamine-Induced Cystitis by Mucosal Protection and Anti-inflammation in a Rat Model. Sci. Rep. 2018, 8, 5795. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release Off. J. Control. Release Soc. 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Tyagi, P.; Li, Z.; Chancellor, M.; De Groat, W.C.; Yoshimura, N.; Huang, L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm. Res. 2004, 21, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Krhut, J.; Navratilova, M.; Sykora, R.; Jurakova, M.; Gartner, M.; Mika, D.; Pavliska, L.; Zvara, P. Intravesical instillation of onabotulinum toxin A embedded in inert hydrogel in the treatment of idiopathic overactive bladder: A double-blind randomized pilot study. Scand. J. Urol. 2016, 50, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, Y.H.; Zisman, A.; Jeshurun-Gutshtat, M.; Gerassi, T.; Hakim, G.; Vinshtok, Y.; Stav, K. Safety and Feasibility of Intravesical Instillation of Botulinum Toxin-A in Hydrogel-based Slow-release Delivery System in Patients With Interstitial Cystitis-Bladder Pain Syndrome: A Pilot Study. Urology 2018, 114, 60–65. [Google Scholar] [CrossRef] [PubMed]
- El Shatoury, M.G.; DeYoung, L.; Turley, E.; Yazdani, A.; Dave, S. Early experimental results of using a novel delivery carrier, hyaluronan-phosphatidylethanolamine (HA-PE), which may allow simple bladder instillation of botulinum toxin A as effectively as direct detrusor muscle injection. J. Pediatric Urol. 2018, 14, 172.e171–172.e176. [Google Scholar] [CrossRef] [PubMed]

| Research and Modalities | No.Pts | Study Design | Follow-up Duration | Patients Criteria | Modalities Utilization and onaBoNTA Dose | Outcome at the End Point |
|---|---|---|---|---|---|---|
| Petrou et al. [22] (DMSO) | 25 | Single arm Prospective cohort | 3 months | Adult females idiopathic DO | DMSO (50% 50mL) plus 300U onaBoNTA | Improve incontinence at 1 month, but not at 3 months |
| Kajbafzadeh et al. [29] (EMDA) | 15 | Single arm Prospective cohort | 9 months | Children MMC-related DO | onaBoNTA (10 U/kg) plus EDMA delivered 10 mA for 15 min | Improve incontinence in 80% cases and decrease VUR grade in 58% cases |
| Kajbafzadeh et al. [30] (EMDA) | 24 | Single arm Prospective cohort | 6 years | Children MMC-related DO | onaBoNTA (10 U/kg) plus EDMA delivered 10 mA for 20 min | Followed up at 1, 2, 3, 5, 6 years, and 75%, 45.5%, 37.5%, 33%, 29.1% of patients remain completely dry |
| Nageib et al. [33] (LESW) | 15 | Single arm Prospective cohort | 3 months | Adults idiopathic DO | 100U onaBoNTA plus LESW 3000 shocks (6.6 mJ/shock, 300 shocks/min) | Improve OABSS at 1, 2 months, but not at 3 months. |
| Kuo et al. [37] (Liposome) | 24 | Double-blind RCT | 3 months | Adults OAB | 200U onaBoNTA plus 80 mg liposomes | Improve frequency at 1 month, but not at 3 months |
| Chuang et al. [38] (Liposome) | 62 | Multicenter double-blind RCT | 4 weeks | Adults OAB | 200U onaBoNTA plus 80 mg liposomes | Decrease micturition events and urgency severity at 4 weeks |
| Chuang and Kuo [39] (Liposome) | 96 | Multicenter double-blind RCT | 4 weeks | Adults IC/BPS | 200U onaBoNTA plus 80 mg liposomes | Improve OSS, ICSI, ICPI, VAS scores, but not superior to placebo at 4 weeks |
| Krhut et al. [43] (Hydrogel) | 39 | Double-blind RCT | 1 month | Adult females OAB | 200U onaBoNTA plus TC-3 gel | Improve urgency, leakage episodes, PPBC, OAB-q scores at 1 month |
| Rappaport et al. [44] (Hydrogel) | 15 | Single arm Prospective cohort | 12 weeks | IC/BPS | 200IU onaBoNTA plus 40 mL TC-3 Gel | Improve ICSI, VAS scores at week 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Lee, W.-C.; Wang, H.-J.; Chuang, Y.-C. Therapeutic Efficacy of onabotulinumtoxinA Delivered Using Various Approaches in Sensory Bladder Disorder. Toxins 2020, 12, 75. https://doi.org/10.3390/toxins12020075
Chen P-Y, Lee W-C, Wang H-J, Chuang Y-C. Therapeutic Efficacy of onabotulinumtoxinA Delivered Using Various Approaches in Sensory Bladder Disorder. Toxins. 2020; 12(2):75. https://doi.org/10.3390/toxins12020075
Chicago/Turabian StyleChen, Po-Yen, Wei-Chia Lee, Hung-Jen Wang, and Yao-Chi Chuang. 2020. "Therapeutic Efficacy of onabotulinumtoxinA Delivered Using Various Approaches in Sensory Bladder Disorder" Toxins 12, no. 2: 75. https://doi.org/10.3390/toxins12020075
APA StyleChen, P.-Y., Lee, W.-C., Wang, H.-J., & Chuang, Y.-C. (2020). Therapeutic Efficacy of onabotulinumtoxinA Delivered Using Various Approaches in Sensory Bladder Disorder. Toxins, 12(2), 75. https://doi.org/10.3390/toxins12020075






