Missiles of Mass Disruption: Composition and Glandular Origin of Venom Used as a Projectile Defensive Weapon by the Assassin Bug Platymeris rhadamanthus
Abstract
:1. Introduction
2. Results
2.1. Venom Proteome
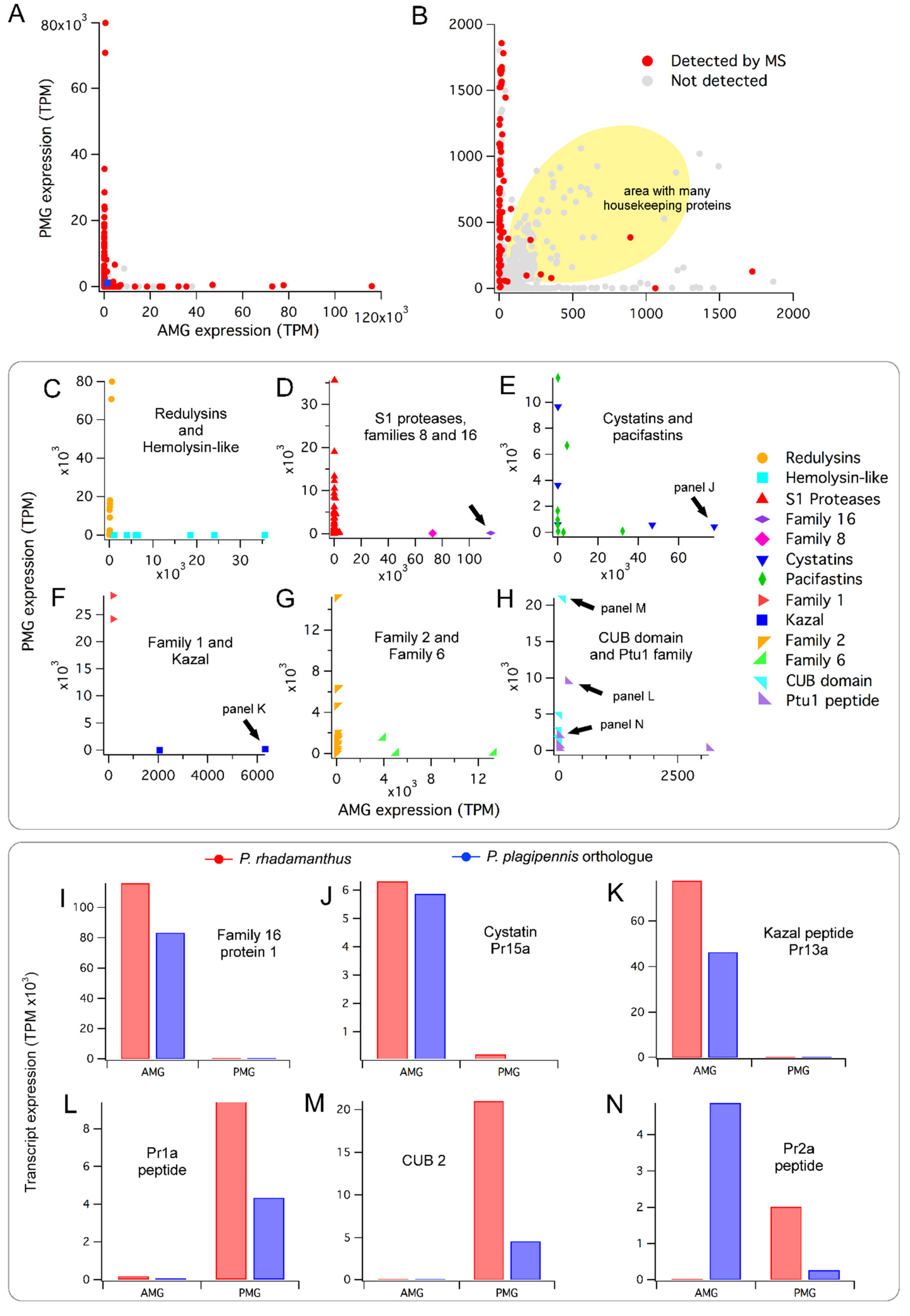
2.2. Conserved Patterns of Venom Gland Specialisation in Assassin Bugs
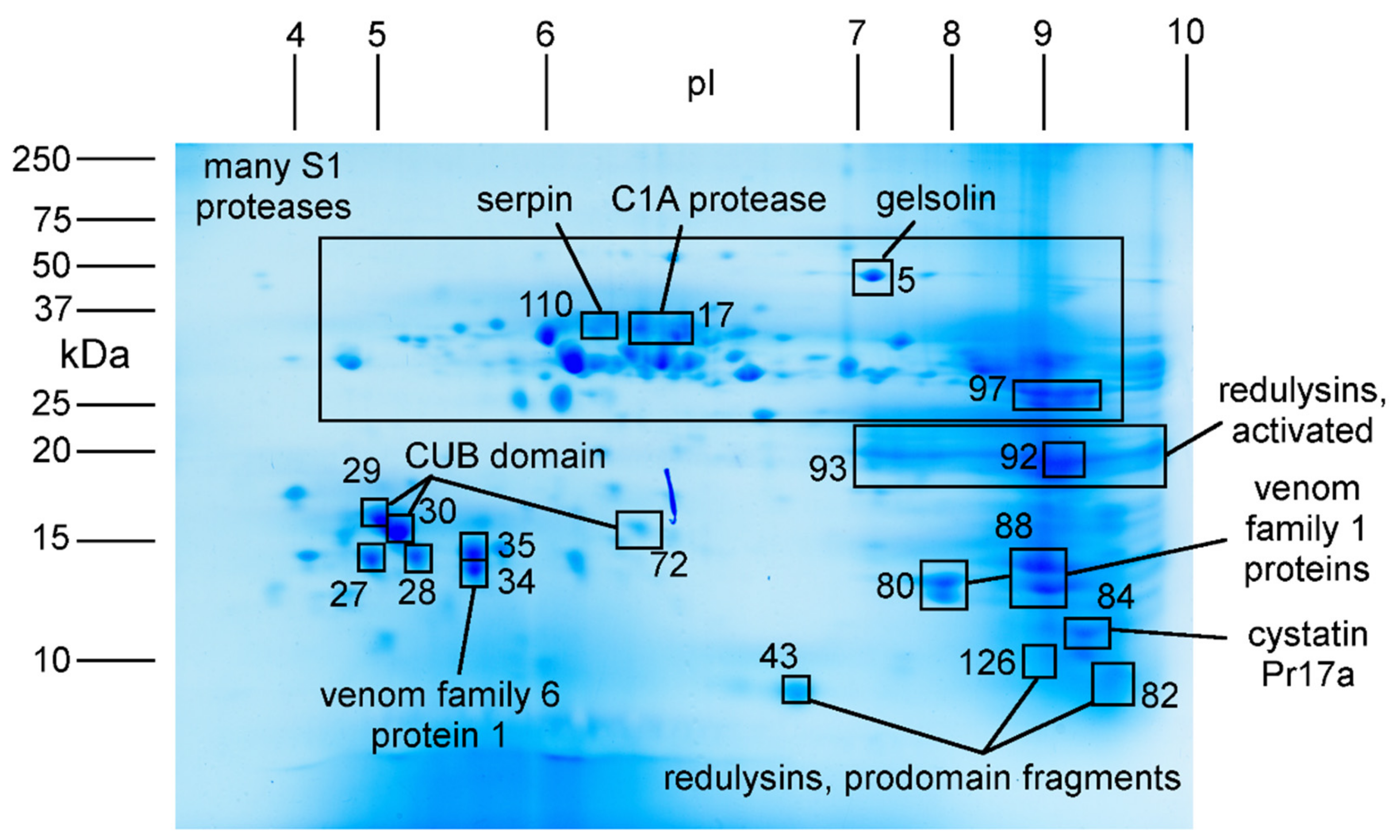
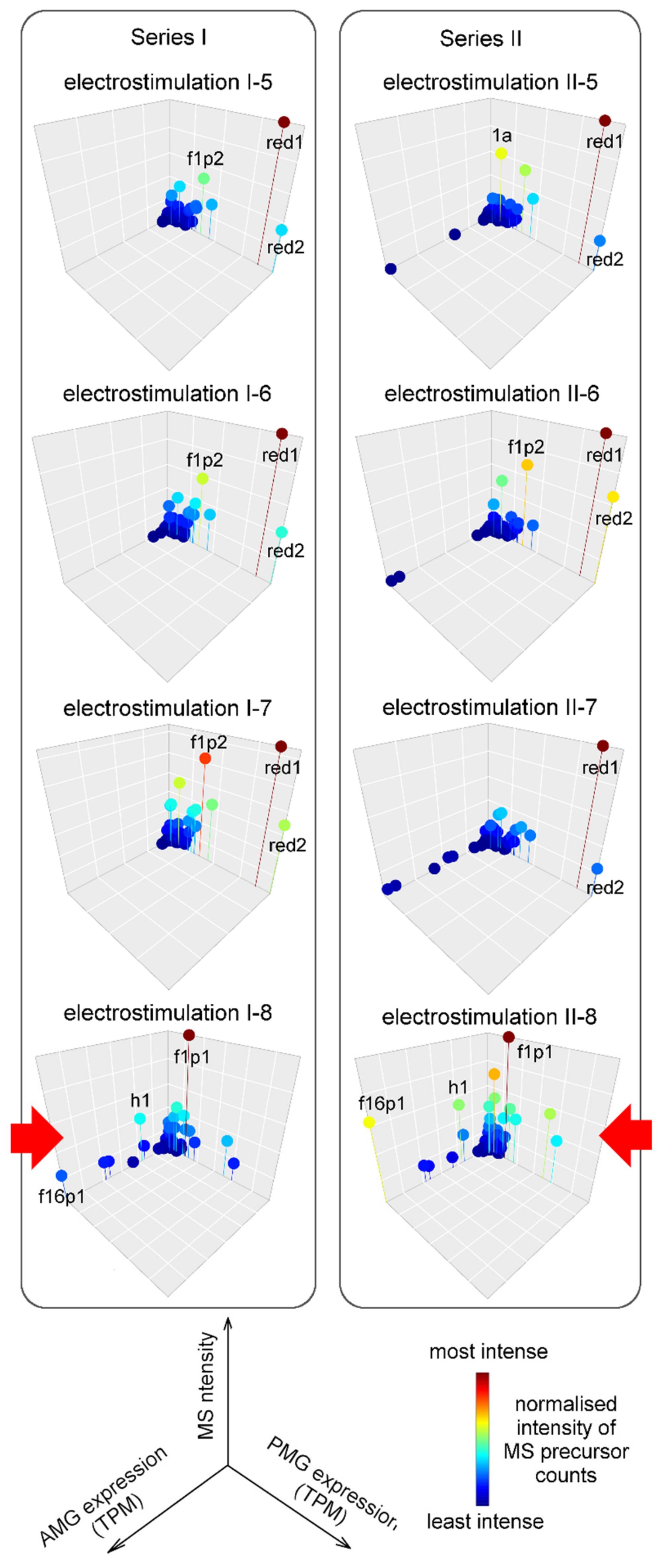
2.3. Major Proteins and Peptides in Venom Obtained by Electrostimulation
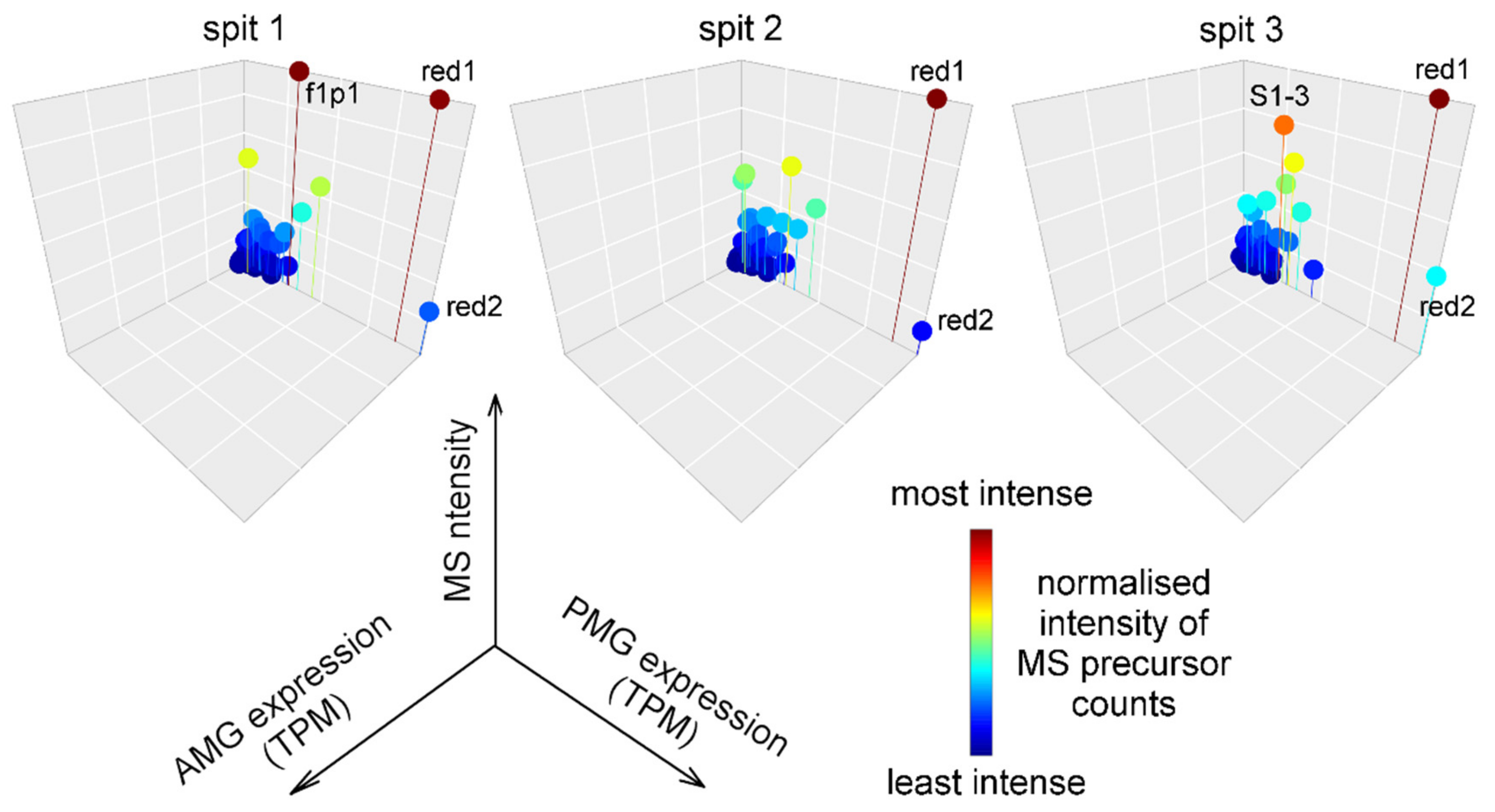
2.4. Composition and Glandular Origin of Defensively Propelled Venom
2.5. Likely Glandular Origin of Venom Obtained by Electrostimulation.
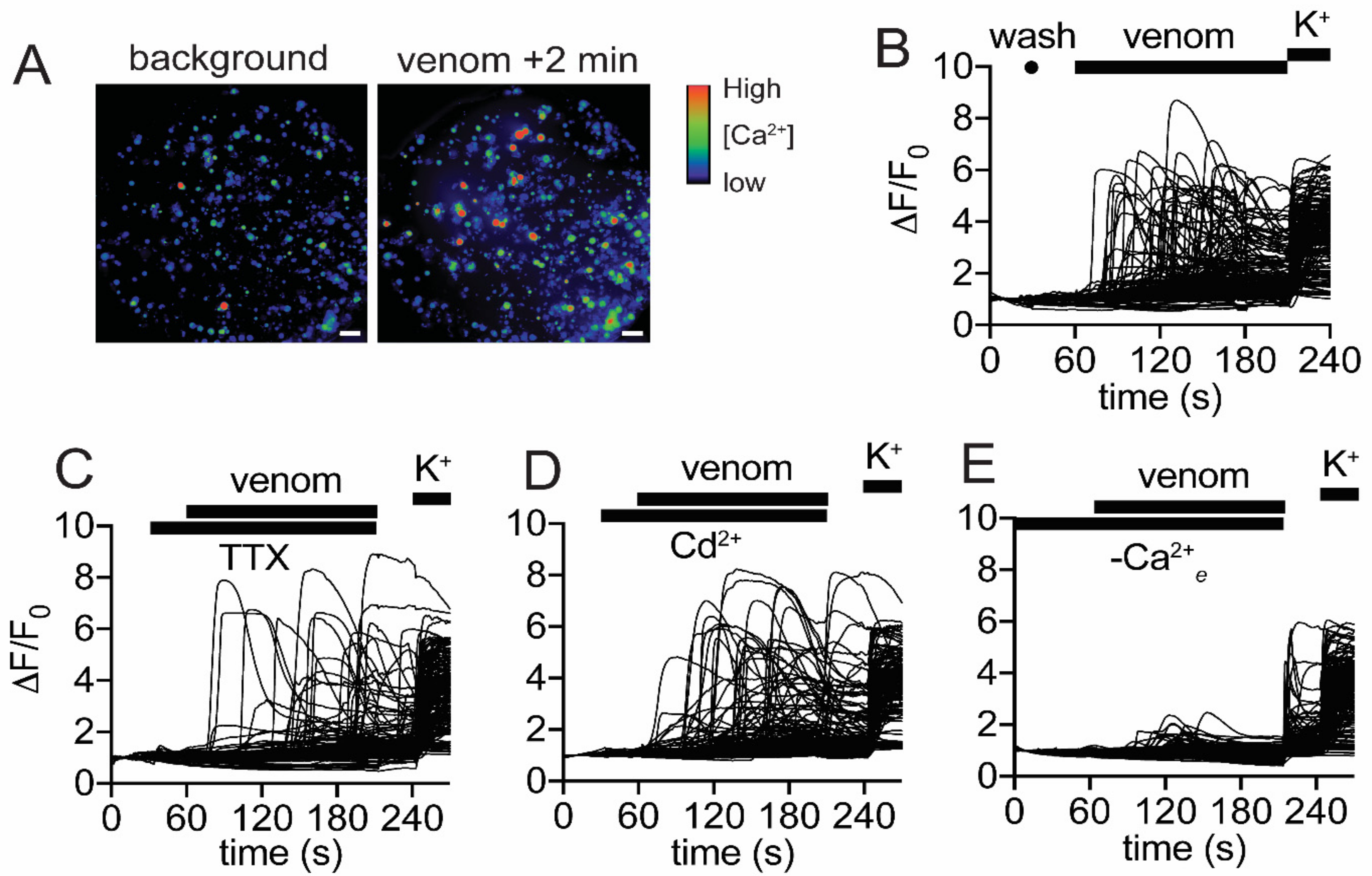
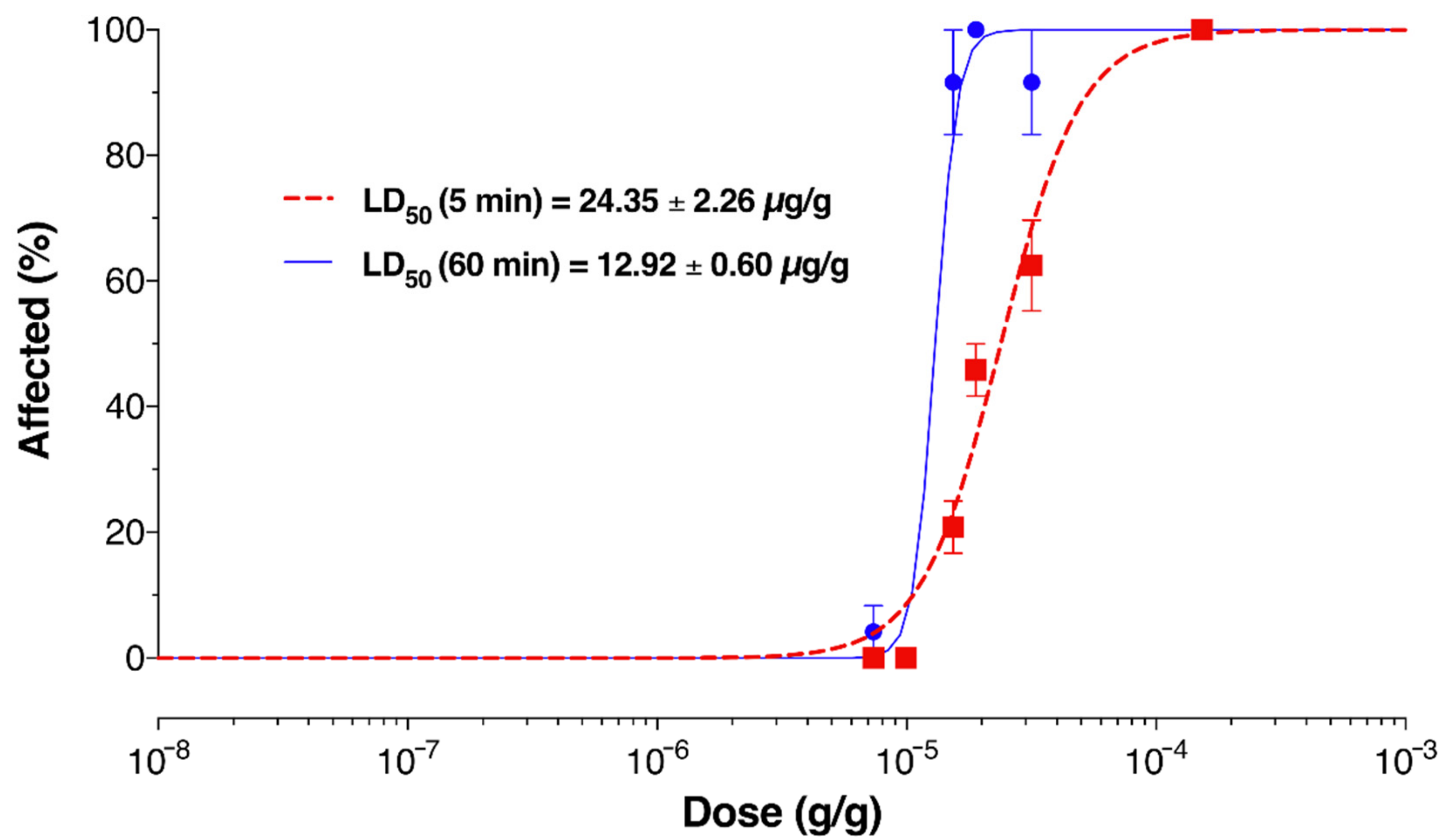
2.6. Venom Activates Mammalian Nociceptors and Kills Insects
3. Discussion
4. Materials and Methods
4.1. Insects and Venom Collection
4.2. Transcriptomics
4.3. Electrophoresis
4.4. Proteomics
4.5. Calcium Imaging of Mouse Sensory Neurons
4.6. Insecticidal Assays
4.7. Data Processing
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panagides, N.; Jackson, T.N.W.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, G.; Tzschätzsch, K.; Bleckmann, H. The spitting behavior of two species of spitting cobras. J. Comp. Physiol. A 2005, 191, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A.; Ormerod, L.D. Snake venom ophthalmia and blindness caused by the spitting cobra (Naja nigricollis) in Nigeria. Am. J. Trop. Med. Hyg. 1976, 25, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bittenbinder, M.A.; Dobson, J.S.; Zdenek, C.N.; op den Brouw, B.; Naude, A.; Vonk, F.J.; Fry, B.G. Differential destructive (non-clotting) fibrinogenolytic activity in Afro-Asian elapid snake venoms and the links to defensive hooding behavior. Toxicology in Vitro 2019, 60, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Bittenbinder, M.A.; Zdenek, C.N.; op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic cobras: Clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef]
