The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study
Abstract
:1. Introduction
2. Results
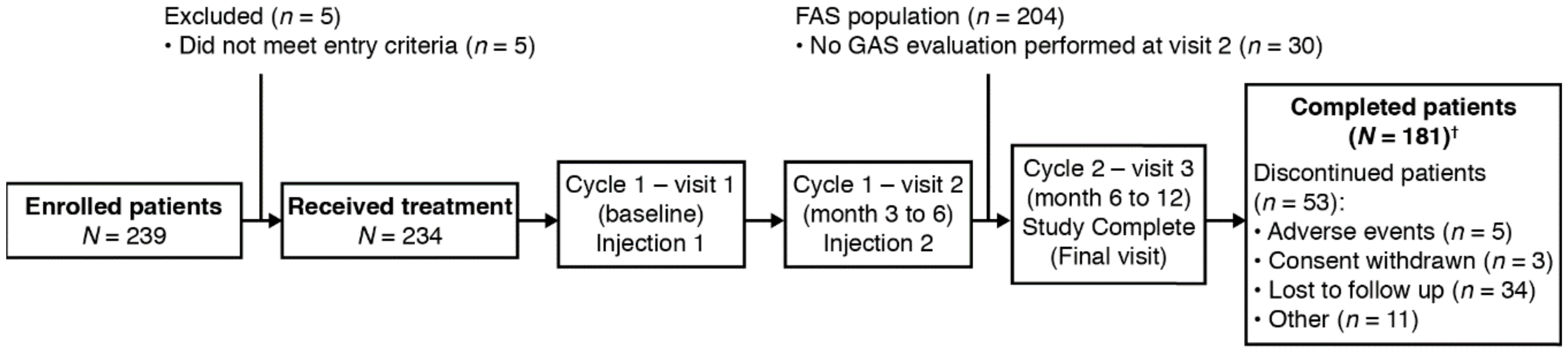
2.1. Study Population
2.2. Primary Efficacy Endpoint
2.3. Secondary Effectiveness Endpoints
2.3.1. Responders at Visit 3
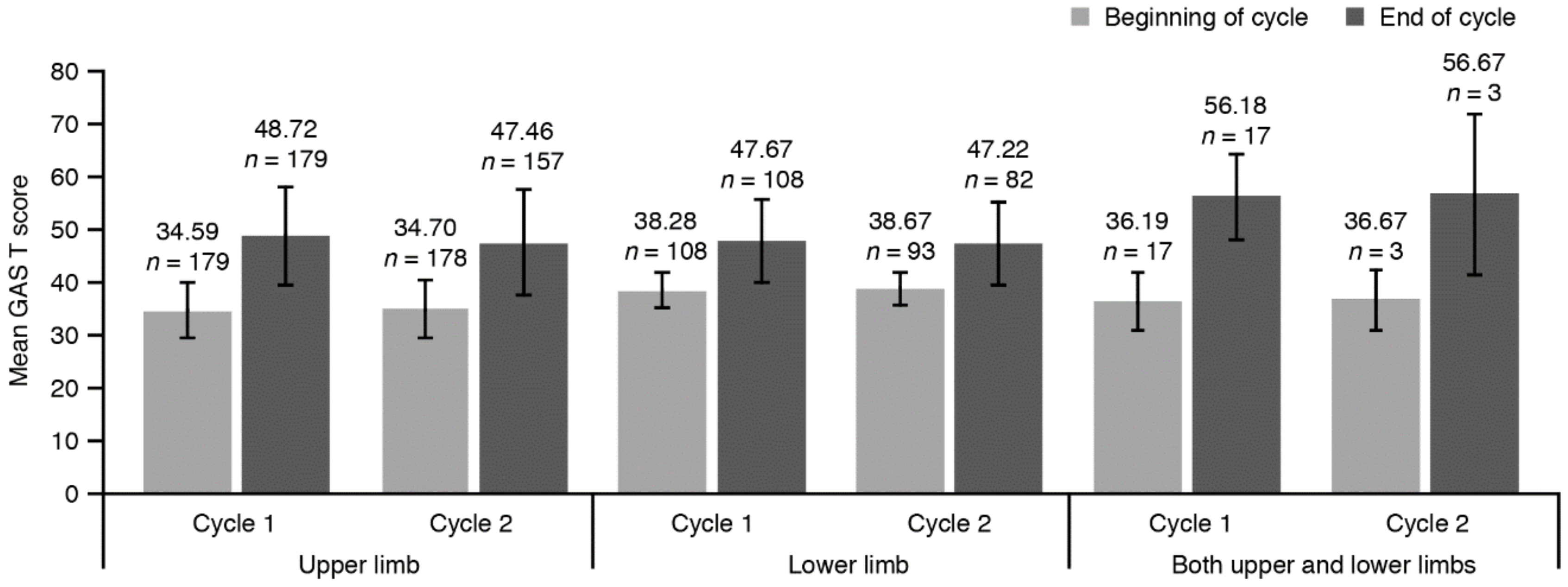
2.3.2. GAS T Score
2.3.3. Pain Scores
2.3.4. Range of Motion
2.3.5. MAS Spasticity Scores
2.3.6. Functional Independence
2.3.7. QoL and Satisfaction
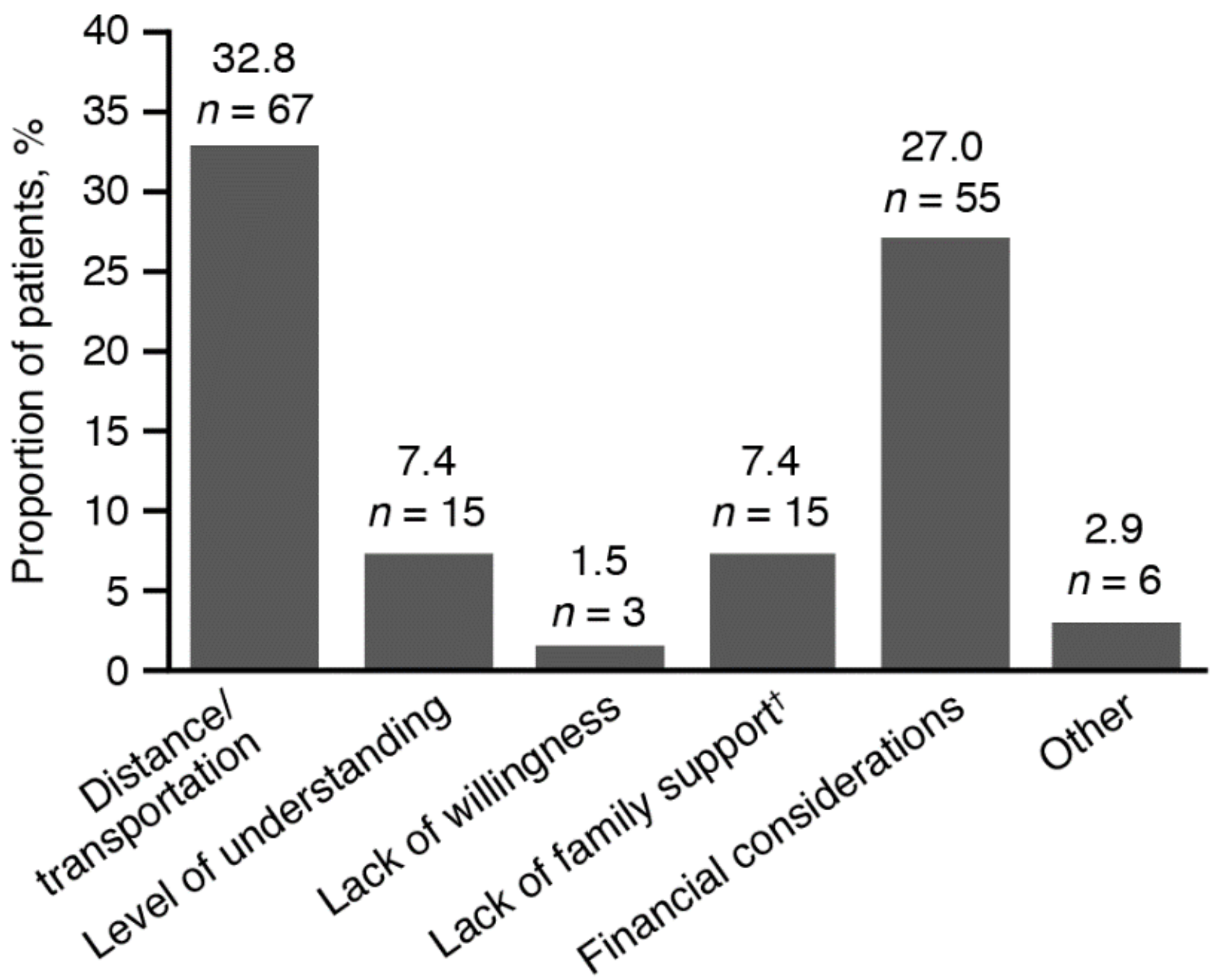
2.4. Socio-Demographic Data
2.5. Injection Practices
2.6. Pharmacoeconomic Impact of BoNT-A Injections
2.7. Safety
3. Discussion
4. Conclusions
5. Methods
5.1. Study Design
5.2. Inclusion and Exclusion Criteria
5.3. Treatment
5.4. Study Assessments and Endpoints
5.5. Statistical Methods
5.6. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sommerfeld, D.K.; Eek, E.U.; Svensson, A.K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of spasticity post stroke. Clin. Rehabil. 2002, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, E.; Terent, A.; Borg, J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur. J. Neurol. 2008, 15, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B.J.; Cerebral Palsy, I. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: International consensus statement. Eur. J. Neurol. 2010, 17, 74–93. [Google Scholar] [CrossRef]
- Royal College of Physicians. Spasticity in Adults: Management Using Botulinum Toxin. National guidelines 2018. Available online: https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin (accessed on 5 June 2018).
