Abstract
Stenotrophomonas maltophilia is a ubiquitous environmental bacterium that has recently emerged as a multidrug-resistant opportunistic pathogen causing bloodstream, respiratory, and urinary tract infections. The connection between the commensal environmental S. maltophilia and the opportunistic pathogen strains is still under investigation. Bacterial toxin–antitoxin (TA) systems have been previously associated with pathogenic traits, such as biofilm formation and resistance to antibiotics, which are important in clinical settings. The same species of the bacterium can possess various sets of TAs, possibly influencing their overall stress response. While the TA systems of other important opportunistic pathogens have been researched, nothing is known about the TA systems of S. maltophilia. Here, we report the identification and characterization of S. maltophilia type II TA systems and their prevalence in the isolates of clinical and environmental origins. We found 49 putative TA systems by bioinformatic analysis in S. maltophilia genomes. Despite their even spread in sequenced S. maltophilia genomes, we observed that relBE, hicAB, and previously undescribed COG3832-ArsR operons were present solely in clinical S. maltophilia isolates collected in Lithuania, while hipBA was more frequent in the environmental ones. The kill-rescue experiments in Escherichia coli proved higBA, hicAB, and relBE systems to be functional TA modules. Together with different TA profiles, the clinical S. maltophilia isolates exhibited stronger biofilm formation, increased antibiotic, and serum resistance compared to environmental isolates. Such tendencies suggest that certain TA systems could be used as indicators of virulence traits.
Keywords:
Stenotrophomonas maltophilia; opportunistic pathogen; clinical origin; environmental origin; toxin-antitoxin system; biofilm; antibiotic resistance Key Contribution:
Previously undescribed Stenotrophomonas maltophilia type II TA systems were identified and their abundance in clinical and environmental S. maltophilia isolates evaluated. A difference in TA content and virulence-related characteristics between S. maltophilia isolates of clinical and environmental origins was observed.
1. Introduction
Stenotrophomonas maltophilia is a bacterium of class Gammaproteobacteria, ubiquitously found in the natural environment (soil, plants, animals, foods, or aqueous habitats) [1]. Recently, S. maltophilia came into attention as a globally emerging multidrug-resistant opportunistic pathogen causing nosocomial and community-acquired infections to immunocompromised patients [2]. The mortality rate of S. maltophilia infection can exceed 35% [3], with the World Health Organization (WHO) listing S. maltophilia as one of the leading multidrug-resistant pathogens [4]. The most known S. maltophilia features contributing to its successful adaptation in clinical environments include intrinsic antibiotic resistance mechanisms and its ability to form biofilms [5], although other less characterized factors (synthesis of extracellular enzymes, bacterial motility, and quorum sensing) are also known to influence its pathogenesis [6]. S. maltophilia is also known as a highly diverse species, since numerous studies have revealed genotypic diversity between clinical S. maltophilia isolates [7,8,9], even when analyzing samples from the same hospital [10,11]. Moreover, no significant genotypic or phenotypic differences between clinical and environment S. maltophilia isolates could be identified [12]. There is still an open question whether all the members of this species can become virulent and is there a specific marker to distinguish clinical and environmental S. maltophilia isolates.
The ability of pathogens to adapt to the clinical environment and to develop resistance to treatment can often be attributed to their ability to quickly exchange genetic information [13]. Gaining antibiotic resistance genes or virulence factors increases the chances of survival and gives rise to nosocomial opportunistic pathogens [14]. The ability of the bacterial species to mobilize their DNA could, therefore, be considered as a virulence factor in itself.
Toxin–antitoxin (TA) systems are abundant genetic elements mostly found in prokaryotes to encode a toxic protein, which inhibits cell growth and an antitoxin molecule (protein or RNA), which protects cells from toxin effects [15]. Human pathogenic bacteria have been shown to possess multiple sets of TAs, the type II TA systems, consisting of toxin and antitoxin proteins, being the most represented module and encoded by both plasmids and chromosomes [16]. Once the bacteria acquires DNA encoding the TA system, it becomes difficult to lose it due to the toxin’s higher stability and eventual suppression of the cell growth in case of loss of the genes [17]. Apart from being addictive, other functions have been proposed for the TA systems, such as a role in virulent phenotype and alleviating bacterial survival under stress conditions [18]. Moreover, the connection between TA systems and virulence traits, such as biofilm formation [19,20] and host colonization [18], was demonstrated. Interestingly, the same species can have various sets of TA systems [21], indicating their ability to be transferred horizontally. Analysis of the prevalence of TA systems in the genomes of 12 dangerous epidemic bacteria has shown that pathogens encoded a higher number of TA systems and reduced genomes compared to their closest non-pathogenic relatives [22]. This suggests that TAs might participate in the manifestation of the evolved pathogenic phenotype. The pathogenic Mycobacterium tuberculosis represents the prominent example, which genome encodes a remarkably high number of TA systems compared with nonpathogenic Mycobacterium strains [23]. Altogether, a possible TA systems role in bacteria pathogenicity is possible.
In this study, we aimed to identify and characterize previously undescribed S. maltophilia type II TA systems, as well as to evaluate and compare the abundance of these TA modules in clinical and environmental S. maltophilia isolates. Additionally, we accessed virulence-related features of S. maltophilia, including antibiotic and serum resistance, biofilm, pellicle, and capsule formation in order to find out if the correlation between the presence of TA systems and virulence-associated traits is apparent in Stenotrophomonas. We observed a difference in TA content and virulence-related characteristics between S. maltophilia isolates of clinical and environmental origins.
2. Results
2.1. Detection of S. maltophilia TA Systems in Genomes
To date, there are several tools available to detect TA systems in bacterial genomes: TADB 2.0 database tool TA-finder [24], RASTA-Bacteria [25], and TASmania [26]. However, only TA-finder was useful in our case, since RASTA-Bacteria limited the size of the analyzed DNA fragments, while TASmania relied on the Ensembl database, which at the time of this search did not have S. maltophilia genomes. On the date of our analysis, 21 fully assembled genomes were available to analyze with TA-finder. If the TA pair contained the same predicted domains, they were considered homologous; if they matched with a protein of a different domain, they were considered a distinct TA pair (e.g., RelE-Xre and RelE-RHH were considered as two distinct TAs). The presence of each predicted TA system was checked against all the genomes using BLAST. The final results for 49 detected TA systems are presented in Figure 1. Interestingly, some of the TA system genes were not identified in the genomes by TA-finder, but their presence was later detected by BLAST. After additional analysis, we found that the gene sequences in those genomes contained mutations that disrupted the reading frame, which could be due to sequencing errors or genuine mutations. The possible pseudogenes belonging to TAs are indicated in Figure 1.
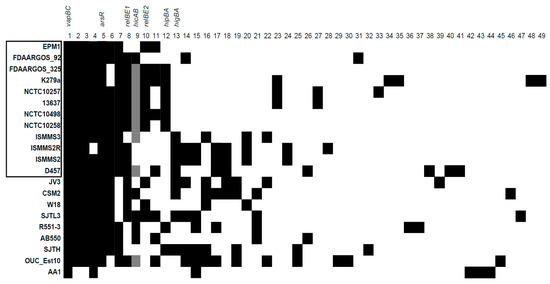
Figure 1.
The predicted toxin–antitoxin (TA) systems in S. maltophilia genomes. The genomes with names bracketed in black rectangle were isolated from clinical sources. Black squares indicate the presence of a TA system, whereas grey squares indicate the presence of a pseudogene version. The information about the presented TA systems (1–49) is described in Table S1. TA systems analyzed in this work are indicated above.