- Jeanne, R.L.; Keeping, M.G. Venom spraying in Parachartergus colobopterus: A novel defensive behavior in a social wasp (Hymenoptera: Vespidae). J. Insect Behav. 1995, 8, 433–442. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Morgan, E.D. Chemicals from the glands of ants. Chem. Soc. Rev. 1984, 13, 245–278. [Google Scholar] [CrossRef]
- Fink, L.S. Venom spitting by the green lynx spider, Peucetia viridans (Araneae, Oxyopidae). J. Arachnol. 1984, 12, 372–373. [Google Scholar]
- Zobel-Thropp, P.A.; Correa, S.M.; Garb, J.E.; Binford, G.J. Spit and venom from Scytodes spiders: A diverse and distinct cocktail. J. Proteome Res. 2014, 13, 817–835. [Google Scholar] [CrossRef]
- Walker, A.A.; Weirauch, C.; Fry, B.G.; King, G.F. Venoms of heteropteran insects: A treasure trove of diverse pharmacological toolkits. Toxins 2016, 8, 43. [Google Scholar] [CrossRef]
- Bedford, G.O. Biology, ecology, and control of palm rhinoceros beetles. Annu. Rev. Entomol. 1980, 25, 309–339. [Google Scholar] [CrossRef]
- Edwards, J.S. Spitting as a defensive mechanism in a predatory reduviid. In Proc XI International Congress on Entomology; ICE: Vienna, Austria, 1960; Vol. 3, pp. 259–263. [Google Scholar]
- Edwards, J.S. The action and compostion of the saliva of an assassin bug Platymeris rhadamanthus Gaerst. (Hemiptera, Reduviidae). J. Exp. Biol. 1961, 38, 61–77. [Google Scholar]
- Walker, A.A.; Madio, B.; Jin, J.; Undheim, E.A.; Fry, B.G.; King, G.F. Melt with this kiss: Paralyzing and liquefying venom of the assassin bug Pristhesancus plagipennis (Hemiptera: Reduviidae). Mol. Cell. Proteomics 2017, 16, 552–566. [Google Scholar] [CrossRef]