- Dashtipour, K.C.; Walker, H.W.; Lee, M.Y. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am. J. Phys. Med. Rehabil. 2015, 94, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic literature review of abobotulinumtoxinA in clinical trials for lower limb spasticity. Medicine 2016, 95, e2468. [Google Scholar] [CrossRef]
- Gracies, J.-M.; Esquenazi, A.; Brashear, A.; Banach, M.; Kocer, S.; Jech, R.; Khatkova, S.; Benetin, J.; Vecchio, M.; McAllister, P.; et al. Efficacy and safety of abobotulinumtoxinA in spastic lower limb: Randomized trial and extension. Neurology 2017, 89, 2245–2253. [Google Scholar] [CrossRef] [Green Version]
- Gracies, J.M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef]
- Cardoso, E.; Rodrigues, B.; Lucena, R.; Oliveira, I.R.; Pedreira, G.; Melo, A. Botulinum toxin type A for the treatment of the upper limb spasticity after stroke: A meta-analysis. Arq. Neuro-Psiquiatr. 2005, 63, 30–33. [Google Scholar] [CrossRef] [Green Version]
- ANVISA-AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Detalhe do Produto: BOTOX. Available online: https://consultas.anvisa.gov.br/#/medicamentos/250000055089118/ (accessed on 3 February 2020).
