Evolution of Asian Corn Borer Resistance to Bt Toxins Used Singly or in Pairs
Abstract
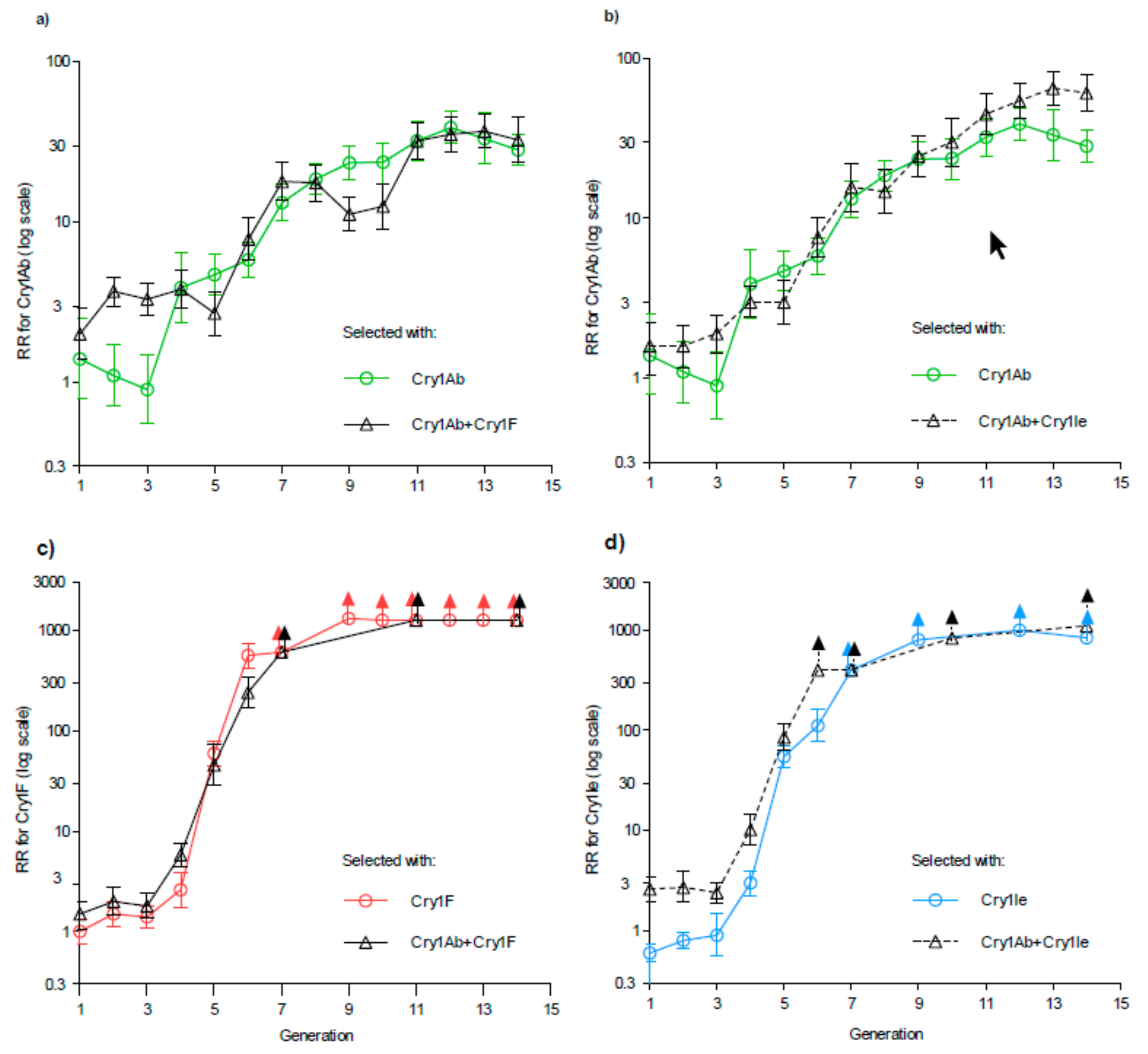
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Bt Toxins
4.2. Insects
4.3. Selection Experiment
4.4. Bioassays
4.5. Cross-Resistance
4.6. Amino Acid Sequence Similarity
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years; ISAAA Brief No. 53; ISAAA: Ithaca, NY, USA, 2017. [Google Scholar]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef] [PubMed]
- Gould, F.; Amasino, R.M.; Brossard, D.; Buell, C.R.; Dixon, R.A.; Falck-Zepeda, J.B.; Gallo, M.A.; Giller, K.; Glenna, L.; Griffin, T.S.; et al. Genetically Engineered Crops: Experiences and Prospects; National Academies of Sciences, Engineering and Medicine; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamson, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Guo, Y.Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lopez, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2012, 37, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.G.; Ponnuraj, J.; Mallappa, B.; Chowdary, L.R.; Zhang, J.W.; Tay, W.T.; Walsh, T.K.; Gordon, K.H.J.; Heckel, D.G.; Downes, S.; et al. ABC transporter mis-splicing associated with resistance to Bt toxin Cry2Ab in laboratory- and field-selected pink bollworm. Sci. Rep. 2018, 8, 13531. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Lepping, M.D.; Rule, D.M.; Farhan, Y.; Schaafsma, A.W. Evidence for field-evolved resistance of Striacosta albicosta (Lepidoptera: Noctuidae) to Cry1F Bacillus thuringiensis protein and transgenic corn hybrids in Ontario, Canada. J. Econ. Entomol. 2017, 110, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China and India. J. Econ. Entomol. 2019, 112, toz173. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Fabrick, J.A.; Tabashnik, B.E. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016, 34, 291–302. [Google Scholar] [CrossRef]
- Roush, R.T. Two-toxin strategies for management of insecticidal transgenic crops: Can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998, 353, 1777–1786. [Google Scholar] [CrossRef]
- Bourguet, D.; Delmotte, F.; Franck, P.; Guillemaud, T.; Reboud, X.; Vacher, C.; Walker, A.S. Heterogeneity of selection and the evolution of resistance. Trends Ecol. Evol. 2013, 28, 110–118. [Google Scholar] [CrossRef]