As can be seen from Figure 1, there were no clear TA distribution patterns characteristic to annotated genomes of S. maltophilia from clinical or environmental origins. The average number of predicted TA systems per genome did not significantly differ between the S. maltophilia of both origins (Figure 2a).
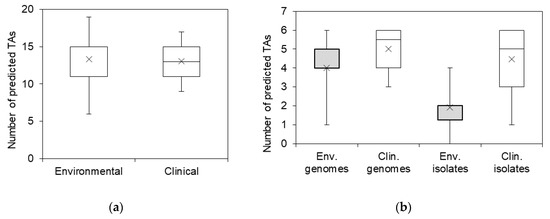
Figure 2.
The prevalence of predicted TA systems in sequenced S. maltophilia genomes and Stenotrophomonas spp. isolates. (a) The distribution of total predicted TA systems in annotated S. maltophilia genomes; (b) the distribution of selected most prevalent TA systems in annotated genomes of S. maltophilia and in isolates of clinical and environmental origin. Boxes indicate upper and lower quartiles, whiskers indicate minimum and maximum values, and crosses indicate mean values.
Not all gene pairs predicted as TA systems are active TA modules [23,27,28]. Therefore, we aimed to check if the prevailing predicted TA systems of S. maltophilia were functional. For functional analysis, the TA systems that were present in at least 1/3 of the analyzed genomes were chosen. To increase the likelihood of the selected operons acting as TA systems, we chose TAs where both genes were no longer than 1 kb [25] (with the exception of hipBA system, where the toxin gene is known to be larger) [29]. The distance between the toxin and antitoxin genes was set to be 50 bp or less, which increased the possibility that the genes belonged to an operon and were expressed from a single transcript. The genes, which were clearly annotated for a function and not related to TAs (e.g., antibiotic resistance genes, membrane transporters), were excluded from further analysis. The final set of seven TAs, selected for functional characterization, is presented in Table 1.

Table 1.
The characteristics of TA systems prevalent in annotated genomes of S. maltophilia.
The majority of the selected systems belonged to well-known type II TA families. There were three TA systems belonging to the relBE family: relBE1 (containing RelE-Xre domains), relBE2 (RelE-RHH domains), and higBA (HigB-Xre domains). However, one of the most common predicted TAs in S. maltophilia, containing COG3832-ArsR domains, was the least described. It was predicted as a TA system bioinformatically more than 10 years ago [30], yet only once was it analyzed thoroughly [23].
The predicted TA systems were conservative not only in S. maltophilia but in Stenotrophomonas spp. with high sequence conservativity (>70%) (Table 1). Therefore, we decided to test the presence and spread of these TAs in both S. maltophilia and Stenotrophomonas spp. isolates from our collection.
2.2. The Prevalence of TA Systems in Stenotrophomonas spp. of Clinical and Environmental Origin
To detect selected TA systems, a set of primers was designed after aligning all available sequences for a TA system in a Stenotrophomonas genus and identifying the most conserved regions (Table S2). Next, the collection of Stenotrophomonas spp. of clinical (n = 21) and environmental (n = 14) origins (Figure 3) was screened for the presence of TAs. All isolates of clinical origin were attributed to S. maltophilia species, while six of the environmental isolates belonged to S. maltophilia. The rest of the environmental isolates were attributed to either S. rhizophila or Stenotrophomonas sp.
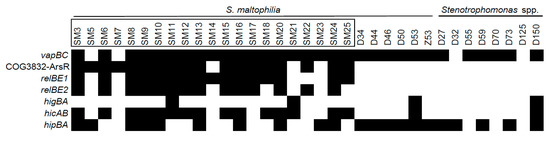
Figure 3.
The prevalence of predicted TA systems in Stenotrophomonas spp. of clinical and environmental origins. The isolates with their names bracketed in black rectangles were derived from clinical sources. The presence of TAs, indicated as black boxes, was detected by PCR as described in the Materials and Methods section.
All of the examined TA systems were detected in at least four isolates (Figure 3). vapC and hipBA modules were the most prevalent (31 and 21 isolates, respectively), and were evenly spread in both clinical and environmental bacteria. However, hipBA was more common in isolates of environmental origin. Three TAs (relBE1, relBE2, and COG3832-ArsR) were prevalent in isolates of clinical origin yet were absent in environmental isolates. hicAB system was detected in the majority of clinical isolates and in only two isolates of environmental origin. Surprisingly, we observed a clear difference in TA profiles between Stenotrophomonas isolates of clinical and environmental origins, contradicting a similar distribution of these TAs in the sequenced genomes. We found that certain TAs were more likely to be present in the clinical isolates of S. maltophilia. Moreover, clinical isolates of S. maltophilia had more TA systems per isolate, compared to the environmental isolates (Figure 2b).
2.3. Functionality of S. maltophilia TA Systems
To test if the predicted TA systems were functional, we cloned toxin and antitoxin genes into separate inducible vectors to perform kill-rescue experiments in E. coli, as described in Materials and methods. The predicted toxin and antitoxin expression was induced with arabinose, and IPTG, respectively.
Only S. maltophilia relBE1, relBE2 and higBA TA systems, belonging to relBE family, were functional in kill-rescue experiments in E. coli, as evident from the growth inhibition upon induction of the toxin and growth restoration, when antitoxin was concomitantly induced (Figure 4a–c). The predicted toxins of hipBA, vapBC, hicAB, and COG3832-ArsR TA systems did not display any toxicity in the kill-rescue assay (Figure 4d–f).
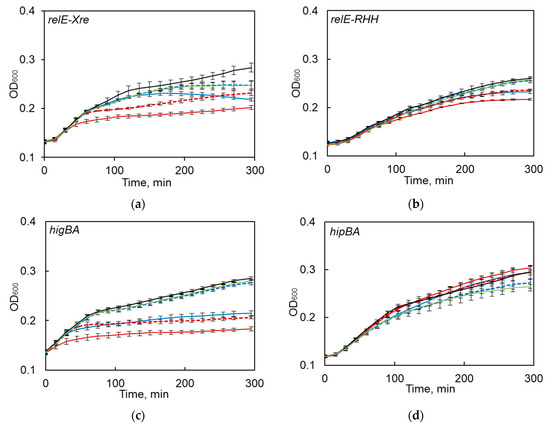
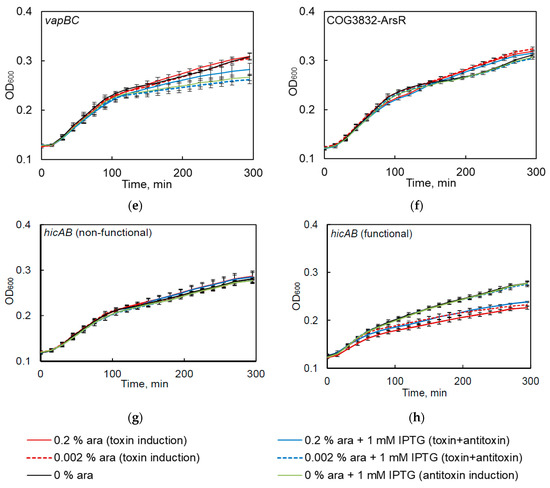
Figure 4.