- Hesse, S.; Mach, H.; Frohlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: A randomized controlled trial. Clin. Rehabil. 2012, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.; Delgado-De Los Santos, M.M.; Chua, K.S.; Abdullah, S.J.; Zakine, B.; Maisonobe, P.; et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: A randomized controlled trial. Neurorehabil. Neural. Repair. 2012, 26, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yan, D.; Li, J.-H.; Shi, Z.-H. Gait improvement by low-dose botulinum toxin A injection treatment of the lower limbs in subacute stroke patients. J. Phys. Ther. Sci. 2015, 27, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Rosales, R.L. Effect of early use of AbobotulinumtoxinA after stroke on spasticity progression: Protocol for a randomised controlled pilot study in adult subjects with moderate to severe upper limb spasticity (ONTIME pilot). Contemp. Clin. Trials Commun. 2017, 6, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Li, C.; Chen, J.; Miranda, J.J.; Luo, R.; Bettger, J.; Zhu, Y.; Feigin, V.; O’Donnell, M.; Zhao, D.; et al. Prevention, management, and rehabilitation of stroke in low- and middle-income countries. eNeurologicalScience 2016, 2, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Ipsen Biopharm Ltd. Dysport Full Prescribing Information. Available online: https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2020/01/09195739/S115_2019_09_25_sBLA_Approval_PMR_Fulfilled_PI_MG_Sept-2019.pdf (accessed on 26 February 2020).
- Allergan‚ Inc. Highlights of Prescribing Information: Botox®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103000s5302lbl.pdf (accessed on 14 August 2018).
