First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil
Abstract
:1. Introduction
2. Results
2.1. Cyanotoxins
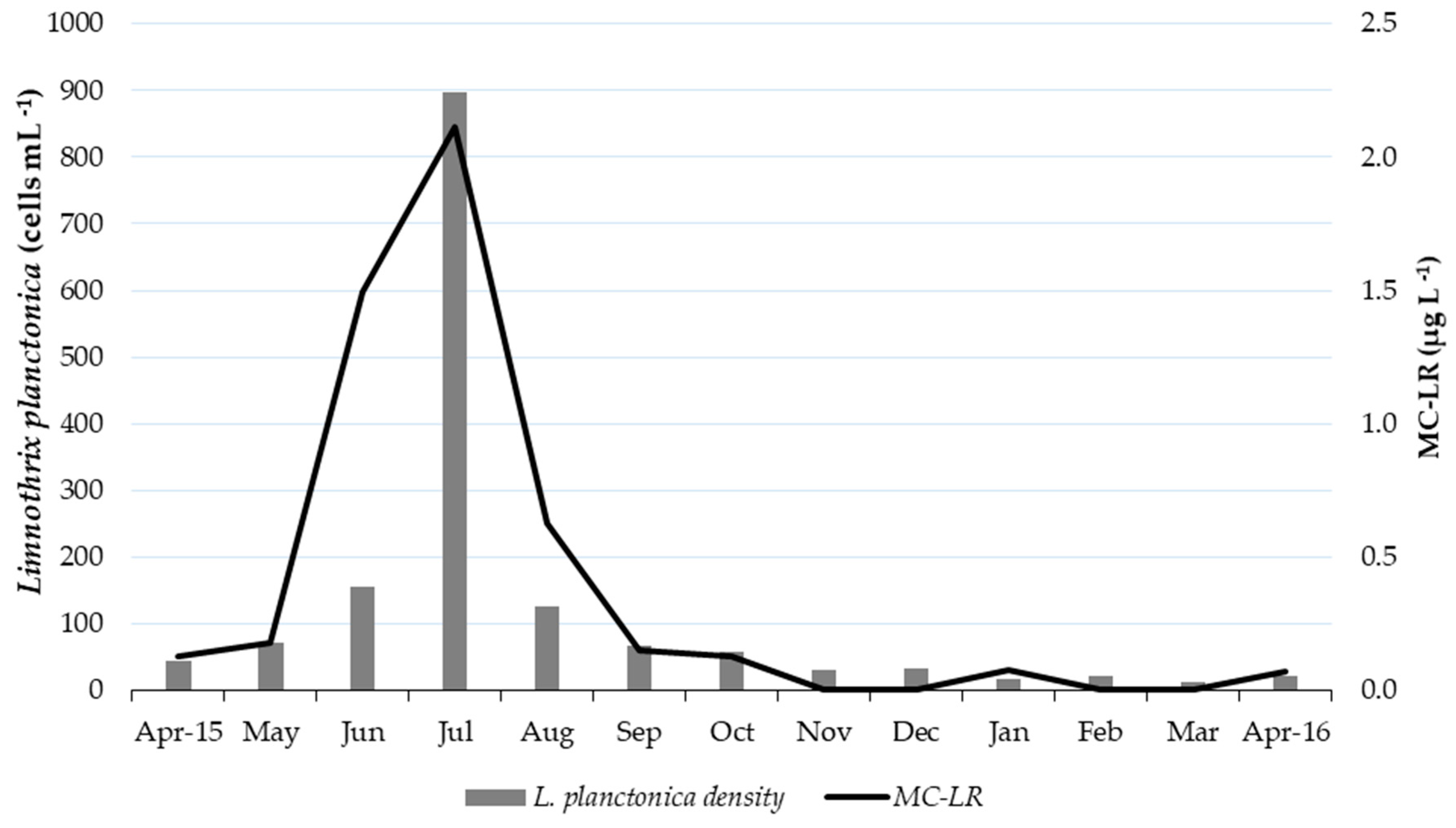
2.1.1. Quantification of Cyanotoxins by ELISA
2.1.2. Molecular Screening for Cyanotoxins
2.1.3. Quantification of MC-LR by LC-ESI-MS/MS
2.2. Environmental Data
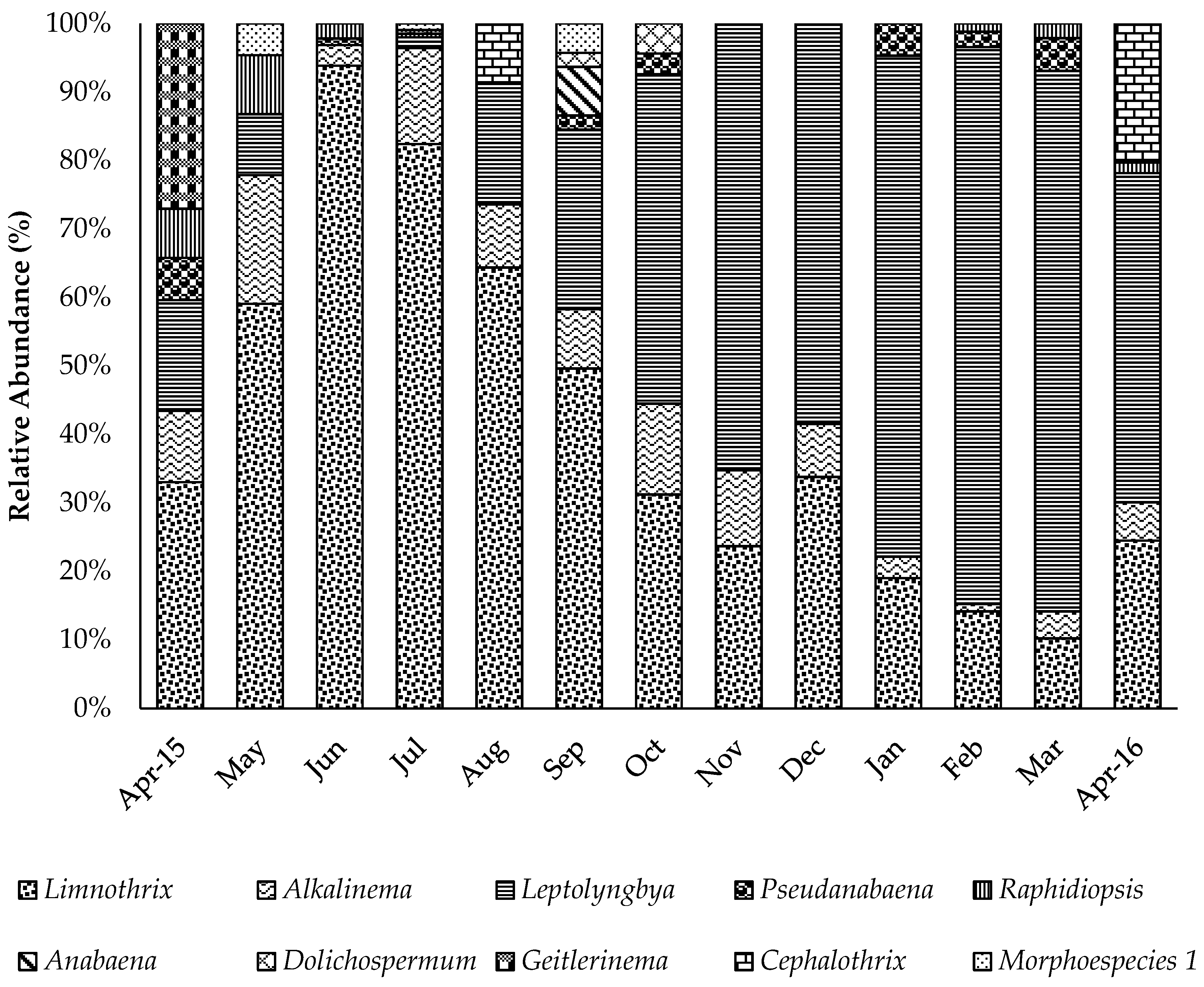
2.3. Cyanobacteria Identification and Counting
2.4. Linkages Between Environmental Factors, Cyanobacteria, and Cyanotoxins
3. Discussion
4. Conclusions
5. Materials and Methods
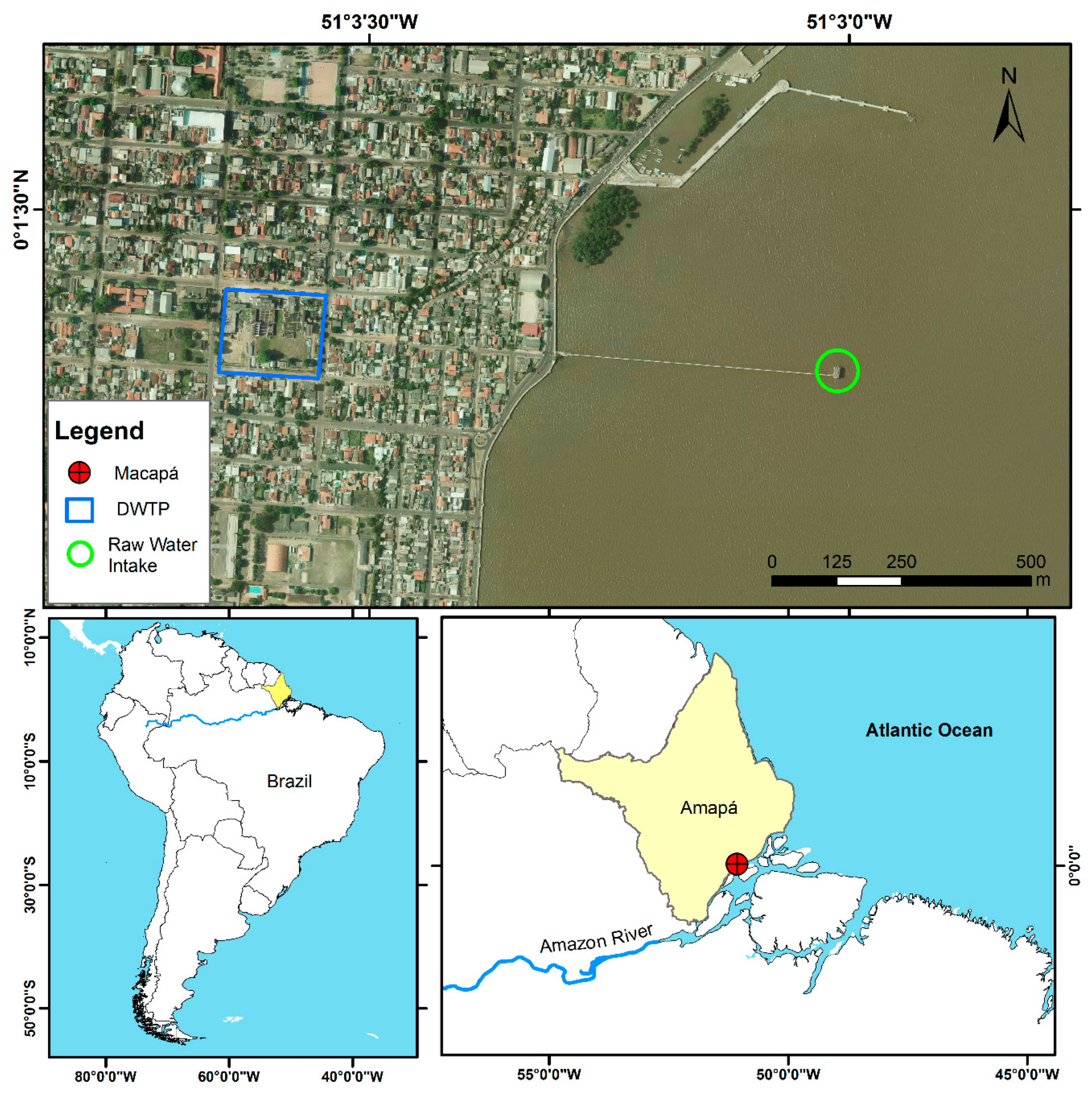
5.1. Study Site
5.2. Water Quality Sampling and Monitoring of Environmental Variables
5.3. Cyanotoxins
5.3.1. Quantification of Cyanotoxins by ELISA
5.3.2. Molecular Screening for Cyanotoxins
5.3.3. Quantification of MC-LR by LC-ESI-MS
5.4. Cyanobacteria Identification and Counting
5.5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Grewe, C.B.; Pulz, O. The Biotechnology of Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 707–739. [Google Scholar]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999. [Google Scholar]