The functionality of predicted S. maltophilia TA systems as tested by the kill-rescue assay in E. coli. (a–c) the growth inhibition and restoration of functional TA systems; (d–f) the kill-rescue assays for the predicted TA that appear to be non-functional in E. coli system; (g,h) representative growth curves for a functional and a non-functional version of hicAB. The toxin and antitoxin genes were cloned into separate inducible plasmids, the toxin was induced by adding arabinose (ara), and its activity counteracted by inducing antitoxins with IPTG. The bacterial growth was measured as OD600, as described in the Materials and Methods section. The experiments were repeated at least three times; error bars indicate standard deviation.
We previously observed that one of the tested TAs (i.e., hicAB) was often present in the sequenced S. maltophilia genomes as a pseudogene (Figure 1). Therefore, when we first observed hicAB to be non-functional in the kill-rescue assay (Figure 4g), we decided to clone it from several other S. maltophilia isolates and to use the kill-rescue assay. Out of eight cloned versions of hicAB, three were non-functional, and five systems displayed a distinct kill-rescue phenotype. The growth of E. coli after the induction of a representative functional S. maltophilia hicAB is presented in Figure 4h. Sequencing of the cloned non-functional versions of hicAB revealed the same frameshift mutation in the toxin gene.
According to our results, S. maltophilia COG3832-ArsR, hipBA, and vapBC predicted TA systems were non-functional in E. coli. To find out, if this was due to the differences of the host or TAs, which were indeed non-functional, we created an expression system for S. maltophilia by using a broad-host range plasmid pBAD1 with an arabinose controlled promoter and cloned the predicted toxins. S. maltophilia relE toxin of the relE-Xre TA system was proven to be functional in E. coli and was thus used as a control. Further, relE toxin showed toxicity upon induction (not shown), while COG3832-ArsR, hipBA, and vapC toxins did not show any toxicity in S. maltophilia under the same induction conditions (not shown).
2.4. Characterization of Stenotrophomonas spp. Isolates
Despite the similar distribution of predicted TAs observed in the S. maltophilia sequenced genomes of clinical and environmental origins (Figure 1), the most prevalent TA modules in bacterial isolates showed characteristic profiles regarding their origin, with clear prevalence for some TAs in clinical isolates (Figure 3). Since the majority of the clinical S. maltophilia isolates were obtained in the same hospital, we wanted to find out if there was a clonality between the analyzed isolates. The genotyping of Stenotrophomonas spp. isolates was undertaken by a random amplified polymorphic DNA (RAPD) assay with two sets of random primers as described in the Materials and Methods section. Each isolate significantly differed and was not grouped into clusters according to their origin (Figure 5). This thereby confirmed that the S. maltophilia of clinical origin were sporadic cases.
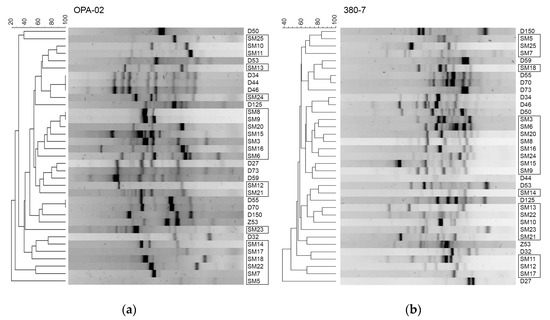
Figure 5.
The genotyping of Stenotrophomonas spp. isolates. Random amplified polymorphic DNA (RAPD) assay was performed as described in the Materials and Methods section using OPA-02 (a) and 380-7 (b) primers (Table S2). The isolates bracketed in black rectangles were isolated from clinical sources.
The genotyping of Stenotrophomonas spp. from clinical and environmental origins showed that isolates could not be grouped into clusters based on their origin of isolation. However, uneven distribution of TAs (i.e., the types of TAs and total TA number per isolate) among the isolates indicated that there were certain traits that distinguished the isolates of different origins. Therefore, we decided to compare other virulence-related traits of the isolates and see if the presence of TAs could be considered as a marker for virulence of the strain.
One of the characteristics that makes S. maltophilia a dangerous opportunistic pathogen is its innate resistance to antibiotics [31]. We tested the resistance of the isolates against a set of antibiotics (trimethoprime, chloramfenicol, gentamicine, ceftazidime, meropenem, and tetracyclin), including trimethoprime-sulfametoxazole and ciprofloxacine, which are commonly used against S. maltophilia infections. Resistance and sensitivity values were determined according EUCAST clinical breakpoints for S. maltophilia or Pseudomonas aeruginosa, if such data for S. maltophilia was not available. We observed significantly higher resistance profiles among the isolates of the clinical origin (Figure 6). Tetracyclin, ceftazidime, meropenem, and gentamicin were effective against most environmental isolates, while the majority of the clinical ones showed resistance.
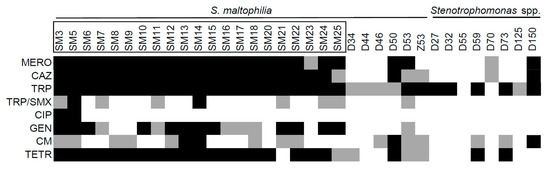
Figure 6.
The resistance to antibiotics of Stenotrophomonas spp. of clinical and environmental origin. The isolates with names bracketed in black rectangles were isolated from clinical sources. Black squares indicate resistance; grey squares indicate intermediate resistance to antibiotic. The antibiotics are as indicated: meropenem (MERO), ceftazidime (CAZ), trimethoprime (TRP), trimethoprime-sulfametoxazole (TRP/SMX), ciprofloxacine (CIP), gentamicine (GEN), chloramphenicol (CM), and tetracyclin (TETR).
The next virulence-related trait we assessed was biofilm formation, a complex community structure composed of adhesive bacteria and matrix components. Various bacteria can form biofilms on medical devices, such as catheters or intubation tubes, since it increases pathogen survival during stress (e.g., antibiotic pressure, desiccation, or laminar flow) [32]. Previous studies have shown the ability of S. maltophilia to form biofilms [33]. We first checked the ability of the isolates to form biofilms at 37 °C, as described in the Materials and Methods section, since this was an expected temperature inside the infected host. The majority of S. maltophilia from clinical origins formed strong biofilms (Figure 7, Figure S1). Only two environmental isolates displayed weak biofilm growth at 37 °C (Figure 7). However, the environmental isolates showed severely reduced growth at 37 °C, indicating that their inability to form biofilms could be due to suboptimal growth conditions. Therefore, we tested the ability of all the isolates to form biofilms at 28 °C, a temperature that resembled environmental (outside the host) conditions. No change in biofilm formation was observed for the clinical strains; however, several of the environmental strains increased their biofilm formation under permissive temperature. Thus, the results demonstrated a clear differentiation of the strains of different in ability to form biofilm at both 37 °C and 28 °C.
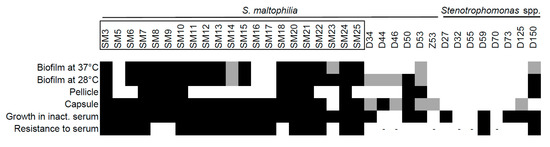
Figure 7.
Biofilm formation and capsule-related characteristics of Stenotrophomonas spp. isolates. The biofilm, pellicle, capsule-forming phenotype, growth in inactivated serum and resistance to serum were determined as described in the Material and Methods section. The isolates with names bracketed in black rectangles were isolated from clinical sources. Black squares indicate the ability to form biofilm, pellicle, the presence of capsular polysaccharides (CPS), or the ability to grow in serum; grey squares indicate the ability to form weak biofilms or weak capsule-forming phenotype. “-” indicates that the isolates were not exposed to non-inactivated serum due to their inability to survive in inactivated serum.