- Crema, C.M.T.; Santos, A.P.B.C.; Magário, L.P.T.; Caldas, C.A.C.T.; Riberto, M. Neuromuscular block practice in the treatment of spasticity in Brazil. Physiatr. Act. 2016, 23, 150–154. [Google Scholar] [CrossRef]
- Prazeres, A.; Lira, M.; Aguiar, P.; Monteiro, L.; Vilasboas, I.; Melo, A. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol. Int. 2018, 10, 7385. [Google Scholar] [CrossRef] [PubMed]
- Fheodoroff, K.; Ashford, S.; Jacinto, J.; Maisonobe, P.; Balcaitiene, J.; Turner-Stokes, L. Factors influencing goal attainment in patients with post-stroke upper limb spasticity following treatment with botulinum toxin A in real-life clinical practice: Sub-analyses from the Upper Limb International Spasticity (ULIS)-II Study. Toxins 2015, 7, 1192–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, E.; Duarte, E.; Vila, J.; Tejero, M.; Guillen, A.; Boza, R.; Escalada, F.; Espadaler, J.M. Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J. Rehabil. Med. 2007, 39, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J. Neurol. Neurosurg. Psychiatry 2007, 78, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, G.; Cardoso, E.; Melo, A. Botulinum toxin type A for refractory post-stroke shoulder pain. Arq. Neuro-Psiquiatr. 2008, 66, 213–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Fu, Y.; Song, H.X.; Ye, Y.; Dong, Y.; Li, J.H. Effectiveness of Botulinum Toxin for Shoulder Pain Treatment: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 2214–2220. [Google Scholar] [CrossRef]
- Gomes, A.L.S.; Mello, F.F.; Cocicov Neto, J.; Benedeti, M.C.; Modolo, L.F.M.; Riberto, M. Can the positions of the spastic upper limb in stroke survivors help muscle choice for botulinum toxin injections? Arq. Neuro-Psiquiatr. 2019, 77, 568–573. [Google Scholar] [CrossRef]
- Francisco, G.E.; McGuire, J.R. Poststroke spasticity management. Stroke 2012, 43, 3132–3136. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, L.H.; Alencar, F.J.; Rodrigues, L.R.; Sousa, F.C.; Teles, J.B. Effects of botulinum toxin type A for spastic foot in post-stroke patients enrolled in a rehabilitation program. Arq. Neuro-Psiquiatr. 2014, 72, 28–32. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: A randomised double blind placebo controlled trial. J. Neurol. Neurosurg Psychiatry 2000, 69, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Jorge, L.L.; de Brito, A.M.d.N.; Marchi, F.H.G.; Hara, A.C.P.; Battistella, L.R.; Riberto, M. New rehabilitation models for neurologic inpatients in Brazil. Disabil Rehabil. 2015, 37, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carod-Artal, F.J.; Medeiros, M.S.; Horan, T.A.; Braga, L.W. Predictive factors of functional gain in long-term stroke survivors admitted to a rehabilitation programme. Brain Inj. 2005, 19, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L. Goal attainment scaling (GAS) in rehabilitation: A practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P.; Zakine, B. Upper limb international spasticity study: Rationale and protocol for a large, international, multicentre prospective cohort study investigating management and goal attainment following treatment with botulinum toxin A in real-life clinical practice. BMJ Open. 2013, 3, e002230. [Google Scholar] [CrossRef] [Green Version]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P. Results from the Upper Limb International Spasticity Study-II (ULISII): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open. 2013, 3, e002771. [Google Scholar] [CrossRef] [Green Version]

