In-Vitro Neutralization of the Neurotoxicity of Coastal Taipan Venom by Australian Polyvalent Antivenom: The Window of Opportunity
Abstract
1. Introduction
2. Results
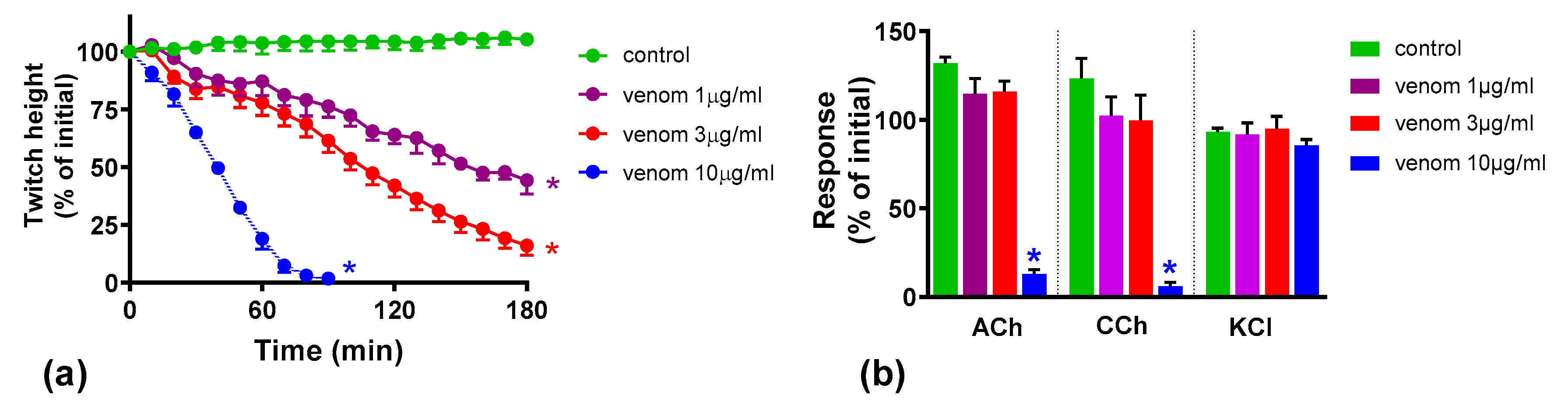
2.1. Concentration-Dependent Neurotoxicity of O. scutellatus Venom
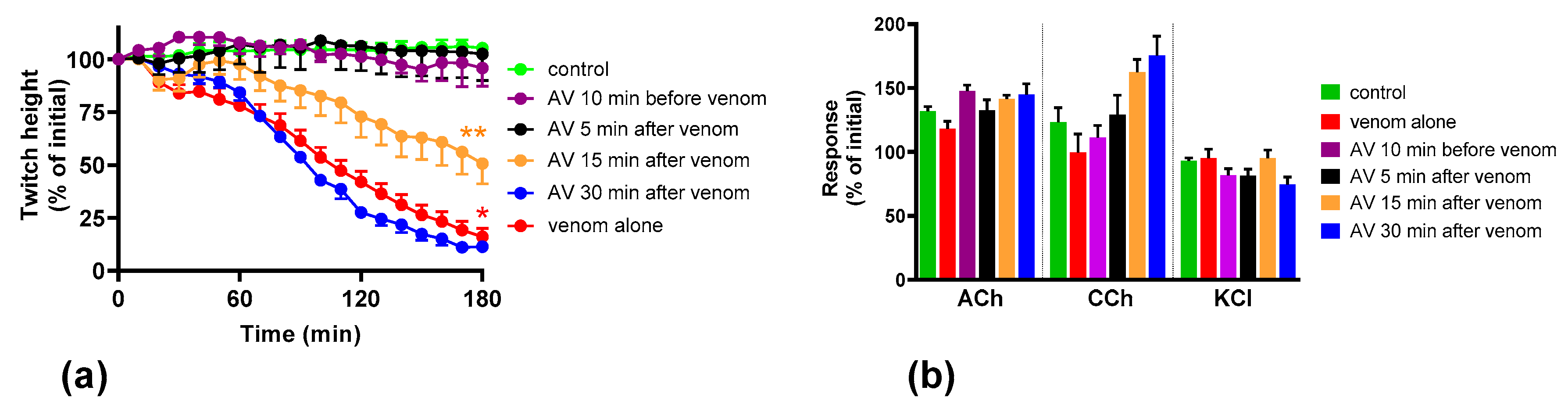
2.2. Effect of Australian Polyvalent Antivenom on the Pre-Synaptic Neurotoxicity by O. scutellatus Venom
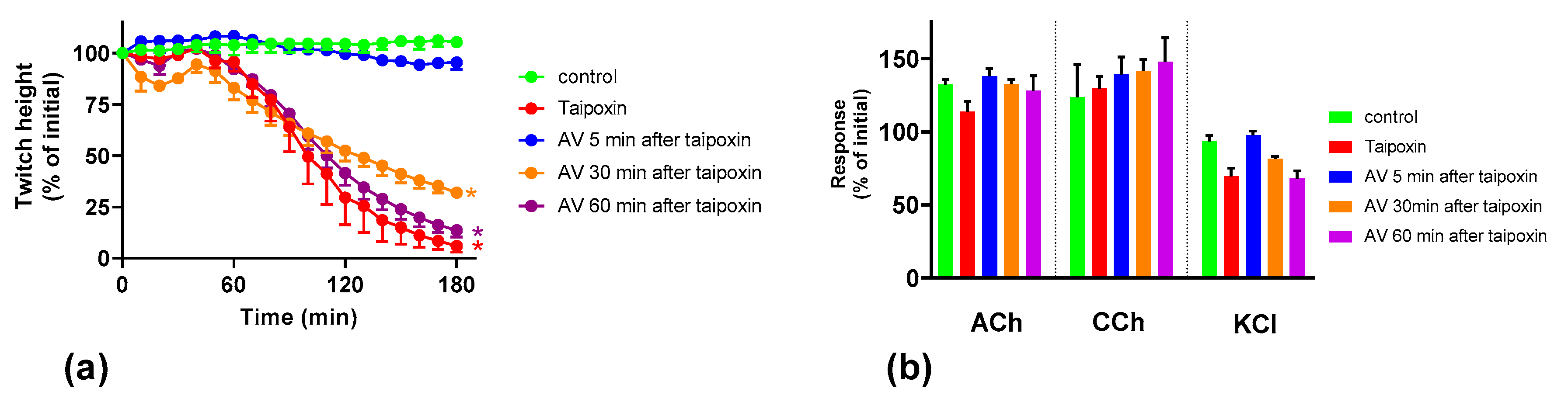
2.3. Effect of Australian Polyvalent Antivenom on Taipoxin
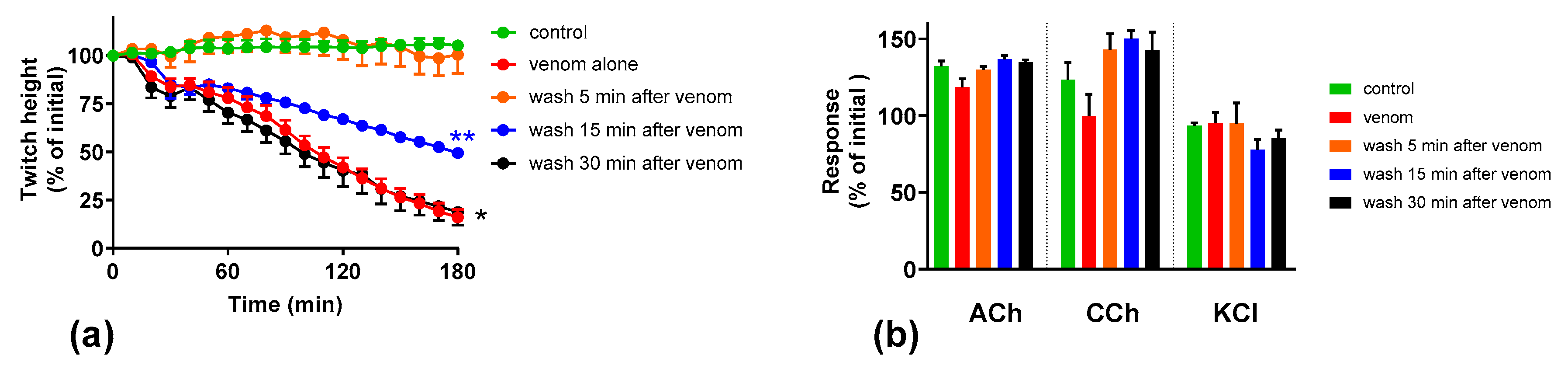
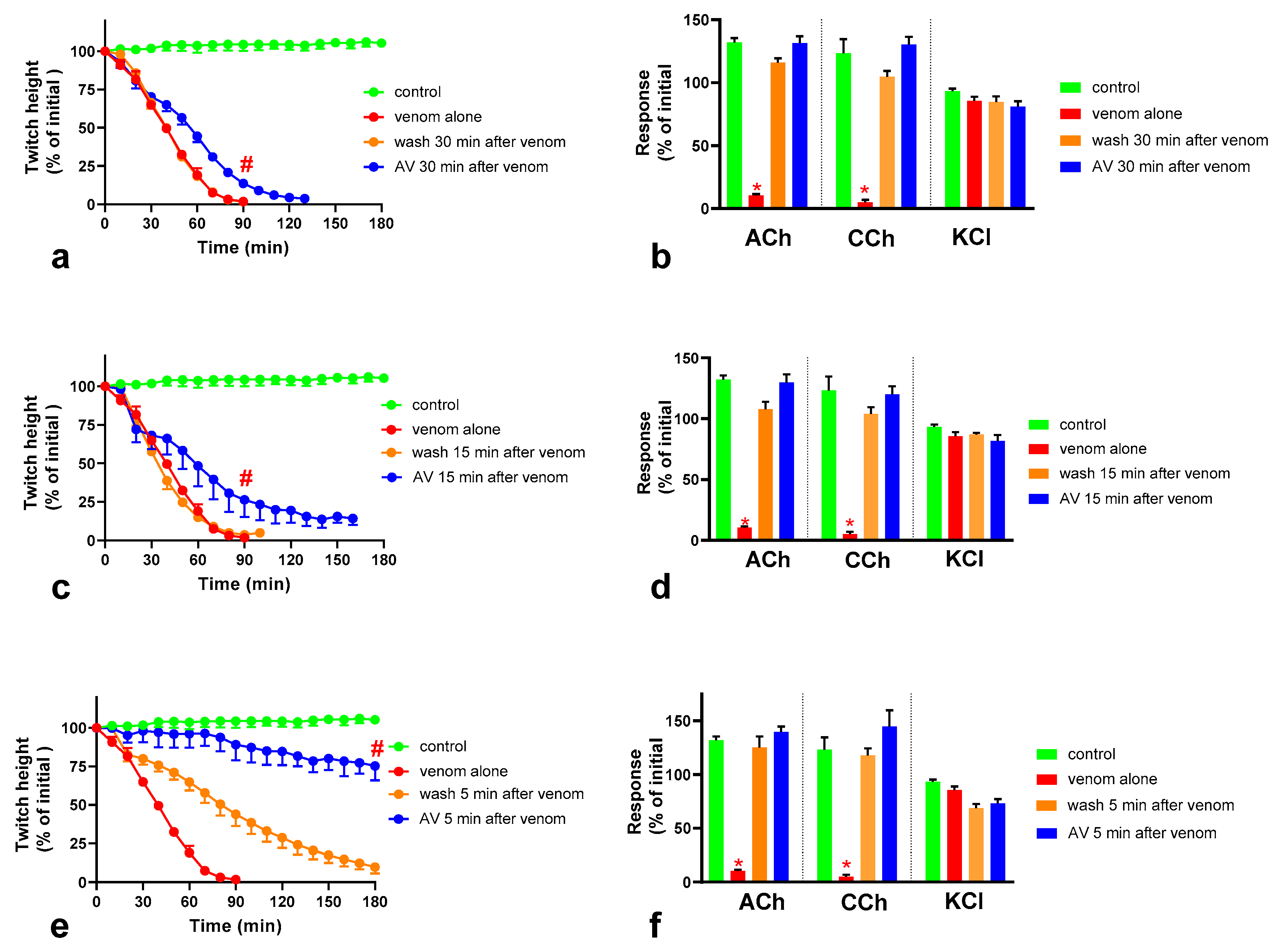
2.4. Effect of Washing the Neuromuscular Preparation on the Neurotoxicity of O. scutellatus Venom
2.5. The Effect of Washing and Australian Polyvalent Antivenom on Neuromuscular Block Caused by O. scutellatus Venom at 10 µg/mL
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms, Toxins and Antivenoms
5.2. Chick-Biventer Cervicis Nerve-Muscle Preparation
5.3. Experimental Design
5.4. Data Analysis and Statistics
5.5. Animal Ethics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, U.K.; Lalloo, D.G.; De Silva, H.J. Neurotoxicity in Snakebite—The Limits of Our Knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Hodgson, W.C.; Isbister, G.K. Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming. Toxins 2017, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Prasarnpun, S.; Walsh, J.; Awad, S.S.; Harris, J.B. Envenoming bites by kraits: The biological basis of treatment-resistant neuromuscular paralysis. Brain 2005, 128, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.W.; Harris, J.B. Nerve Terminal Damage by β-Bungarotoxin. Am. J. Pathol. 