- Walker, A.A.; Mayhew, M.L.; Jin, J.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Meritt, D.J.; King, G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Haridass, E.T.; Ananthakrishnan, T.N. Functional morphology of the salivary system in some reduviids (Insecta-Heteroptera-Reduviidae). P. Indian A. S. Anim. Sci. 1981, 90, 145–160. [Google Scholar]
- Moore, E.L. A Biochemical and Molecular Analysis of Venom with Distinct Physiological Actions from Two Arthropod Sources: The Parasitoid Jewel Wasp, Ampulex Compressa, of the Insect Order Hymenoptera and the Obligate Entomophagous Assassin Bug, Platymeris Biguttata, of the Insect Order Hemiptera. Ph.D. Thesis, University of California, Riverside, CA, USA, 2003. [Google Scholar]
- Assumpção, T.C.F.; Francischetti, I.M.; Andersen, J.F.; Schwarz, A.; Santana, J.M.; Ribeiro, J.M. An insight into the sialome of the blood-sucking bug Triatoma infestans, a vector of Chagas’ disease. Insect Biochem. Mol. Biol. 2008, 38, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Hernández-Vargas, M.J.; Corzo, G.; Fry, B.G.; King, G.F. Giant fish-killing water bug reveals ancient and dynamic venom evolution in Heteroptera. Cell. Mol. Life Sci. 2018, 75, 3215–3229. [Google Scholar] [CrossRef]
- Liang, Z.; Sottrup-Jensen, L.; Aspán, A.; Hall, M.; Söderhäll, K. Pacifastin, a novel 155-kDa heterodimeric proteinase inhibitor containing a unique transferrin chain. Proc. Natl. Acad. Sci. USA 1997, 94, 6682. [Google Scholar] [CrossRef]
- Corzo, G.; Adachi-Akahane, S.; Nagao, T.; Kusui, Y.; Nakajima, T. Novel peptides from assassin bugs (Hemiptera: Reduviidae): Isolation, chemical and biological characterization. FEBS Letters 2001, 499, 256–261. [Google Scholar] [CrossRef]