We then analyzed the ability of the isolates to form pellicle, a type of biofilm, which formed at the air–liquid interface. This specific structure is associated with the increased virulence of bacteria [34]. As can be seen in Figure 7, only the isolates with biofilm forming capacity were able to form pellicle, though this structure was not common to all such isolates and was more frequent in the clinical strains.
The ability to form capsule is considered as an important virulence trait in bacteria, together with resistance to antibiotics, biofilm formation, adhesiveness, desiccation, and other features [35,36,37]. The role of capsular exopolysaccharides in S. maltophilia virulence is still unknown. Therefore, we were interested to discover if bacterial isolates could produce polysaccharides that were indicative of capsule formation. The capsular polysaccharides were extracted and analyzed as described in the Materials and Methods section. The vast majority of clinical S. maltophilia isolates (95%) produced high molecular mass polysaccharides, which were associated with capsular polysaccharides (CPS). This phenotype was demonstrated by only a third (36%) of environmental Stenotrophomonas spp. isolates (Figure 7, Figure S2). However, S. maltophilia environmental isolates tended to produce capsule-associated polysaccharides more often than Stenotrophomonas of other species.
The ability to produce exopolysaccharide capsule is indicative of a strain’s ability to avoid killing by a host’s immune system [36,38]. We aimed to test if isolates differed in their ability to survive serum-mediated killing. First, we tested the ability of isolates to grow in a heat-treated serum with an inactivated complement system. All of the clinical isolates were able to grow, yet only half of the environmental isolates grew in the presence of inactivated serum (Figure 7). Next, we assessed the growth of Stenotrophomonas isolates in the presence of the active serum. The majority of clinical S. maltophilia strains were resistant to complement components when grown in an active serum, whereas only two of the environmental isolates showed resistance, thereby demonstrating an inability of environmental strains to resist a host immune system and cause an infection.
3. Discussion
In this study, we aimed to investigate the prevalence and profiles of TA systems in a newly emerging opportunistic pathogen, S. maltophilia, as well as to compare the TA presence with the manifestation of the virulence features of clinical and environmental isolates.
While the role of plasmid-borne TA systems is known as plasmid maintenance [39], the biological function of chromosome encoded TA systems is far less clear. It has been shown that chromosome encoded TA systems help stabilize genome integrity by preventing large scale genome reductions, which keeps genes needed for survival in unfavorable conditions [40,41] and causes growth arrest that helps bacteria survive environmental stress [39]. The most widespread type II TA system families affect the translation apparatus of a cell by cleaving or otherwise disrupting the functionality of mRNA (relBE (including higBA) [42,43], hicAB [44], and mazEF [45]) or tRNA (vapBC [46] and hipBA [29]). It has also been reported that TA systems function as virulence factors. Thus, a higBA TA system in Pseudomonas aeruginosa influences different virulence factor expression and biofilm formation [20]. Mycobacterium tuberculosis MazEF type TA systems play a role in pathogen intracellular survival inside the host [47]. A Staphylococcus aureus SavRS TA system functions as a regulator of virulence traits [48]. Moreover, previous studies have shown that the number of TA systems is enlarged in pathogenic strains compared with closely related nonpathogenic bacteria (M. tuberculosis encodes 88 putative TA systems [49], while non-harmful M. smegmatis encodes only three TA modules [50]). In this study, we investigated whether the same trend was characteristic to TA systems in the opportunistic pathogen S. maltophilia. The data observed on vapBC and relBE families, which are two major type II TA families [51,52], were in line with the observations on the prevalence of TA systems.
The amplicon detection-based screen of the TA systems in bacterial isolates revealed unexpected results, since relBE1, relBE2, and COG3832-ArsR TA systems were found solely in clinical S. maltophilia isolates and were not detected in environmental samples. Moreover, some preferences were observed for hicAB and hipBA TAs. The dissimilarity between observations of bioinformatic TA analysis in genomes available in the databases and our detection might be explained by the different geographic locations of bacterial sources. The bacteria analyzed in our study were isolated from soil in Lithuania, while the origin of S. maltophilia genomes in the databases varied greatly. However, the genotyping assay proved that the analyzed isolates did not belong to the same clonal group and showed high genetic diversity. Similar results were previously reported for S. maltophilia [53,54], supporting our findings about genotypic diversity among clinical and environmental S. maltophilia isolates from Lithuania.
In the present study, the putative COG3832-ArsR TA system was found in nearly all analyzed S. maltophilia genomes. COG3832-ArsR operon was previously proposed to be a TA system on the basis of comparative prokaryotic genomic analysis [30]. This module was found among 10 of the most induced TA systems in drug tolerant persister cells for M. tuberculosis [23], though functional analysis of this operon was not done in any TA system research. However, S. maltophilia COG3832-ArsR did not show toxicity in the kill-rescue assay and therefore its attribution to TA systems remains questionable. Altogether, functional analysis revealed that four of the seven analyzed S. maltophilia TA systems were functionally active TA operons in E. coli. The confirmed TAs belonged to previously described classes. To exclude the possibility of host incompatibility, we transferred the inactive toxins into S. maltophilia, yet no toxicity was observed. Interestingly, we identified two types of the same S. maltophilia hicAB TA systems via bioinformatic analysis in bacterial isolates. Previous studies have reported that TA system genes may contain mutations, which can result in nonfunctional pseudogene formation [55]. Loss of function mutations in virulence-associated genes can indicate genomic plasticity and adaptation to different environments [56], suggesting that hicAB TA systems may be favorable or, otherwise, unfavorable when living under particular conditions.
In our study, only the chromosome sequences of S. maltophilia were used for TA system prediction, however, the detected TA systems might be located on both chromosomes and mobile elements. TA systems are known to be transmitted via horizontal gene transfer [21,57]. It was shown that in S. maltophilia, trimethoprim/sulfamethoxazole resistance genes were found in class 1 integron and plasmid [58], indicating the ability S. maltophilia to acquire genetic information through horizontal gene transfer. Therefore, it is possible that some of the analyzed TA systems can spread via mobile elements, yet further studies are needed to confirm this possibility.
In this study, we demonstrated that clinical and environmental Stenotrophomonas isolates possessed not only distinct TA profiles but other important virulence-related traits. S. maltophilia is notorious for its multidrug resistance associated with intrinsic resistance factors, such as low membrane permeability, multidrug resistance efflux pumps, antibiotic-modifying enzymes, and ability to acquire resistance via mutations or acquisition of resistance genes through horizontal gene transfer [31]. Our study revealed a high antibiotic resistance of clinical S. maltophilia isolates, all of which displayed resistance to at least five out of eight antimicrobials. Environmental Stenotrophomonas isolates were more sensitive to antibiotics compared to bacteria of clinical origins, including meropenem (though S. maltophilia is known to have metallo-β-lactamases, providing resistance to various β-lactam antibiotics including carbapenems) [59,60]. The data on the antibiotic resistance level of clinical and environmental S. maltophilia isolates are contradictory [60,61,62], although it has been proposed that some mechanisms of antibiotic resistance occur in the environment and could remain in clinical isolates [63].