1999, 154, 447–455. [Google Scholar] [CrossRef]
- Rigoni, M.; Paoli, M.; Milanesi, E.; Caccin, P.; Rasola, A.; Bernardi, P.; Montecucco, C. Snake phospholipase A2 neurotoxins enter neurons, bind specifically to mitochondria, and open their transition pores. J. Biol. Chem. 2008, 283, 34013–34020. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rossetto, O. How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem. Sci. 2000, 25, 266–270. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic α-neurotoxins in paralysis due to snake envenoming in humans. Cell. Mol. Life Sci. 2018, 75, 4465–4478. [Google Scholar] [CrossRef]
- Silva, A.; Isbister, G.K. Current research into snake antivenoms, their mechanisms of action and applications. Biochem. Soc. Trans. 2020, 48, 537–546. [Google Scholar] [CrossRef]
- Boulain, J.; Ménez, A. Neurotoxin-Specific Immunoglobulins Accelerate Dissociation of the Neurotoxin-Acetylcholine Receptor Complex. Science 1982, 217, 732–733. [Google Scholar] [CrossRef]
- Hodgson, W.C.; Wickramaratna, J.C. In-vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol. 2002, 29, 807–814. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.A.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy—Australian Snakebite Project (ASP-25). Clin. Toxicol. 2017, 55, 115–122. [Google Scholar] [CrossRef]