- Bernard, C.; Corzo, G.; Mosbah, A.; Nakajima, T.; Darbon, H. Solution structure of Ptu1, a toxin from the assassin bug Peirates turpis that blocks the voltage-sensitive calcium channel N-type. Biochemistry 2001, 40, 12795–12800. [Google Scholar] [CrossRef]
- Undheim, E.A.; Sunagar, K.; Hamilton, B.R.; Jones, A.; Venter, D.J.; Fry, B.G.; King, G.F. Multifunctional warheads: Diversification of the toxin arsenal of centipedes via novel multidomain transcripts. J Proteomics 2014, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kromer, E.; Nakakura, N.; Lagueux, M. Cloning of a Locusta cDNA encoding a precursor peptide for two structurally related proteinase inhibitors. Insect Biochem. Mol. Biol. 1994, 24, 329–331. [Google Scholar] [CrossRef]
- Simonet, G.; Claeys, I.; Huybrechts, J.; De Loof, A.; Broeck, J.V. Bacterial production and purification of SGPI-1 and SGPI-2, two peptidic serine protease inhibitors from the desert locust, Schistocerca gregaria. Protein Expression Purif. 2003, 31, 188–196. [Google Scholar] [CrossRef]
- Simonet, G.; Claeys, I.; Vanderperren, H.; November, T.; De Loof, A.; Vanden Broeck, J. cDNA cloning of two different serine protease inhibitor precursors in the migratory locust, Locusta migratoria. Insect Mol. Biol. 2002, 11, 249–256. [Google Scholar] [CrossRef]
- Breugelmans, B.; Simonet, G.; van Hoef, V.; Van Soest, S.; Vanden Broeck, J. Pacifastin-related peptides: Structural and functional characteristics of a family of serine peptidase inhibitors. Peptides 2009, 30, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Amino, R.; Martins, R.M.; Procopio, J.; Hirata, I.Y.; Juliano, M.A.; Schenkman, S. Trialysin, a novel pore-forming protein from saliva of hematophagous insects activated by limited proteolysis. J. Biol. Chem. 2002, 277, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Amino, R.; Daghastanli, K.R.; Cuccovia, I.M.; Juliano, M.A.; Schenkman, S. A short proregion of trialysin, a pore-forming protein of Triatoma infestans salivary glands, controls activity by folding the N-terminal lytic motif. FEBS J. 2008, 275, 994–1002. [Google Scholar] [CrossRef]
