Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom
Abstract
:1. Introduction
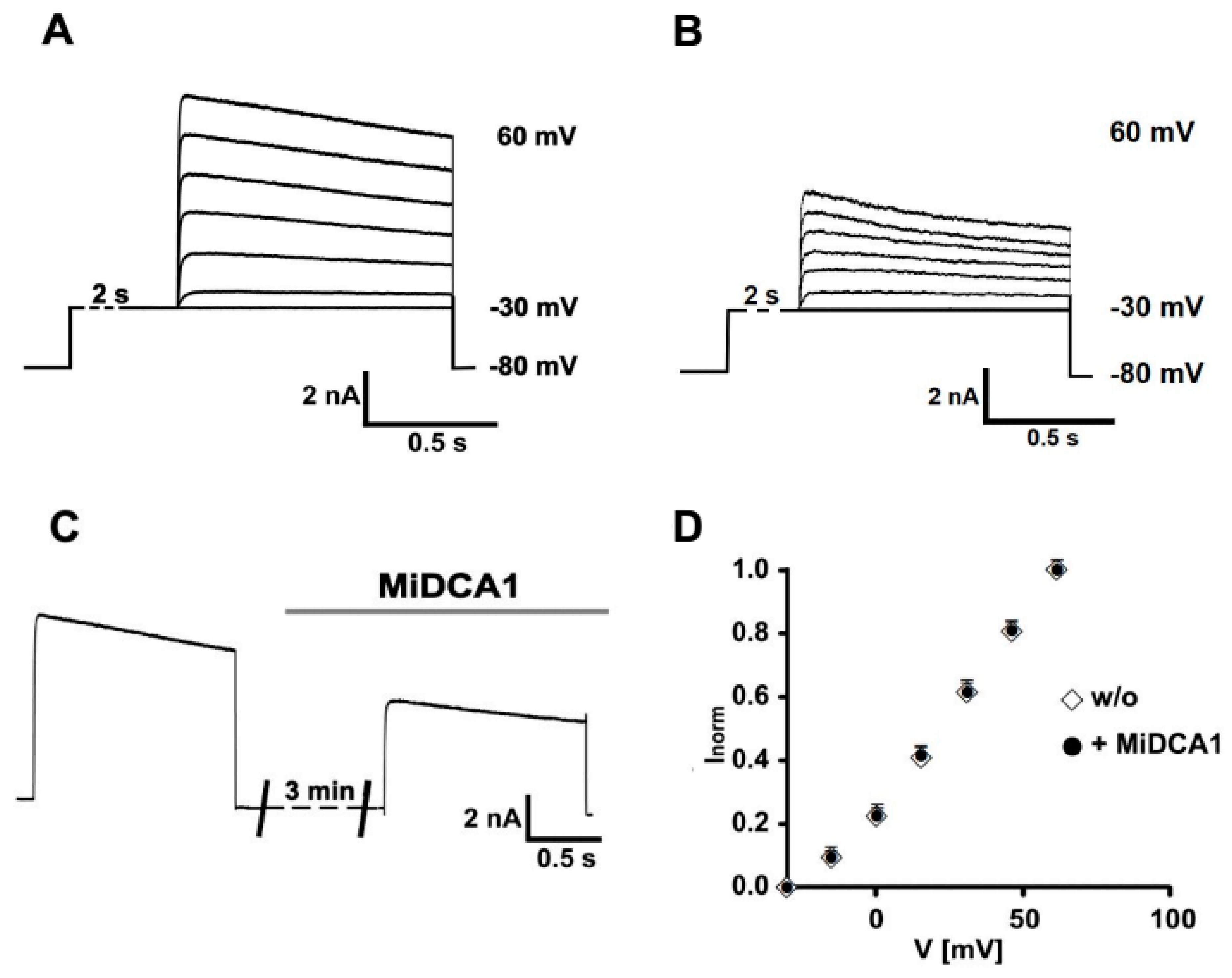
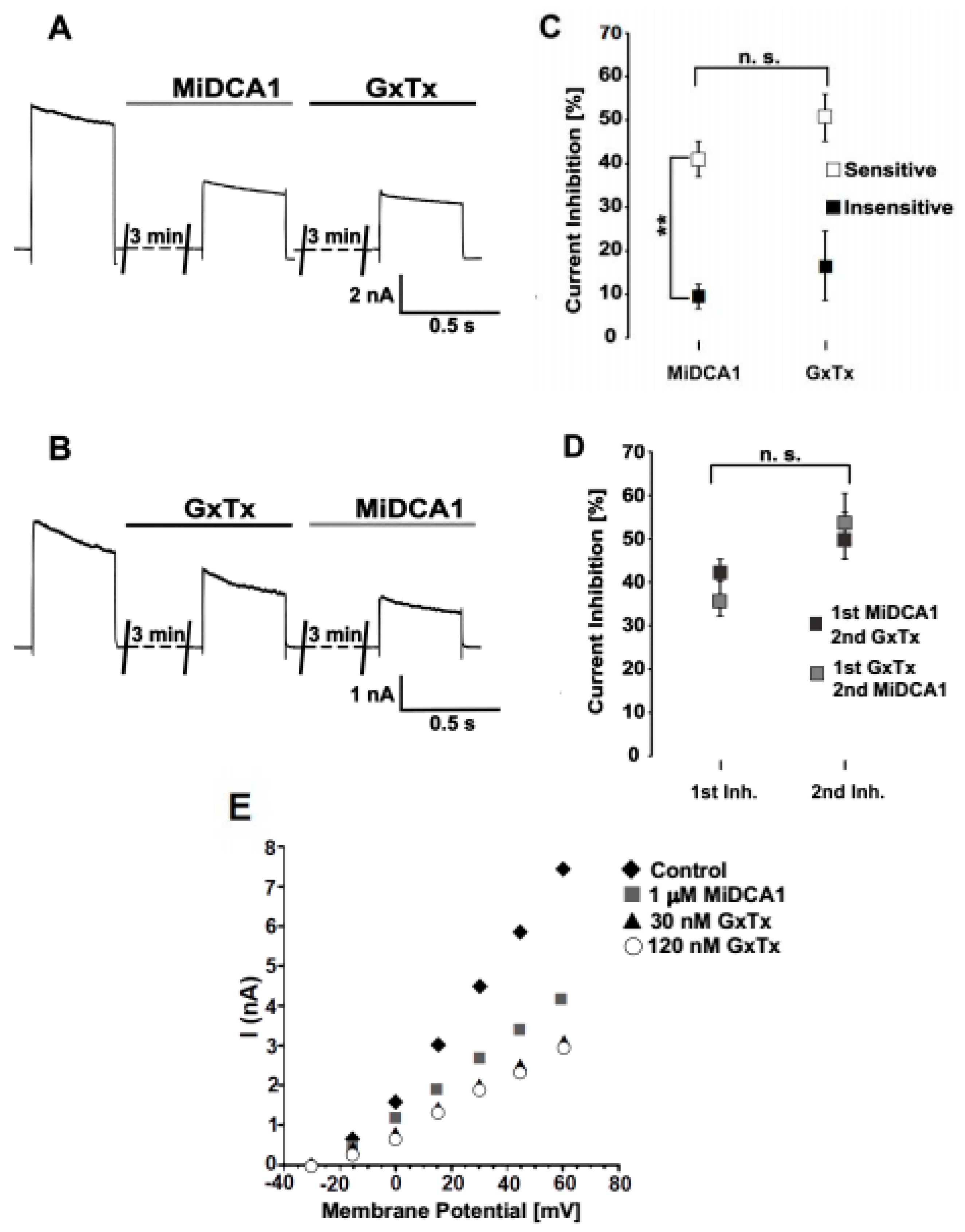
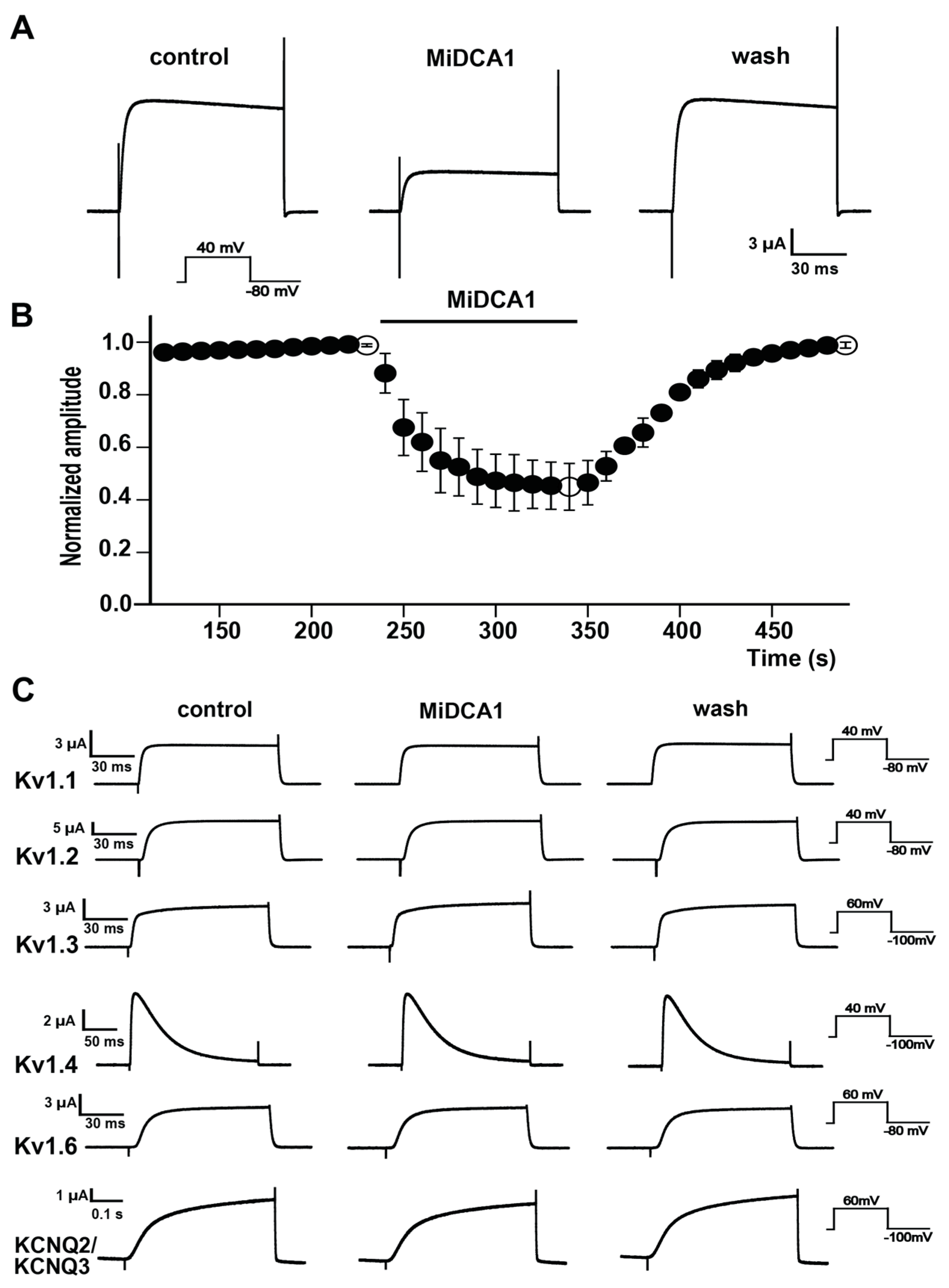
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alabi, A.A.; Bahamonde, M.I.; Jung, H.J.; Kim, J.I.; Swartz, K.J. Portability of paddle motif function and pharmacology in voltage sensors. Nature 2007, 450, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.; Forsythe, I.D.; Kopp-Scheinpflug, C. Going native: Voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 2010, 588, 3187–3200. [Google Scholar] [CrossRef] [PubMed]
- Flink, M.T.; Atchison, W.D. Iberiotoxin-induced block of Ca2+-activated K+ channels induces dihydropyridine sensitivity of ACh release from mammalian motor nerve terminals. J. Pharmacol. Exp. Ther. 2003, 305, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Zoga, V.; Kawano, T.; Liang, M.-Y.; Bienengraeber, M.; Weihrauch, D.; McCallum, B.; Gemes, G.; Hogan, Q.; Sarantopoulos, C. KATP channel subunits in rat dorsal root ganglia: Alterations by painful axotomy. Mol. Pain 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; Zhu, L.; Yip, P.; Grist, J.; Michael, G.J.; McMahon, S.B. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp. Neurol. 2014, 251, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, S.; Jouirou, B.; Mosbah, A.; De Waard, M.; Sabatier, J.-M. Diversity of folds in animal toxins acting on ion channels. Biochem. J. 2004, 378, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Swartz, K.J. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon 2007, 49, 213–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, M.; Tsuboi, Y.; Kitagawa, J.; Nakagawa, K.; Iwata, K.; Matsumoto, S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain 2011, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H. Molecular aspects of neuronal voltage-dependent K+ channels. Eur. J. Biochem. 1991, 202, 701–713. [Google Scholar] [CrossRef]
- Pelchen-Matthews, A.; Dolly, J.O. Distribution in the rat central nervous system of acceptor sub-types for dendrotoxin, a K+ channel probe. Neuroscience 1989, 29, 347–361. [Google Scholar] [CrossRef]
- Dreyer, F.; Penner, R. The actions of presynaptic snake toxins on membrane currents of mouse motor nerve terminals. J. Physiol. 1987, 386, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rowan, E.G.; Harvey, A.L. Potassium channel blocking actions of β-bungarotoxin and related toxins on mouse and frog motor nerve terminals. Br. J. Pharmacol. 1988, 94, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Dal Belo, C.A.; Leite, G.B.; Toyama, M.H.; Marangoni, S.; Corrado, A.P.; Fontana, M.D.; Southan, A.; Rowan, E.G.; Hyslop, S.; Rodrigues-Simioni, L. Pharmacological and structural characterization of a novel phospholipase A2 from Micrurus dumerilii carinicauda venom. Toxicon 2005, 46, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Dal Belo, C.A.; Toyama, M.H.; Toyama, D.O.; Marangoni, S.; Moreno, F.B.; Cavada, B.S.; Fontana, M.D.; Hyslop, S.; Carneiro, E.M.; Boschero, A.C. Determination of the amino acid sequence of a new phospholipase A2 (MiDCA1) isolated from Micrurus dumerilii carinicauda venom. Protein J. 2005, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins (Basel) 2013, 5, 2533–2571. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, H.; Huang, C.; Liao, K.; Pan, H. Snake venom PLA2, a promising target for broad-spectrum antivenom drug development. Biomed. Res. Int. 2017, 2017, 1–10. [Google Scholar]