- Falconer, I.; Bartram, J.; Chorus, I.; Kuiper-Goodman, T.; Utkilen, H.; Burch, M.; Codd, G. Safe levels and safe practices. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 161–182. [Google Scholar]
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Msagati, T.A.M.; Siame, B.A.; Shushu, D.D. Evaluation of methods for the isolation, detection and quantification of cyanobacterial hepatotoxins. Aquat. Toxicol. 2006, 78, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Djediat, C.; Malécot, M.; de Luze, A.; Bernard, C.; Puiseux-Dao, S.; Edery, M. Localization of microcystin-LR in medaka fish tissues after cyanotoxin gavage. Toxicon 2010, 55, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Catherine, A. Appendix 3: Tables of Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J.A.O., Spoof, L.E.M., Codd, G.A., Eds.; Wiley: Chichester, UK, 2017; pp. 526–537. [Google Scholar]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Cyanotoxins. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 651–675. [Google Scholar]
- Chen, G.; Yu, S.; Wei, G. Studies on microcystin contents in different drinking water in highly endemic area of liver cancer. Chin. J. Prev. Med. 1996, 30, 6–9. [Google Scholar]
- Sanches, S.M.; Vieira, E.M.; Prado, E.L.; Benetti, F.; Takayanagui, A.M.M. Estudo da presença da toxina microcistina-LR em água utilizada em clínica de hemodiálise e validação de um método análítico. Eclet. Quim. 2007, 32, 43–48. [Google Scholar] [CrossRef]
- McDermott, C.; Nho, C.; Howard, W.; Holton, B. The cyanobacterial toxin, microcystin-LR, can induce apoptosis in a variety of cell types. Toxicon 1998, 36, 1981–1996. [Google Scholar] [CrossRef]
- Lankoff, A.; Carmichael, W.W.; Grasman, K.A.; Yuan, M. The uptake kinetics and immunotoxic effects of microcystin-LR in human and chicken peripheral blood lymphocytes in vitro. Toxicology 2004, 204, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Sicińska, P.; Bukowska, B.; Michałowicz, J.; Duda, W. Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon 2006, 47, 387–397. [Google Scholar] [CrossRef]
- Azevedo, S.M.F.O.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 181, 441–446. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- Seção, I. Portaria de Consolidação Federal no 5. In Consolidação das normas sobre as ações e os serviços de saúde do Sistema Único de Saúde; Ministério da Saúde: Brasília, Brasil, 2017; pp. 1–444. [Google Scholar]