- Fohlman, J.; Eaker, D.; Karlsson, E.; Thesleff, S. Taipoxin, an Extremely Potent Presynaptic Neurotoxin from the Venom of the Australian Snake Taipan (Oxyuranus s. scutellatus): Isolation, Characterization, Quaternary Structure and Pharmacological Properties. Eur. J. Biochem. 1976, 68, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, F.; Wolf, K.M.; Martin, B.M.; Possani, L.D.; Chiappinelli, V.A. Two Novel α-Neurotoxins Isolated from the Taipan Snake, Oxyuranus scutellatus, Exhibit Reduced Affinity for Nicotinic Acetylcholine Receptors in Brain and Skeletal Muscle. Biochemistry 2001, 2960, 7910–7916. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, R.; Hart, A.J.; Reeve, S.; Smith, A.I.; Fry, B.G.; Hodgson, W.C. Variations in the pharmacological profile of post-synaptic neurotoxins isolated from the venoms of the Papuan (Oxyuranus scutellatus canni) and coastal (Oxyuranus scutellatus scutellatus) taipans. Neurotoxicology 2010, 31, 239–243. [Google Scholar] [CrossRef]
- Harris, J. Neuromuscular Junction (NMJ): A Target for Natural and Environmental Toxins in Humans. In Encyclopedia of Neuroscience; Elsevier Academic Press: Boston, MA, USA, 2009; pp. 539–549. [Google Scholar]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 1, 2533–2571. [Google Scholar] [CrossRef]
- Šribar, J.; Oberčkal, J.; Križaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2: An update. Toxicon 2014, 89, 9–16. [Google Scholar] [CrossRef]