- Robinson, S.D.; Mueller, A.; Clayton, D.; Starobova, H.; Hamilton, B.R.; Payne, R.J.; Vetter, I.; King, G.F.; Undheim, E.A.B. A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 2018, 4, eaau4640. [Google Scholar] [CrossRef]
- Vetter, I. Development and optimization of FLIPR high throughput calcium assays for ion channels and GPCRs. In Calcium Signaling; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 740, pp. 45–82. [Google Scholar]
- Walker, A.; Dobson, J.; Jin, J.; Robinson, S.; Herzig, V.; Vetter, I.; King, G.; Fry, B. Buzz kill: Function and proteomic composition of venom from the giant assassin fly Dolopus genitalis (Diptera: Asilidae). Toxins 2018, 10, 456. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The rise and fall of an evolutionary innovation: Contrasting strategies of venom evolution in ancient and young animals. PLoS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Qian, C.; Liang, D.; Liu, Y.; Wang, P.; Kausar, S.; Wei, G.; Zhu, B.; Wang, L.; Liu, C. Identification of a small pacifastin protease inhibitor from Nasonia vitripennis venom that inhibits humoral immunity of host (Musca domestica). Toxicon 2017, 131, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ruder, T.; Sunagar, K.; Undheim, E.A.B.; Ali, S.A.; Wai, T.-C.; Low, D.H.W.; Jackson, T.N.W.; King, G.F.; Antunes, A.; Fry, B.G. Molecular phylogeny and evolution of the proteins encoded by coleoid (cuttlefish, octopus, and squid) posterior venom glands. J. Mol. Evol. 2013, 76, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Scherfer, C.; Korayem, A.M.; Zhao, Z.; Schmidt, O.; Theopold, U. Insect hemolymph clotting: Evidence for interaction between the coagulation system and the prophenoloxidase activating cascade. Insect Biochem. Mol. Biol. 2002, 32, 919–928. [Google Scholar] [CrossRef]