Biofilm formation is one of the best characterized S. maltophilia virulence traits. S. maltophilia forms biofilms on glass and different types of plastic [33], including medical devices (indwelling venous catheters [64], prosthetic valves [65], and lens implants [66]). Biofilm formation on biotic surfaces (e.g., human epithelial respiratory cells) has also been reported [67,68]. In this study, we showed that clinical and environmental S. maltophilia isolates differed in biofilm formation abilities, which was in line with previous studies [9,69]. Pellicle is a type of biofilm, formed at the air–liquid interface [70] and protecting bacterial population from environmental stresses. Further, it can increase survival rates by increasing accessibility to oxygen [71]. Less than a half of both clinical and environmental isolates were able to form pellicle, indicating that this phenotype may not be directly linked to virulence traits in S. maltophilia.
Although the ability of S. maltophilia to form capsule has not been described, studies of capsule functions in other bacterial pathogens showed its crucial role in the protection from unfavorable environmental conditions, including antibiotic treatment, host immune response, and resistance to disinfectants and heat [72,73,74,75]. Based on the separation of exopolysaccharides on SDS-PAGE gel, in some of the strains we observed the fraction of high molecular mass polysaccharides, as well as fractions resembling lipopolysaccharides [76]. Typically, such fractions represent capsular exopolysaccharides [37]. We demonstrated that CPS associated high molecular mass exopolysaccharides were detected in nearly all clinical isolates, while only 36% of environmental Stenotrophomonas spp. isolates displayed this phenotype. However, the majority of environmental isolates belonging to the S. maltophilia species did synthesize extracellular polysaccharides. Therefore, further studies are needed to investigate the importance of capsules in environmental isolates. Nevertheless, the presence of the capsule phenotypes among clinical S. maltophilia isolates supports the idea that capsule is beneficial to helping unfavorable conditions and host immune responses survive [38]. The hospital-related strains were also distinguished by increased resistance to serum-induced stress, especially when compared to strains of environmental origin. The ability of isolates to grow at 37 ºC (temperature of the host) and resist the complement system of the active serum indicates the increased infectivity potential of these strains. Some of the environmental isolates did not grow even in the heat-inactivated serum, which indicates the inability of these strains to multiply inside the host even before the encounter with the immune system. This could hint at the inability of environmental isolates to persist and grow due to improper nutrients and blood serum composition.
Based on the phenotypes of clinical and environmental S. maltophilia and genotyping results, we can assume that the environment could be the primary source of opportunistic S. maltophilia infections. However, further development of virulence potential might include an exchange of genetic information and switching on potential virulence genes.
4. Conclusions
Both clinical and environmental S. maltophilia genomes contain a variety of type II TA systems, the most common of which belong to vapBC, relBE, hipBA, hicAB, and putative COG3832-ArsR families. While bioinformatics assay did not show differences in TA distribution between clinical and environmental TAs, the screening of our collection of isolates did. relBE and COG3832-ArsR operons were present solely in clinical S. maltophilia isolates, while hipBA was more frequent in the environmental isolates. Together with different TA composition, the clinical S. maltophilia isolates exhibited clearly stronger biofilm formation, as well as increased antibiotic and serum resistance compared to environmental isolates.
5. Materials and Methods
5.1. Bioinformatic Assays
In total, 21 complete S. maltophilia genomes from NCBI database (date of accession 2018-10-13) (Table S3) were analyzed using the TADB 2.0 database tool TA-finder [24]. To identify TA systems in S. maltophilia genomes, a basic local alignment search tool (BLAST) [77] was used. The Clustal Omega tool [78] was used for sequence alignments.
5.2. The Bacteria Used in the Study and Growth Conditions
Plasmids and strains used in this study are listed in Table 2. Clinical and environmental Stenotrophomonas spp. isolates are listed in Table 3. Stenotrophomonas spp. and E. coli were grown in an LB medium, unless indicated otherwise. E. coli and clinical S. maltophilia isolates were grown at 37 °C. Environmental S. maltophilia and Stenotrophomonas spp. isolates were grown at 28 °C, unless indicated otherwise. DNA was transformed to E. coli by heat shock [79] and electroporation was used for S. maltophilia [80]. Antibiotics (ampicillin 100 mg/L, chloramphenicol 30 mg/L, and kanamycin 60 mg/L) were used to maintain plasmids when needed.

Table 2.
Strains and plasmids used in this study.

Table 3.
Clinical and environmental Stenotrophomonas spp. isolates used in this study.
5.3. Detection of TA Systems in Stenotrophomonas
For the detection of S. maltophilia TA systems, primers targeting conservative gene fragments were designed (Table S2) and PCR was performed using DreamTaq polymerase (Thermo Fisher Scientific).
5.4. Cloning of TA System Genes
All enzymes used for cloning were from Thermo Fisher Scientific and were used according to the manufacturer’s recommendations. For gene cloning, a high-fidelity Phusion polymerase was used with the primers listed in Table S2. A hicAB TA system was cloned using SM6, SM9, SM11, SM12, SM13, SM16, SM20, and SM24 isolate DNA as the template. For relBE2 and higBA the isolate SM11 was used, for relBE1, hipBA, vapBC, and COG3832-ArsR the isolate SM9 DNA was used as a template. For TA functional analysis in E. coli, all toxin genes were cloned into pBAD30 plasmid; antitoxin genes were cloned into pUHcat plasmid. Both vectors were hydrolyzed with SphI, then overhangs were blunted with T4 polymerase. Final restriction was performed with HindIII. To clone the toxins into a broad host range pBAD1 vector, pBAD1_GFP plasmid was used. First, the pBAD1_GFP vector was hydrolyzed with XbaI and other steps were performed as described above.
5.5. Kill-Rescue Assay
The kill-rescue assay was performed as described previously [86]. Briefly, E. coli BW25113 F‘ strains harboring plasmids pBAD_tox and pUHEcat_antitox were grown overnight, diluted 1:500 into fresh medium supplemented with 0.2% glucose, and grown to its early exponential phase (optical density at 600 nm (OD600) = 0.12). The expression of toxin and/or antitoxin was then induced with arabinose (0.002% and 0.2%) and/or IPTG (1 mM), respectively. Growth was measured as OD600 in a Tecan Infinite M200 Pro plate reader at 37 °C with shaking.
5.6. Random Amplified Polymorphic DNA (RAPD) Assay
For the RAPD assay, DNA amplification was performed at the same time on the same thermocycler in a final reaction volume of 25 µL, containing 2.5 µL of 10× polymerase reaction buffer with KCl: 0.5 µL 10 mM dNTP, 1.25 µL 50 mM MgCl2, 2 µL 10 pmol/µL OPA-02 or 380-7 primer, 0.15 µL DreamTaq polymerase, and 5 µL of bacteria lysate.
Cycling conditions are as follows: initial denaturation at 95 °C for 2 min followed by 39 cycles of denaturation at 95 °C for 30 s, annealing at 36 °C/34 °C for 1 min (OPA-02 and 380-7 primers, respectively), extension at 72 °C for 2 min and final extension at 72 °C for 5 min.
PCR products were loaded on a 1% (w/v) agarose gel with 0.5 mg/mL of ethidium bromide (1 × TAE buffer, 7 V/cm voltage, 1.5 h). Electrophoresis gels were visualized with Bio Rad Molecular Imager and analyzed using GelCompar II software (Applied Maths) with the Dice coefficient set at 1% and band tolerance set at 1.5% using the UPGMA method.