- Carrière, Y.; Crickmore, N.; Tabashnik, B.E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 2015, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Cao, J.; Collins, H.L.; Bates, S.L.; Roush, R.T.; Earle, E.D.; Shelton, A.M. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Cao, J.; Li, Y.X.; Collins, H.L.; Roush, R.T.; Earle, E.D.; Shelton, A.M. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 2003, 21, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Brevault, T.; Heuberger, S.; Zhang, M.; Ellers-Kirk, C.; Ni, X.Z.; Masson, L.; Li, X.C.; Tabashnik, B.E.; Carrière, Y. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA 2013, 110, 5806–5811. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Ma, W.; Wang, X.; Gao, M.; Dai, Y.; Wei, X.; Zhang, L.; Peng, Y.; Chen, S.; Ding, L.; et al. Next-generation transgenic cotton: Pyramiding RNAi and Bt counters insect resistance. Plant Biotechnol. J. 2017, 15, 1204–1213. [Google Scholar] [CrossRef]
- Afidchao, M.M.; Musters, C.J.; de Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef]
- Li, J.; Coates, B.S.; Kim, K.S.; Bourguet, D.; Ponsard, S.; He, K.L.; Wang, Z.Y. The genetic structure of Asian corn borer, Ostrinia furnacalis, populations in China: Haplotype variance in northern populations and potential impact on management of resistance to transgenic maize. J. Hered. 2014, 105, 642–655. [Google Scholar] [CrossRef]
- Nafus, D.M.; Schreiner, I.H. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Int. J. Pest Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Xie, H.C.; Li, D.S.; Zhang, H.G.; Mason, C.E.; Wang, Z.Y.; Lu, X.; Cai, W.Z.; He, K.L. Seasonal and geographical variation in diapause and cold hardiness of the Asian corn borer, Ostrinia furnacalis. Insect Sci. 2014, 22, 578–586. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, T.T.; Bai, S.X.; Wang, Z.Y.; He, K.L. Evaluation of Bt corn with pyramided genes on efficacy and insect resistance management for the Asian corn borer in China. PLoS ONE 2016, 11, e0168442. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.Z.; Quan, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K. Characterization of the Cry1Ah resistance in Asian corn borer and its cross-resistance to other Bacillus thuringiensis toxins. Sci. Rep. 2018, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, Y.D.; Wang, Z.Y.; Bravo, A.; Soberón, M.; He, K.L. Genetic basis of Cry1F-resistance in a laboratory selected Asian corn borer strain and its cross-resistance to other Bacillus thuringiensis toxins. PLoS ONE 2016, 11, e0161189. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yang, J.; Quan, Y.D.; Wang, Z.Y.; Cai, W.Z.; He, K.L. Characterization of Asian corn borer resistance to Bt toxin Cry1Ie. Toxins 2017, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Z.; Zhang, J.; He, K.; Ferry, N.; Gatehouse, A.M.R. Cross-resistance of Cry1Ab-selected Asian corn borer to other Cry toxins. J. Appl. Entomol. 2010, 134, 429–438. [Google Scholar] [CrossRef]