- King, G.F.; Hardy, M.G. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.S.; Weirauch, C. Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): Insights from divergence dating and ancestral state reconstruction. PLoS ONE 2012, 7, e45523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.A.; Rosenthal, M.; Undheim, E.A.B.; King, G.F. Harvesting venom toxins from assassin bugs and other heteropteran insects. JoVE 2018, e57729. [Google Scholar] [CrossRef]
- Manzoli-Palma, M.F.; Gobbi, N.; Palma, M.S. Insects as biological models to assay spider and scorpion venom toxicity. J. Venom. Anim. Tox. Incl. Trop. Dis. 2003, 9, 174–185. [Google Scholar] [CrossRef]
- Sher, D.; Fishman, Y.; Zhang, M.; Lebendiker, M.; Gaathon, A.; Mancheño, J.-M.; Zlotkin, E. Hydralysins, a new category of β-pore-forming toxins in Cnidaria. J. Biol. Chem. 2005, 280, 22847–22855. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Claassen, M.; Schmidt, A.; Aebersold, R. Estimation of absolute protein quantities of unlabeled samples by selected reaction monitoring mass spectrometry. Mol. Cell. Proteom. 2012, 11, M111.013987. [Google Scholar]
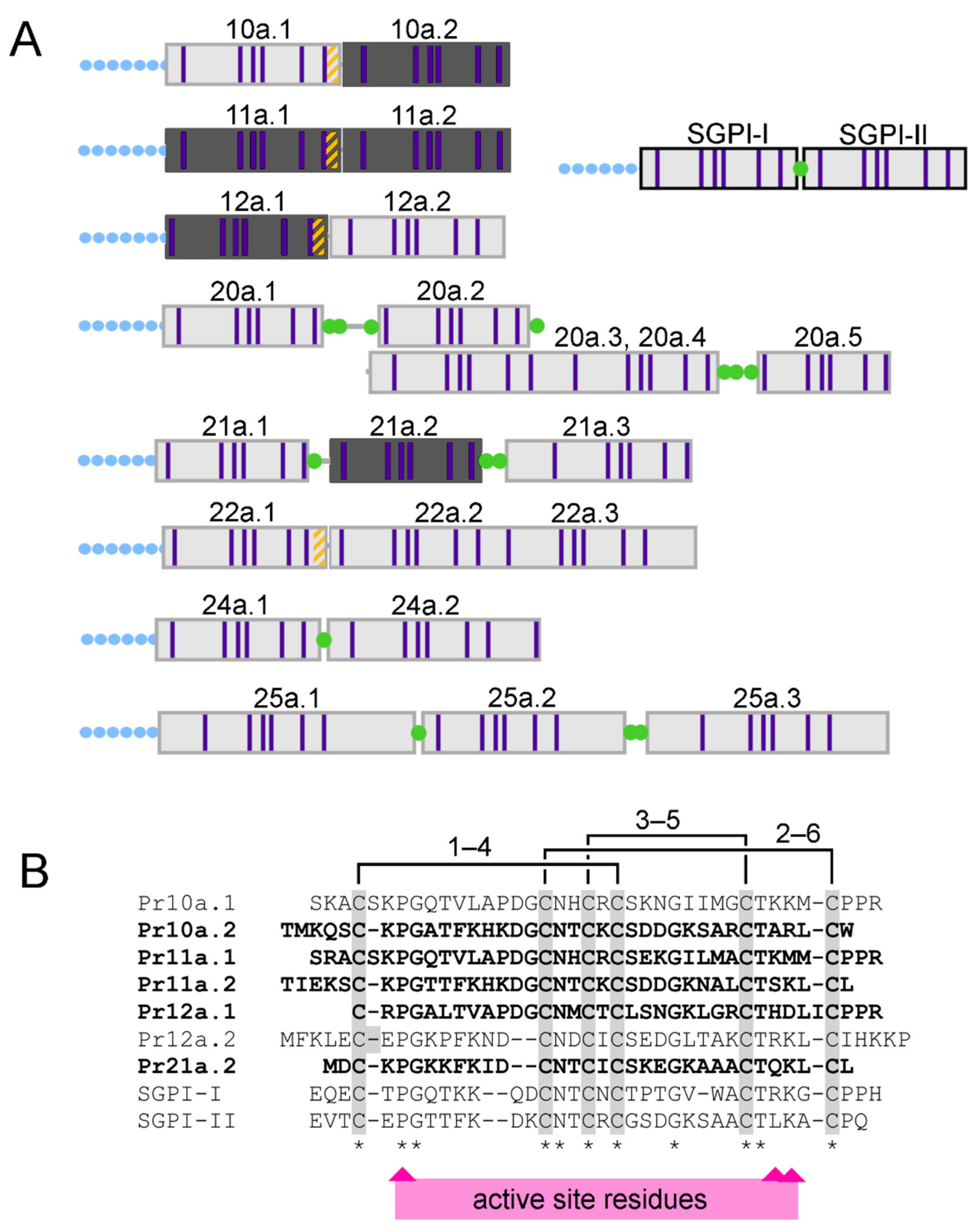
| Amino Acid Sequence | Precursor | Putative Fold | No. Cys | Theoretical Native Mass (Da) | Observed Native Mass (Da) | Theoretical RAa Mass (Da) | Observed RA Mass (Da) |
|---|---|---|---|---|---|---|---|
| DEKDCIARGQKCVGENKPCCKGTTCMYYANRCVGV | U-RDTX b-Pr1a | ICK c | 6 | 3835.69 | 3835.62 | 4105.89 | 4105.81 |
| TIEKSCKPGTTFKHKDGCNTCKCSDDGKNALCTSKLCL | U-RDTX-Pr11a.2 | Pacifastin | 6 | 4070.88 | 4070.81 | 4341.09 | 4340.99 |