- Pungercar, J.; Krizaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon 2007, 50, 871–892. [Google Scholar] [CrossRef] [PubMed]
- Vardjan, N.; Mattiazzi, M.; Rowan, E.G.; Križaj, I.; Petrovič, U.; Petan, T. Neurotoxic phospholipase A2 toxicity model: An insight from mammalian cells. Commun. Integr. Biol. 2013, 6, 6–8. [Google Scholar] [CrossRef]
- Prasarnpun, S.; Walsh, J.; Harris, J.B. Beta-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology 2004, 47, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Johnston, C.; Siribaddana, S.; Isbister, G.K. Neuromuscular Effects of Common Krait (Bungarus caeruleus) Envenoming in Sri Lanka. PLoS Negl. Trop. Dis. 2016, 10, e0004368. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.; Buckley, N.; Siribaddana, S.; Isbister, G.K. Neurotoxicity in Russell’s viper (Daboia russelii) envenoming in Sri Lanka: A clinical and neurophysiological study. Clin. Toxicol. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Herrera, M.; De Cássia, R.; Collaço, D.O.; Villalta, M.; Segura, Á.; Vargas, M.; Wright, C.E.; Paiva, O.K.; Matainaho, T.; Jensen, S.D.; et al. Neutralization of the neuromuscular inhibition of venom and taipoxin from the taipan (Oxyuranus scutellatus) by F(ab’)2 and whole IgG antivenoms. Toxicol. Lett. 2016, 241, 175–183. [Google Scholar] [CrossRef]