| Parameter | All Patients (n = 204) |
|---|---|
| Age in years, mean (SD); median [range] | 56.4 (13.2); 59.0 [18−79] |
| Sex, n (%) Male Female | 105 (51.5) 99 (48.5) |
| Handedness, n (%) Ambidextrous Left Right | 4 (2.0) 14 (6.9) 186 (91.2) |
| Time since last CVA (months), mean (SD); median [range] | 51.3 (61.4); 28.1 [12−374] |
| Etiology of last CVA, n (%) Hemorrhage Infarct Infarct and hemorrhage | 45 (22.1) 151 (74.0) 8 (3.9) |
| Location of last CVA, n (%) Left hemisphere Posterior circulation Right hemisphere | 99 (48.5) 3 (1.5) 102 (50.0) |
| Number of CVA episodes (previous and studied), n (%) 1 2 3 >3 | 159 (77.9) 32 (15.7) 8 (3.9) 5 (2.5) |
| Time since onset of stroke (months), mean (SD); median [range] | 61.6 (69.4); 32.0 [12−374] |
| Time since onset of spasticity (months), mean (SD); median [range] | 40.2 (53.1); 22.7 [0−350] |
| Time between first CVA and onset of spasticity (months), mean (SD); median [range] | 25.7 (53.2); 3.6 [0−349] |
| UL affected by spasticity, n (%) Right Left Bilateral | 204 (100) 93 (45.6) 105 (51.5) 6 (2.9) |
| LL affected by spasticity, n (%) Right Left Bilateral | 185 (90.7) 84 (45.4) 95 (51.4) 6 (3.2) |
| UL spasticity patterns, n (%) † Adducted internally rotated shoulder Flexed elbow Flexed wrist Pronated forearm Clenched fist Thumb-in-palm | 169 (82.8) 168 (82.4) 144 (70.6) 133 (65.2) 113 (55.4) 101 (49.5) |
| LL spasticity patterns, n (%) † Equinovarus foot Knee extension Claw toes Knee flexion Hip adduction Hip flexion Striatal toe | 147 (79.5) 89 (48.1) 70 (37.8) 52 (28.1) 36 (19.5) 31 (16.8) 23 (12.4) |
| Visit 2 | n (%) | 95% CI |
|---|---|---|
| Primary goal limb | ||
| UL (n = 154) | 89 (57.8) | 49.9, 65.3 |
| LL (n = 39) | 25 (64.1) | 48.4, 77.3 |
| Both limbs (n = 11) | 11 (100.0) | 70.0, 100.0 |
| Overall (n = 204) | 125 (61.3) | 54.4, 67.7 |
| Domain | Level | Visit 1, n (%) [95% CI] | Visit 2, n (%) [95% CI] | Visit 3, n (%) [95% CI] |
|---|---|---|---|---|
| Mobility | I have no problem in walking about | 7 (5.1) [2.3, 10.4] | 13 (10.2) [6.0, 16.9] | 17 (15.0) [9.5, 22.9] |
| I have slight problems in walking about | 35 (25.5) [19.0, 33.5] | 39 (30.7) [23.3, 39.2] | 40 (35.4) [27.2, 44.6] | |
| I have moderate problems in walking about | 38 (27.7) [20.9, 35.8] | 38 (29.9) [22.6, 38.4] | 32 (28.3) [20.8, 37.3] | |
| I have severe problems in walking about | 36 (26.3) [19.6, 34.2] | 19 (15.0) [9.7, 22.3] | 9 (8.0) [4.1, 14.6] | |
| I am unable to walk about | 21 (15.3) [10.2, 22.4] | 18 (14.2) [9.1, 21.4] | 15 (13.3) [8.1, 20.9] | |
| Missing | 67 | 77 | 91 | |
| Self-care | I have no problem washing or dressing myself | 25 (18.2) [12.6, 25.6] | 34 (26.8) [19.8, 35.1] | 45 (39.8) [31.3, 49.0] |
| I have slight problems washing or dressing myself | 31 (22.6) [16.4, 30.4] | 32 (25.2) [18.4, 33.4] | 23 (20.4) [13.9, 28.8] | |