| TMKQSCKPGATFKHKDGCNTCKCSDDGKSARCTARLCW | U-RDTX-Pr10a.2 | Pacifastin | 6 | 4158.86 | 4158.78 | 4429.06 | 4428.96 |
| EEHGCIPPFQPCEGVNSRCCGLYVCFNKICLATP | U-RDTX-Pr2a | ICK | 6 | 3763.65 b | 3763.59 d | 4033.86 d | 4033.77 b |
| SRACSKPGQTVLAPDGCNHCRCSEKGILMACTKMMCPPR | U-RDTX-Pr11a.1 | Pacifastin | 6 | 4172.89 | 4172.82 | 4443.10 | 4442.99 |
| HGCIPPFQPCEGVNSRCCGLYVCFNKICLATP | U-RDTX-Pr2b | ICK | 6 | 3505.57 b | 3505.51 d | 3775.77 d | 3775.68 b |
| GGCIQRYGKCSTENSNCCAPSECYFSFNQCF | U-RDTX-Pr7a | ICK | 6 | 3439.33 | 3439.26 | 3709.53 | 3709.43 |
| CIPAANPCRGNAKCCGNYVCKNGRCLPRS | U-RDTX-Pr5a | ICK | 6 | 3061.39 | 3061.31 | 3331.59 | 3331.52 |
| MMPVCFEGEKLNKDQTKCIKA | U-RDTX-Pr9a | Unknown | 2 | 2410.16 | 2410.13 | 2500.23 | 2500.19 |
| GEDVCIPSGQKCGPYMNMGCCKGLVCMSYAARCVSMGGIPR | U-RDTX-Pr4a | ICK | 6 | 4264.84 | 4264.77 | 4535.05 | 4534.09 |
| MDCKPGKKFKIDCNTCICSKEGKAAACTQKLCLK | U-RDTX-Pr21.2 | Pacifastin | 6 | 3699.79 | 3699.72 | 3970.00 | - |
| CRPGALTVAPDGCNMCTCLSNGKLGRCTHDLICPPR | U-RDTX-Pr12a.1 | Pacifastin | 6 | 3765.73 | - | 4035.93 | 4035.85 |
| Spot | Protein | Theoretical pI | Observed pI | Theoretical Mass (kDa) | Observed Mass (kDa) | ProteinPilot Score a |
|---|---|---|---|---|---|---|
| 5 | Gelsolin 1 | 7.2 | 7.1 | 39.3 | 49 | 60.9 |
| 17 | C1A protease 1 | 8.0 | 6.4 | 35.2 | 35 | 28.8 |
| 27 | Gelsolin 1, subdomain 1 | 5.2 | 4.8 | 14.1 | 14 | 15.0 |
| 28 | Gelsolin 1, subdomain 1 | 5.2 | 5.2 | 14.1 | 14 | 20.0 |
| 29 | CUB domain protein 2 | 5.0 | 4.7 | 12.6 | 16 | 4.0 |
| 30 | CUB domain protein 1 | 5.0 | 5.1 | 12.1 | 15 | 18.1 |
| 34 | Family 4 protein 1 | 8.9 | 6.4 | 17.1 | 16 | 32.0 |
| 35 | Gelsolin 1, subdomain 1 | 5.2 | 5.6 | 14.1 | 15 | 26.3 |
| 43/82/126 | Redulysins, N-terminal fragments | Nd b | 6–10 | nd | <10 | 14.5–18.9 |