- Rigoni, M.; Caccin, P.; Gschmeissner, S.; Koster, G.; Postle, A.D.; Rossetto, O.; Schiavo, G.; Montecucco, C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science 2005, 310, 1678–1680. [Google Scholar] [CrossRef]
- Bocksteins, E.; Raes, A.L.; Van de Vijver, G.; Bruyns, T.; Van Bogaert, P.-P.; Snyders, D.J. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am. J. Physiol. Physiol. 2009, 296, C1271–C1278. [Google Scholar] [CrossRef]
- Feinshreiber, L.; Singer-Lahat, D.; Friedrich, R.; Matti, U.; Sheinin, A.; Yizhar, O.; Nachman, R.; Chikvashvili, D.; Rettig, J.; Ashery, U.; et al. Non-conducting function of the Kv2.1 channel enables it to recruit vesicles for release in neuroendocrine and nerve cells. J. Cell Sci. 2010, 123, 1940–1947. [Google Scholar] [CrossRef] [Green Version]
- Herrington, J.; Zhou, Y.-P.; Bugianesi, R.M.; Dulski, P.M.; Feng, Y.; Warren, V.A.; Smith, M.M.; Kohler, M.G.; Garsky, V.M.; Sanchez, M.; et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 2006, 55, 1034–1042. [Google Scholar] [CrossRef]
- Pongs, O.; Schwarz, J.R. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 2010, 90, 755–796. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Netzer, R.; Böhlke, K.; Liu, Q.; Pongs, O. Structural and functional characterization of Kv6.2, a new gamma-subunit of voltage-gated potassium channel. Recept. Channels 1999, 6, 337–350. [Google Scholar] [PubMed]