- Fontana Oliveira, I.C.; Gutiérrez, J.M.; Lewin, M.R.; Oshima-Franco, Y. Varespladib (LY315920) inhibits neuromuscular blockade induced by Oxyuranus scutellatus venom in a nerve-muscle preparation. Toxicon 2020, 187, 101–104. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Solving the “Brown snake paradox”: In vitro characterisation of Australasian snake presynaptic neurotoxin activity. Toxicol. Lett. 2012, 210, 318–323. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhushani, U.; Isbister, G.K.; Tasoulis, T.; Hodgson, W.C.; Silva, A. In-Vitro Neutralization of the Neurotoxicity of Coastal Taipan Venom by Australian Polyvalent Antivenom: The Window of Opportunity. Toxins 2020, 12, 690. https://doi.org/10.3390/toxins12110690
Madhushani U, Isbister GK, Tasoulis T, Hodgson WC, Silva A. In-Vitro Neutralization of the Neurotoxicity of Coastal Taipan Venom by Australian Polyvalent Antivenom: The Window of Opportunity. Toxins. 2020; 12(11):690. https://doi.org/10.3390/toxins12110690
Chicago/Turabian StyleMadhushani, Umesha, Geoffrey K. Isbister, Theo Tasoulis, Wayne C. Hodgson, and Anjana Silva. 2020. "In-Vitro Neutralization of the Neurotoxicity of Coastal Taipan Venom by Australian Polyvalent Antivenom: The Window of Opportunity" Toxins 12, no. 11: 690. https://doi.org/10.3390/toxins12110690
APA StyleMadhushani, U., Isbister, G. K., Tasoulis, T., Hodgson, W. C., & Silva, A. (2020). In-Vitro Neutralization of the Neurotoxicity of Coastal Taipan Venom by Australian Polyvalent Antivenom: The Window of Opportunity. Toxins, 12(11), 690. https://doi.org/10.3390/toxins12110690