- Zhao, C.; Jurat-Fuentes, J.L.; Abdelgaffar, H.M.; Pan, H.Y.; Song, F.P.; Zhang, J. Identification of a new cry1I-type gene as a candidate for gene pyramiding in corn to control Ostrinia species larvae. Appl. Environ. Microbiol. 2015, 81, 3699–3705. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.D.; Dalmacio, S.C.; Criador, A.R.; Alvarez, E.R.; Hechanova, R.F. Field performance of TC1507 transgenic corn hybrids against Asian corn borer in the Philippines. Philipp. Agric. Sci. 2010, 93, 375–383. [Google Scholar]
- Feng, D.M.; Chen, Z.; Wang, Z.W.; Zhang, C.L.; He, K.L.; Guo, S.Y. Domain III of Bacillus thuringiensis Cry1Ie toxin plays an important role in binding to peritrophic membrane of Asian corn borer. PLoS ONE 2015, 10, e0136430. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Unnithan, G.C.; Masson, L.; Crowder, D.W.; Li, X.; Carrière, Y. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Natl. Acad. Sci. USA 2009, 106, 11889–11894. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Zhang, C.L.; Lin, X.Y.; Zhang, Y.R.; He, K.L.; Song, F.P.; Zhang, J. Purification of an active fragment of Cry1Ie toxin from Bacillus thuringiensis. Protein Expr. Purif. 2011, 78, 204–208. [Google Scholar] [CrossRef]
- Song, Y.Y.; Zhou, D.R.; He, K.L. Studies on mass rearing of Asian corn borer: Development of a satisfactory non-agar semi-artificial diet and its use. Acta Phytophyl. Sin. 1999, 26, 324–328. [Google Scholar] [CrossRef]
- Zhou, D.R.; Ye, Z.H.; Wang, Z.Y. Artificial rearing technique for Asian corn borer, Ostrinia furnacalis (Guenée) and its applications in pest management research. In Advances in Insect Rearing for Research and Pest Management; Anderson, T.E., Leppla, N.C., Eds.; Westview Press: Boulder, CO, USA, 1992; pp. 173–193. [Google Scholar]
- He, K.L.; Zhou, D.R.; Song, Y.Y. Utilization of CIMMYT’s MBR population for ACB resistance and maize inbred development in China. In Proceedings of the International Symposium, International Maize and Wheat Improvement Center (CIMMYT), Mexico City, Mexico, 27 November–3 December 1994. [Google Scholar]