5.7. The Evaluation of Antibiotic Resistance
Antimicrobial resistance analysis was performed by growing Stenotrophomonas isolates on agarose LB plates with selected concentrations of antimicrobial agents for 16 h at 28 °C. Control growth was considered as growth of isolates on the LB plates without antimicrobial agents. The isolate was considered as resistant to tested antibiotics only if growth visually looked the same as the control. In the case of significantly weaker growth, the isolate was referred to as an intermediate resistant and in the absence of growth, the isolate was considered as sensitive.
5.8. Biofilm Formation Assay
Biofilm formation experiments were performed as described elsewhere [38]. Briefly, overnight Stenotrophomonas spp. cultures (grown in TSB medium (Oxoid) at 37 ◦C for 16 h) were inoculated into the 150 μL TSB medium containing wells of 96 U-form polystyrene plate (30-fold dilution), then incubated stationary at 28 °C or 37 °C for 24 h. The OD600 of planktonic culture was measured and wells were washed 3 times with PBS buffer. Adherent cells were stained with 0.1% crystal violet dye for 15 min, then washed 5 times with the PBS buffer. Dye was dissolved in 96% ethanol for OD580 measurements. The OD580/OD600 ratio was calculated to normalize the number of biofilm forming cells to the total cell number.
5.9. Pellicle Formation Assay
The pellicle formation was performed as previously described [87]. Briefly, overnight cultures Stenotrophomonas spp. (grown 16 h in TSB medium at 37 °C) were inoculated into the TSB medium containing wells of a flat-bottom 12 well polystyrene microplate in a total volume of 3 mL (1000-fold dilution). The cultures were incubated stationary at 28 °C for 30 h. Results were evaluated qualitatively by monitoring the presence/absence of bacterial biomass formed in the air–liquid contact.
5.10. Extraction of Capsular Polysaccharides
The bacteria were grown on LB agar plates for 16 h and suspended in the PBS buffer. Capsular polysaccharide extraction and analysis were performed as described earlier [38]. Briefly, polysaccharides were released by vortexing for 30 s. After centrifugation at 9000× g for 10 min, polysaccharides were precipitated in 75% ice-cold ethanol. The pellet was resuspended in SDS sample buffer and boiled for 5 min. Samples were loaded on the 12% SDS-PAGE gels. After electrophoresis, gels were stained overnight with 0.1% (w/v) Alcian Blue.
5.11. Serum Resistance
Serum resistance was evaluated by measuring bacterial growth in LB medium, heat inactivated FBS (fetal bovine serum (Gibco, 12657029)), and active FBS. FBS was inactivated by incubation at 56 °C for 30 min with constant shaking. Overnight cultures were inoculated at ×1000 dilution to LB medium and 80% FBS (20% of LB medium) or inactive 80% FBS. Growth curves were measured at 37 °C with periodic shaking every 20 min using a Tecan Infinite M200 Pro microplate reader.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/12/10/635/s1, Figure S1: Biofilm formation of clinical and environmental Stenotrophomonas spp. isolates, Figure S2: The polysaccharide production of Stenotrophomonas spp. isolates of clinical and environmental origin, Table S1: TA systems detected by TA-finder in annotated S. maltophilia genomes, Table S2: Primers used in this study, Table S3: S. maltophilia genomes used in this study.
Author Contributions
J.A. and E.S. conceived and designed the experiments; L.K. and J.S. performed the experiments; L.K., J.S. and J.A. analyzed the data; J.A. and L.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Modestas Ružauskas for Stenotrophomonas spp., Jolanta Miciulevičienė, R. Plančiūnienė and K. Sužiedėlis for the clinical S. maltophilia isolates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brooke, J.S. Stenotrophomonas maltophilia: An Emerging Global Opportunistic Pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kastoris, A.C.; Vouloumanou, E.K.; Rafailidis, P.I.; Kapaskelis, A.M.; Dimopoulos, G. Attributable mortality of Stenotrophomonas maltophilia infections: A systematic review of the literature. Future Microbiol. 2009, 4, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- WHO | World Health Organization. Available online: https://www.who.int/drugresistance/AMR_Importance/en/ (accessed on 28 July 2020).
- Pompilio, A.; Savini, V.; Fiscarelli, E.; Gherardi, G.; Di Bonaventura, G. Clonal Diversity, Biofilm Formation, and Antimicrobial Resistance among Stenotrophomonas maltophilia Strains from Cystic Fibrosis and Non-Cystic Fibrosis Patients. Antibiotics 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, A.; Strateva, T. Stenotrophomonas maltophilia—A low-grade pathogen with numerous virulence factors. Infect. Dis. 2019, 51, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Overhage, J.; Bathe, S.; Winter, J.; Fischer, R.; Schwartz, T. Genotyping of Environmental and Clinical Stenotrophomonas maltophilia Isolates and their Pathogenic Potential. PLoS ONE 2011, 6, e27615. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Ghalavand, Z.; Fallah, F.; Yadegar, A.; Ardebili, A.; Tarashi, S.; Pournajaf, A.; Mardaneh, J.; Shams, S.; Hashemi, A. Characterization of Phenotypic and Genotypic Diversity of Stenotrophomonas maltophilia Strains Isolated From Selected Hospitals in Iran. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Pompilio, A.; Pomponio, S.; Crocetta, V.; Gherardi, G.; Verginelli, F.; Fiscarelli, E.; Dicuonzo, G.; Savini, V.; D’Antonio, D.; Di Bonaventura, G. Phenotypic and genotypic characterization of Stenotrophomonas maltophiliaisolates from patients with cystic fibrosis: Genome diversity, biofilm formation, and virulence. BMC Microbiol. 2011, 11, 159. [Google Scholar] [CrossRef]
- Madi, H.; Lukić, J.; Vasiljević, Z.; Biočanin, M.; Kojić, M.; Jovčić, B.; Lozo, J. Genotypic and Phenotypic Characterization of Stenotrophomonas maltophilia Strains from a Pediatric Tertiary Care Hospital in Serbia. PLoS ONE 2016, 11, e0165660. [Google Scholar] [CrossRef]
- Valdezate, S.; Vindel, A.; Martin-Davila, P.; Del Saz, B.S.; Baquero, F.; Canton, R. High Genetic Diversity among Stenotrophomonas maltophilia Strains Despite Their Originating at a Single Hospital. J. Clin. Microbiol. 2004, 42, 693–699. [Google Scholar] [CrossRef]
- Lira, F.; Berg, G.; Martínez, J.L. Double-Face Meets the Bacterial World: The Opportunistic Pathogen Stenotrophomonas maltophilia. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, S.; Bedeković, V. Horizontal gene transfer-emerging multidrug resistance in hospital bacteria. Acta Pharmacol. Sin. 2003, 24, 519–526. [Google Scholar] [PubMed]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, B.-J. Structure, Biology, and Therapeutic Application of Toxin-Antitoxin Systems in Pathogenic Bacteria. Toxins 2016, 8, 305. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Park, J.-H.; Inouye, M. Toxin-Antitoxin Systems in Bacteria and Archaea. Annu. Rev. Genet. 2011, 45, 61–79. [Google Scholar] [CrossRef]