| I have moderate problems washing or dressing myself | 29 (21.2) [15.1, 28.8] | 30 (23.6) [17.0, 31.8] | 25 (22.1) [15.4, 30.7] | |
| I have severe problems washing or dressing myself | 17 (12.4) [7.8, 19.1] | 11 (8.7) [4.8, 15.0] | 10 (8.8) [4.7, 15.7] | |
| I am unable to wash or dress myself | 35 (25.5) [19.0, 33.5] | 20 (15.7) [10.4, 23.2] | 10 (8.8) [4.7, 15.7] | |
| Missing | 67 | 77 | 91 | |
| Usual activity | I have no problems doing my usual activities | 9 (6.6) [3.4, 12.3] | 15 (11.8) [7.2, 18.7] | 21 (18.6) [12.4, 26.8] |
| I have slight problems doing my usual activities | 22 (16.2) [10.9, 23.3] | 36 (28.3) [21.2, 36.8] | 34 (30.1) [22.4, 39.1] | |
| I have moderate problems doing my usual activities | 38 (27.9) [21.1, 36.0] | 34 (26.8) [19.8, 35.1] | 23 (20.4) [13.9, 28.8] | |
| I have severe problems doing my usual activities | 28 (20.6) [14.6, 28.2] | 16 (12.6) [7.8, 19.6] | 17 (15.0) [9.5, 22.9] | |
| I am unable to do my usual activities | 39 (28.7) [21.7, 36.8] | 26 (20.5) [14.3, 28.4] | 18 (15.9) [10.2, 23.9] | |
| Missing | 68 | 77 | 91 | |
| Pain/discomfort | I have no pain or discomfort | 40 (29.2) [22.2, 37.2] | 47 (37.0) [29.1, 45.7] | 53 (46.9) [38.0, 56.1] |
| I have slight pain or discomfort | 35 (25.5) [19.0, 33.5] | 41 (32.3) [24.8, 40.8] | 32 (28.3) [20.8, 37.3] | |
| I have moderate pain or discomfort | 34 (24.8) [18.3, 32.7] | 24 (18.9) [13.0, 26.6] | 18 (15.9) [10.2, 23.9] | |
| I have severe pain or discomfort | 20 (14.6) [9.6, 21.6] | 13 (10.2) [6.0, 16.9] | 9 (8.0) [4.1, 14.6] | |
| I am extreme pain or discomfort | 8 (5.8) [2.8, 11.3] | 2 (1.6) [0.1, 5.9] | 1 (0.9) [0.0, 5.3] | |
| Missing | 67 | 77 | 91 | |
| Anxiety/depression | I am not anxious or depressed | 47 (34.3) [26.9, 42.6] | 39 (30.7) [23.3, 39.2] | 43 (38.1) [29.6, 47.3] |
| I am slightly anxious or depressed | 36 (26.3) [19.6, 34.2] | 38 (29.9) [22.6, 38.4] | 36 (31.9) [24.0, 40.9] | |
| I am moderately anxious or depressed | 30 (21.9) [15.8, 29.6] | 32 (25.2) [18.4, 33.4] | 21 (18.6) [12.4, 26.8] | |
| I am severely anxious or depressed | 10 (7.3) [3.9, 13.1] | 12 (9.4) [5.4, 15.9] | 10 (8.8) [4.7, 15.7] | |
| I am extremely anxious or depressed | 14 (10.2) [6.1, 16.5] | 6 (4.7) [2.0, 10.1] | 3 (2.7) [0.6, 7.9] | |
| Missing | 67 | 77 | 91 |
| Visit | Treatment Satisfaction | Investigators’ Global Assessment of Benefit, n (%) [95% CI] | Patients’ Global Assessment of Benefit, n (%) [95% CI] | Caregivers’ Global Assessment of Benefit, n (%) [95% CI] |
|---|---|---|---|---|
| End of cycle 1 | Great benefit | 61 (29.9) [24.0, 36.5] | 81 (39.7) [33.2, 46.6] | 72 (35.3) [29.1, 42.1] |
| Some benefit | 135 (66.2) [59.4, 72.3] | 92 (45.1) [38.4, 52.0] | 80 (39.2) [32.8, 46.1] | |
| Same | 7 (3.4) [1.5, 7.0] | 22 (10.8) [7.2, 15.8] | 16 (7.8) [4.8, 12.4] | |
| Worse | 0 (0) [0.0, 2.2] | 2 (1.0) [0.0, 3.7] | 0 (0) [0.0, 2.2] | |
| Much worse | 1 (0.5) [0.0, 3.0] | 0 (0) [0.0, 2.2] | 0 (0) [0.0, 2.2] | |
| Not done | 0 (0) [0.0, 2.2] | 7 (3.4) [1.5, 7.0] | 36 (17.6) [13.0, 23.5] | |
| Missing | 0 | 0 | 0 | |
| End of cycle 2 | Great benefit | 67 (37.2) [30.5, 44.5] | 79 (43.9) [36.8, 51.2] | 65 (36.1) [29.4, 43.4] |