- Zhang, T.T.; He, M.X.; Gatehouse, A.M.R.; Wang, Z.Y.; Edwards, M.G.; Li, Q.; He, K.L. Inheritance patterns, dominance and cross-resistance of Cry1Ab-and Cry1Ac-selected Ostrinia furnacalis (Guenée). Toxins 2014, 6, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, Z.; Wen, L.; Bai, S.; Ma, X.; Yao, Z. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lep., Crambidae). J. Appl. Entomol. 2005, 129, 407–412. [Google Scholar] [CrossRef]
- Robertson, J.L.; Preisler, H.K.; Russell, R.M. PoloPlus: Probit and Logit Analysis; User’s Guide; LeOra Software: Petaluma, CA, USA, 2003. [Google Scholar]
| Selected with a | Response to | n b | LC50 (95% FL) c (μg toxin/g diet) | RR (95% CI) d |
|---|---|---|---|---|
| None (S) e | Cry1Ab | 672 | 0.36 (0.28–0.46) | 1.0 (0.7–1.4) |
| None (S) | Cry1F | 672 | 0.62 (0.46–0.79) | 1.0 (0.7–1.5) |
| None (S) | Cry1Ie | 672 | 5.15 (4.18–6.18) | 1.0 (0.8–1.3) |
| Cry1Ab | Cry1Ab | 672 | 5.30 (3.68–6.88) * | 15 (9.9–22) * |
| Cry1Ab | Cry1F | 768 | 1.00 (0.68–1.36) * | 1.6 (1.1–2.5) * |
| Cry1Ab | Cry1Ie | 672 | 4.64 (3.09–6.29) | 0.9 (0.6–1.4) |
| Cry1F | Cry1Ab | 672 | 0.88 (0.68–1.08) * | 2.4 (1.7–3.4) * |
| Cry1F | Cry1F | 96 | >1000 | >1600 |
| Cry1F | Cry1Ie | 672 | 4.16 (3.24–5.22) | 0.8 (0.6–1.1) |
| Cry1Ie | Cry1Ab | 768 | 0.92 (0.77–1.12) * | 2.5 (1.9–3.5) * |
| Cry1Ie | Cry1F | 672 | >100 | >160 |
| Cry1Ie | Cry1Ie | 96 | >2047 | >840 |
| Amino Acid Sequence Similarity (%) | |||||
|---|---|---|---|---|---|
| Toxin Pair | Domain I | Domain II | Domain III | Overall | |
| Cry1Ab | Cry1F | 74 | 50 | 63 | 63 |
| Cry1Ab | Cry1Ie | 62 | 44 | 80 | 59 |
| Cry1F | Cry1Ie | 62 | 40 | 70 | 55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Quan, Y.; Yang, J.; Shu, C.; Wang, Z.; Zhang, J.; Gatehouse, A.M.R.; Tabashnik, B.E.; He, K. Evolution of Asian Corn Borer Resistance to Bt Toxins Used Singly or in Pairs. Toxins 2019, 11, 461. https://doi.org/10.3390/toxins11080461
Wang Y, Quan Y, Yang J, Shu C, Wang Z, Zhang J, Gatehouse AMR, Tabashnik BE, He K. Evolution of Asian Corn Borer Resistance to Bt Toxins Used Singly or in Pairs. Toxins. 2019; 11(8):461. https://doi.org/10.3390/toxins11080461
Chicago/Turabian StyleWang, Yueqin, Yudong Quan, Jing Yang, Changlong Shu, Zhenying Wang, Jie Zhang, Angharad M. R. Gatehouse, Bruce E. Tabashnik, and Kanglai He. 2019. "Evolution of Asian Corn Borer Resistance to Bt Toxins Used Singly or in Pairs" Toxins 11, no. 8: 461. https://doi.org/10.3390/toxins11080461
APA StyleWang, Y., Quan, Y., Yang, J., Shu, C., Wang, Z., Zhang, J., Gatehouse, A. M. R., Tabashnik, B. E., & He, K. (2019). Evolution of Asian Corn Borer Resistance to Bt Toxins Used Singly or in Pairs. Toxins, 11(8), 461. https://doi.org/10.3390/toxins11080461