- Lobato-Márquez, D.; Díaz-Orejas, R.; García-del Portillo, F. Toxin-antitoxins and bacterial virulence. FEMS Microbiol. Rev. 2016, 40, 592–609. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Hay, A.J.; Zhong, Z.; Zhu, J.; Kan, B. Functional RelBE-Family Toxin-Antitoxin Pairs Affect Biofilm Maturation and Intestine Colonization in Vibrio cholerae. PLoS ONE 2015, 10, e0135696. [Google Scholar] [CrossRef]
- Wood, T.L.; Wood, T.K. The HigB/HigA toxin/antitoxin system of Pseudomonas aeruginosa influences the virulence factors pyochelin, pyocyanin, and biofilm formation. Microbiol. Open 2016, 5, 499–511. [Google Scholar] [CrossRef]
- Leplae, R.; Geeraerts, D.; Hallez, R.; Guglielmini, J.; Drèze, P.; Van Melderen, L. Diversity of bacterial type II toxin–antitoxin systems: A comprehensive search and functional analysis of novel families. Nucleic Acids Res. 2011, 39, 5513–5525. [Google Scholar] [CrossRef]
- Georgiades, K.; Raoult, D. Genomes of the Most Dangerous Epidemic Bacteria Have a Virulence Repertoire Characterized by Fewer Genes but More Toxin-Antitoxin Modules. PLoS ONE 2011, 6, e17962. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Bordes, P.; Genevaux, P. Multiple Toxin-Antitoxin Systems in Mycobacterium tuberculosis. Toxins 2014, 6, 1002–1020. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, Y.; Shen, Y.; Li, X.; Zhou, H.; Tai, C.; Deng, Z.; Ou, H.-Y. TADB 2.0: An updated database of bacterial type II toxin–antitoxin loci. Nucleic Acids Res. 2018, 46, D749–D753. [Google Scholar] [CrossRef] [PubMed]
- Sevin, E.W.; Barloy-Hubler, F. RASTA-Bacteria: A web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome Biol. 2007, 8, R155. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, H.; Bordes, P.; Mansour, M.; Bigot, D.-J.; Genevaux, P.; Falquet, L. TASmania: A bacterial Toxin-Antitoxin Systems database. PLoS Comput. Biol. 2019, 15, e1006946. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Müller, C.; Harmer, N.; Titball, R.W. Identification of type II toxin–antitoxin modules in Burkholderia pseudomallei. FEMS Microbiol. Lett. 2013, 338, 86–94. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, N.; Cao, M.; Ren, S.; Zeng, T.; Qin, M.; Zhao, X.; Yuan, F.; Chen, H.; Bei, W. Identification of Three Type II Toxin-Antitoxin Systems in Streptococcus suis Serotype 2. Toxins 2018, 10, 467. [Google Scholar] [CrossRef]
- Coussens, N.P.; Daines, D.A. Wake me when it’s over—Bacterial toxin-antitoxin proteins and induced dormancy. Exp. Biol. Med. 2016, 241, 1332–1342. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol. Direct 2009, 4, 19. [Google Scholar] [CrossRef]
- Sánchez, M.B. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 658. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. Apmis. Suppl. 2013, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Spedicato, I.; D’Antonio, D.; Robuffo, I.; Piccolomini, R. Biofilm formation by Stenotrophomonas maltophilia: Modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob. Agents Chemother. 2004, 48, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Armitano, J.; Méjean, V.; Jourlin-Castelli, C. Gram-negative bacteria can also form pellicles. Environ. Microbiol. Rep. 2014, 6, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A.G. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Niu, T.; Guo, L.; Luo, Q.; Zhou, K.; Yu, W.; Chen, Y.; Huang, C.; Xiao, Y. Wza gene knockout decreases Acinetobacter baumannii virulence and affects Wzy-dependent capsular polysaccharide synthesis. Virulence 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015, 11, e1004691. [Google Scholar] [CrossRef]
- Skerniškytė, J.; Krasauskas, R.; Péchoux, C.; Kulakauskas, S.; Armalytė, J.; Sužiedėlienė, E. Surface-Related Features and Virulence Among Acinetobacter baumannii Clinical Isolates Belonging to International Clones I and II. Front. Microbiol. 2018, 9, 3116. [Google Scholar] [CrossRef]
- Van Melderen, L.; De Bast, M.S. Bacterial Toxin–Antitoxin Systems: More Than Selfish Entities? PLoS Genet. 2009, 5, e1000437. [Google Scholar] [CrossRef]
- Rowe-Magnus, D.A. Comparative Analysis of Superintegrons: Engineering Extensive Genetic Diversity in the Vibrionaceae. Genome Res. 2003, 13, 428–442. [Google Scholar] [CrossRef]
- Szekeres, S.; Dauti, M.; Wilde, C.; Mazel, D.; Rowe-Magnus, D.A. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection: Chromosomal addiction loci minimize gene loss. Mol. Microbiol. 2007, 63, 1588–1605. [Google Scholar] [CrossRef]
- Gotfredsen, M.; Gerdes, K. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 1998, 29, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Schureck, M.A.; Repack, A.; Miles, S.J.; Marquez, J.; Dunham, C.M. Mechanism of endonuclease cleavage by the HigB toxin. Nucleic Acids Res. 2016, 44, 7944–7953. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Grishin, N.V.; Koonin, E.V. The HicAB cassette, a putative novel, RNA-targeting toxin-antitoxin system in archaea and bacteria. Bioinformatics 2006, 22, 2581–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; Zhang, J.; Inouye, M. Characterization of ChpBK, an mRNA interferase from Escherichia coli. J. Biol. Chem. 2005, 280, 26080–26088. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.S.; Gerdes, K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc. Natl. Acad. Sci. USA 2011, 108, 7403–7407. [Google Scholar] [CrossRef]
- Tiwari, P.; Arora, G.; Singh, M.; Kidwai, S.; Narayan, O.P.; Singh, R. MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Wen, W.; Liu, B.; Xue, L.; Zhu, Z.; Niu, L.; Sun, B. Autoregulation and Virulence Control by the Toxin-Antitoxin System SavRS in Staphylococcus aureus. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Ramage, H.R.; Connolly, L.E.; Cox, J.S. Comprehensive Functional Analysis of Mycobacterium tuberculosis Toxin-Antitoxin Systems: Implications for Pathogenesis, Stress Responses, and Evolution. PLoS Genet. 2009, 5. [Google Scholar] [CrossRef]
- Frampton, R.; Aggio, R.B.M.; Villas-Bôas, S.G.; Arcus, V.L.; Cook, G.M. Toxin-Antitoxin Systems of Mycobacterium smegmatis Are Essential for Cell Survival. J. Biol. Chem. 2012, 287, 5340–5356. [Google Scholar] [CrossRef]
- Lopes, A.P.Y.; Lopes, L.M.; Fraga, T.R.; Chura-Chambi, R.M.; Sanson, A.L.; Cheng, E.; Nakajima, E.; Morganti, L.; Martins, E.A.L. VapC from the Leptospiral VapBC Toxin-Antitoxin Module Displays Ribonuclease Activity on the Initiator tRNA. PLoS ONE 2014, 9, e101678. [Google Scholar] [CrossRef]
- Chan, W.T.; Espinosa, M.; Yeo, C.C. Keeping the Wolves at Bay: Antitoxins of Prokaryotic Type II Toxin-Antitoxin Systems. Front. Mol. Biosci. 2016, 3. [Google Scholar] [CrossRef]