| 80 | Family 1 protein 1 | 7.9 | 7.8 | 13.8 | 13 | 21.3 |
| 88 | Family 1 protein 2 | 9.0 | 8.9 | 14.8 | 14 | 56.0 |
| 93 | Redulysins 2,3,7, active form | 9.1–9.6 | 7–10 | 21.0–23.5 | 18–22 | >15 |
| 96 | Redulysin 2, active form | 9.3 | 9.1 | 21.5 | 20 | 54.6 |
| 97 | Gelsolin 1, subdomains 2 and 3 | 9.0 | 9.0 | 25.2 | 25 | 69.9 |
| 110 | Serpin 1 | 5.5 | 6.4 | 42.6 | 35 | 36.6 |
| various | S1 proteases | nd | 4–10 | nd | 23–50 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, A.A.; Robinson, S.D.; Undheim, E.A.B.; Jin, J.; Han, X.; Fry, B.G.; Vetter, I.; King, G.F. Missiles of Mass Disruption: Composition and Glandular Origin of Venom Used as a Projectile Defensive Weapon by the Assassin Bug Platymeris rhadamanthus. Toxins 2019, 11, 673. https://doi.org/10.3390/toxins11110673
Walker AA, Robinson SD, Undheim EAB, Jin J, Han X, Fry BG, Vetter I, King GF. Missiles of Mass Disruption: Composition and Glandular Origin of Venom Used as a Projectile Defensive Weapon by the Assassin Bug Platymeris rhadamanthus. Toxins. 2019; 11(11):673. https://doi.org/10.3390/toxins11110673
Chicago/Turabian StyleWalker, Andrew A., Samuel D. Robinson, Eivind A. B. Undheim, Jiayi Jin, Xiao Han, Bryan G. Fry, Irina Vetter, and Glenn F. King. 2019. "Missiles of Mass Disruption: Composition and Glandular Origin of Venom Used as a Projectile Defensive Weapon by the Assassin Bug Platymeris rhadamanthus" Toxins 11, no. 11: 673. https://doi.org/10.3390/toxins11110673
APA StyleWalker, A. A., Robinson, S. D., Undheim, E. A. B., Jin, J., Han, X., Fry, B. G., Vetter, I., & King, G. F. (2019). Missiles of Mass Disruption: Composition and Glandular Origin of Venom Used as a Projectile Defensive Weapon by the Assassin Bug Platymeris rhadamanthus. Toxins, 11(11), 673. https://doi.org/10.3390/toxins11110673