- Stocker, M.; Krause, M.; Pedarzani, P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 4662–4667. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.C.; Begenisich, T. Pharmacology and surface electrostatics of the K+ channel outer pore vestibule. J. Membr. Biol. 2006, 212, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ramu, Y.; Xu, Y.; Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 2006, 442, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Vacher, H.; Mohapatra, D.P.; Misonou, H.; Trimmer, J.S. Regulation of Kv1 channel trafficking by the mamba snake neurotoxin dendrotoxin K. FASEB J. 2007, 21, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Bocksteins, E.; Labro, A.J.; Snyders, D.J.; Mohapatra, D.P. The electrically silent Kv6.4 subunit confers hyperpolarized gating charge movement in Kv2.1/Kv6.4 heterotetrameric channels. PLoS ONE 2012, 7, e37143. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, J.; Zhang, F.; Liu, Z. Isolation and characterization of SsmTx-I, a specific Kv2.1 blocker from the venom of the centipede Scolopendra subspinipes mutilans L. Koch. J. Pept. Sci. 2014, 20, 159–164. [Google Scholar] [CrossRef]
- Davletov, B.; Connell, E.; Darios, F. Regulation of SNARE fusion machinery by fatty acids. Cell. Mol. Life Sci. 2007, 64, 1597–1608. [Google Scholar] [CrossRef]
- Poling, J.S.; Karanian, J.W.; Salem, N.; Vicini, S. Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol. Pharmacol. 1995, 47, 381–390. [Google Scholar]