| Some benefit | 102 (56.7) [49.4, 63.7] | 72 (40.0) [33.1, 47.3] | 64 (35.6) [28.9, 42.8] | |
| Same | 7 (3.9) [1.7, 8.0] | 19 (10.6) [6.8, 16.0] | 12 (6.7) [3.7, 11.4] | |
| Worse | 4 (2.2) [0.7, 5.8] | 2 (1.1) [0.0, 4.2] | 1 (0.6) [0.0, 3.4] | |
| Much worse | 0 (0) [0.0, 2.5] | 1 (0.6) [0.0, 3.4] | 0 (0) [0.0, 2.5] | |
| Not done | 0 (0) [0.0, 2.5] | 7 (3.9) [1.7, 8.0] | 38 (21.1) [15.8, 27.7] | |
| Missing | 24 | 24 | 24 |
| Injection Parameters | AbobotulinumtoxinA | OnabotulinumtoxinA | Chinese Type A Botulinum Toxin | Other Unspecified BoNT-A Preparation | Overall |
|---|---|---|---|---|---|
| Number of patients injected at visit 1, n (%) | 173 (84.8) | 6 (2.9) | 3 (1.5) | 22 (10.8) | 204 (100) |
| Dose administered at visit 1, median (range) units | |||||
| UL | 600.0 (100.0−1500.0) | 300.0 (190.0−315.0) | 205.0 (205.0−360.0) | 197.5 (37.0−355.0) | - |
| LL | 450.0 (80.0−950.0) | 300.0 (100.0−350.0) | 220.0 (200.0−260.0) | 56.0 (20.0−400.0) | - |
| Both limbs | 1000.0 (400.0−1904.0) | 600.0 (300.0−600.0) | 425.0 (405.0−620.0) | 207.5 (99.0−730.0) | - |
| Number of muscles injected at visit 1, mean (SD) | 7.7 (3.2) | 9.3 (1.8) | 15.0 (1.0) | 9.9 (2.4) | 8.1 (3.2) |
| Injection guidance technique used at visit 1, n (%) | |||||
| Palpation/anatomic landmarks | 156 (90.2) | 2 (33.3) | 3 (100) | 22 (100) | 183 (89.7) |
| Electric stimulation | 19 (11.0) | 4 (66.7) | 0 | 0 | 23 (11.3) |
| Echography/ultrasound | 0 | 0 | 0 | 1 (4.5) | 1 (0.5) |
| Number of patients injected at visit 2, n (%) | 170 (84.6) | 6 (3.0) | 3 (1.5) | 22 (10.9) | 201 (100) |
| Dose administered at visit 2, median (range) units | |||||
| UL | 600.0 (50.0−1500.0) | 307.5 (185.0−400.0) | 245.0 (201.0−460.0) | 149.5 (37.0−265.0) | - |
| LL | 500.0 (25.0−1100.0) | 237.5 (100.0−315.0) | 250.0 (190.0−290.0) | 40.0 (20.0−100.0) | - |
| Both limbs | 1000.0 (175.0−1960.0) | 550.0 (380.0−600.0) | 495.0 (491.0−650.0) | 180.0 (99.0−275.0) | - |
| Number of muscles injected at visit 2, mean (SD) | 7.4 (3.4) | 9.8 (0.8) | 14.7 (1.5) | 8.0 (3.0) | 7.7 (3.4) |
| Injection guidance technique used at visit 2, n (%) | |||||
| Palpation/anatomic landmarks | 154 (90.6) | 3 (50.0) | 3 (100) | 22 (100) | 182 (90.5) |
| Electric stimulation | 18 (10.6) | 4 (66.7) | 0 | 0 | 22 (10.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, P.; Riberto, M.; Frances, J.A.; Chueire, R.; Amorim, A.C.F.G.; Xerez, D.; Chung, T.M.; Mercuri, L.H.C.; Longo, A.L.; Lianza, S.; et al. The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study. Toxins 2020, 12, 770. https://doi.org/10.3390/toxins12120770
Khan P, Riberto M, Frances JA, Chueire R, Amorim ACFG, Xerez D, Chung TM, Mercuri LHC, Longo AL, Lianza S, et al. The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study. Toxins. 2020; 12(12):770. https://doi.org/10.3390/toxins12120770
Chicago/Turabian StyleKhan, Patricia, Marcelo Riberto, João Amaury Frances, Regina Chueire, Ana Cristina Ferreira Garcia Amorim, Denise Xerez, Tae Mo Chung, Lucia Helena Costa Mercuri, Alexandre Luiz Longo, Sérgio Lianza, and et al. 2020. "The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study" Toxins 12, no. 12: 770. https://doi.org/10.3390/toxins12120770