- Strateva, T.; Trifonova, A.; Savov, E.; Dimov, S. An update on the antimicrobial susceptibility and molecular epidemiology of Stenotrophomonas maltophilia in Bulgaria: A 5-year study (2011–2016). Infect. Dis. 2019, 51, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.H.; Pinheiro, M.N.; Coelho, F.S.; Sampaio, J.L.M.; Merquior, V.L.C.; Marques, E.A. Phenotypic properties, drug susceptibility and genetic relatedness of Stenotrophomonas maltophilia clinical strains from seven hospitals in Rio de Janeiro, Brazil. J. Appl. Microbiol. 2004, 96, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Weigel, W.; Sztukowska, M.; Demuth, D.R. Identification and functional characterization of type II toxin/antitoxin systems in Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2018, 33, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Bliven, K.A.; Maurelli, A.T. Antivirulence Genes: Insights into Pathogen Evolution through Gene Loss. Infect. Immun. 2012, 80, 4061–4070. [Google Scholar] [CrossRef] [PubMed]
- Lima-Mendez, G.; Alvarenga, D.O.; Ross, K.; Hallet, B.; Melderen, L.V.; Varani, A.M.; Chandler, M. Toxin-Antitoxin Gene Pairs Found in Tn3 Family Transposons Appear To Be an Integral Part of the Transposition Module. mBio 2020, 11. [Google Scholar] [CrossRef]
- Hu, L.-F.; Chang, X.; Ye, Y.; Wang, Z.-X.; Shao, Y.-B.; Shi, W.; Li, X.; Li, J.-B. Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int. J. Antimicrob. Agents 2011, 37, 230–234. [Google Scholar] [CrossRef]
- Walsh, T.R.; Macgowan, A.P.; Bennett, P.M. Sequence Analysis and Enzyme Kinetics of the L2 Serine β-Lactamase from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1997, 41, 5. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, W.; Cui, Q.; Niu, W.; Li, H.; Zhao, X.; Wei, X.; Wang, X.; Huang, S.; Dong, D.; et al. Prevalence and detection of Stenotrophomonas maltophilia carrying metallo-Î2-lactamase blaL1 in Beijing, China. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Youenou, B.; Favre-Bonté, S.; Bodilis, J.; Brothier, E.; Dubost, A.; Muller, D.; Nazaret, S. Comparative Genomics of Environmental and Clinical Stenotrophomonas maltophilia Strains with Different Antibiotic Resistance Profiles. Genome Biol. Evol. 2015, 7, 2484–2505. [Google Scholar] [CrossRef]
- Deredjian, A.; Alliot, N.; Blanchard, L.; Brothier, E.; Anane, M.; Cambier, P.; Jolivet, C.; Khelil, M.N.; Nazaret, S.; Saby, N.; et al. Occurrence of Stenotrophomonas maltophilia in agricultural soils and antibiotic resistance properties. Res. Microbiol. 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Muder, R.R.; Harris, A.P.; Muller, S.; Edmond, M.; Chow, J.W.; Papadakis, K.; Wagener, M.W.; Bodey, G.P.; Steckelberg, J.M. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: A prospective, multicenter study of 91 episodes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1996, 22, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Mehta, N.J. Stenotrophomonas maltophilia endocarditis: A systematic review. Angiology 2002, 53, 49–55. [Google Scholar] [CrossRef]
- Horio, N.; Horiguchi, M.; Murakami, K.; Yamamoto, E.; Miyake, Y. Stenotrophomonas maltophilia endophthalmitis after intraocular lens implantation. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2000, 238, 299–301. [Google Scholar] [CrossRef]
- de Vidipó, L.A.; de Marques, E.A.; Puchelle, E.; Plotkowski, M.-C. Stenotrophomonas maltophilia Interaction with Human Epithelial Respiratory Cells In Vitro. Microbiol. Immunol. 2001, 45, 563–569. [Google Scholar] [CrossRef]
- De Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Alcántara, N.; Martinez, M.B.; Girón, J.A. Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell. Microbiol. 2003, 5, 625–636. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhao, Q.; Xiao, S. The Impact of spgM, rpfF, rmlA Gene Distribution on Biofilm Formation in Stenotrophomonas maltophilia. PLoS ONE 2014, 9, e108409. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Yamamoto, K.; Arai, H.; Ishii, M.; Igarashi, Y. Trade-off between oxygen and iron acquisition in bacterial cells at the air–liquid interface. FEMS Microbiol. Ecol. 2011, 77, 83–94. [Google Scholar] [CrossRef]
- Campa, M.; Bendinelli, M.; Friedman, H. Pseudomonas Aeruginosa as an Opportunistic Pathogen; Springer Science & Business Media: New York, NY, USA, 2012; ISBN 978-1-4615-3036-7. [Google Scholar]
- Tipton, K.A.; Dimitrova, D.; Rather, P.N. Phase-Variable Control of Multiple Phenotypes in Acinetobacter baumannii Strain AB5075. J. Bacteriol. 2015, 197, 2593–2599. [Google Scholar] [CrossRef]
- Chin, C.Y.; Tipton, K.A.; Farokhyfar, M.; Burd, E.M.; Weiss, D.S.; Rather, P.N. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat. Microbiol. 2018, 3, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Boulnois, G.J.; Roberts, I.S. Genetics of Capsular Polysaccharide Production in Bacteria. In Proceedings of the Bacterial Capsules; Jann, K., Jann, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 1–18. [Google Scholar]
- McKay, G.A.; Woods, D.E.; MacDonald, K.L.; Poole, K. Role of phosphoglucomutase of Stenotrophomonas maltophilia in lipopolysaccharide biosynthesis, virulence, and antibiotic resistance. Infect. Immun. 2003, 71, 3068–3075. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol. 2014, 1079, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Morita, M.; Nishimura, Y.; Sugino, Y. A rapid and highly efficient method for preparation of competent Escherichia coli cells. Nucleic Acids Res. 1990, 18, 6169. [Google Scholar] [CrossRef]
- Ye, X.; Dong, H.; Huang, Y.-P. Highly efficient transformation of Stenotrophomonas maltophilia S21, an environmental isolate from soil, by electroporation. J. Microbiol. Methods 2014, 107, 92–97. [Google Scholar] [CrossRef]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Motiejūnaitė, R.; Armalytė, J.; Markuckas, A.; Sužiedėlienė, E. Escherichia coli dinJ-yafQ genes act as a toxin-antitoxin module. FEMS Microbiol. Lett. 2007, 268, 112–119. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Petkevičius, V.; Vaitekūnas, J.; Stankevičiūtė, J.; Gasparavičiūtė, R.; Meškys, R. Catabolism of 2-Hydroxypyridine by Burkholderia sp. Strain MAK1: A 2-Hydroxypyridine 5-Monooxygenase Encoded by hpdABCDE Catalyzes the First Step of Biodegradation. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Armalytė, J.; Skerniškytė, J.; Bakienė, E.; Krasauskas, R.; Šiugždinienė, R.; Kareivienė, V.; Kerzienė, S.; Klimienė, I.; Sužiedėlienė, E.; Ružauskas, M. Microbial Diversity and Antimicrobial Resistance Profile in Microbiota From Soils of Conventional and Organic Farming Systems. Front. Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Jurėnaitė, M.; Markuckas, A.; Sužiedėlienė, E. Identification and Characterization of Type II Toxin-Antitoxin Systems in the Opportunistic Pathogen Acinetobacter baumannii. J. Bacteriol. 2013, 195, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Krasauskas, R.; Skerniškytė, J.; Armalytė, J.; Sužiedėlienė, E. The role of Acinetobacter baumannii response regulator BfmR in pellicle formation and competitiveness via contact-dependent inhibition system. BMC Microbiol. 2019, 19, 241. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).