- MacKinnon, R.; Miller, C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science 1989, 245, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; Hellwig, M.; Kerschensteiner, D. Subunit assembly and domain analysis of electrically silent K+ channel α-subunits of the rat Kv9 subfamily. J. Neurochem. 1999, 72, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Lazdunski, M. Sea anemone toxins affecting potassium channels. In Marine Toxins as Research Tools; Springer: Berlin/Heidelberg, Germany, 2009; Volume 46, pp. 99–122. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ma, L.; Lin, C.; Teng, S.; Chai, Y.; Bahring, R.; Vardanyan, V.; Li, L.; Pongs, O.; Hui, R. Characterization of a novel long QT syndrome mutation G52R-KCNE1 in a Chinese family. Cardiovasc. Res. 2003, 59, 612–619. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schütter, N.; Barreto, Y.C.; Vardanyan, V.; Hornig, S.; Hyslop, S.; Marangoni, S.; Rodrigues-Simioni, L.; Pongs, O.; Dal Belo, C.A. Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom. Toxins 2019, 11, 335. https://doi.org/10.3390/toxins11060335
Schütter N, Barreto YC, Vardanyan V, Hornig S, Hyslop S, Marangoni S, Rodrigues-Simioni L, Pongs O, Dal Belo CA. Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom. Toxins. 2019; 11(6):335. https://doi.org/10.3390/toxins11060335
Chicago/Turabian StyleSchütter, Niklas, Yuri Correia Barreto, Vitya Vardanyan, Sönke Hornig, Stephen Hyslop, Sérgio Marangoni, Léa Rodrigues-Simioni, Olaf Pongs, and Cháriston André Dal Belo. 2019. "Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom" Toxins 11, no. 6: 335. https://doi.org/10.3390/toxins11060335
APA StyleSchütter, N., Barreto, Y. C., Vardanyan, V., Hornig, S., Hyslop, S., Marangoni, S., Rodrigues-Simioni, L., Pongs, O., & Dal Belo, C. A. (2019). Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom. Toxins, 11(6), 335. https://doi.org/10.3390/toxins11060335