- Daniel, Mariely Helena Barbosa; Resende, Rodrigo Matias de Sousa. Avaliação da Vigilância da Qualidade da Água no Estado do Amapá—Ano base 2011; Ministério da Saúde: Brasília, Brasil, 2012. [Google Scholar]
- Oliveira, E.D.C.; da Cunha, A.C.; da Silva, N.B.; Castelo-Branco, R.; Morais, J.; Schneider, M.P.C.; Faustino, S.M.M.; Ramos, V.; Vasconcelos, V. Morphological and molecular characterization of cyanobacterial isolates from the mouth of the Amazon River. Phytotaxa 2019, 387, 269–288. [Google Scholar] [CrossRef]
- Da Cunha, A.C.; Brito, D.C.; Junior, A.C.B.; dos Pinheiro, L.A.R.; Cunha, H.F.A.; Santos, E.; Krusche, A.V. Challenges and solutions for hydrodynamic and water quality in rivers in the Amazon Basin. Hydrodyn. Nat. Water Bodies 2012, 3, 67–88. [Google Scholar]
- Pinheiro, L.A.R.; Cunha, A.C.; Cunha, H.F.A.C.; Souza, L.R.; Bilhalva, J.S.; Brito, D.C.; Brasil Júnior, A.C.P. Aplicação de simulação computacional à dispersão de poluentes no baixo rio Amazonas: Potenciais riscos à captação de água na orla de Macapá-Amapá. Amaz; Ciência e Desenvolv: Belém, Brasil, 2008; Volume 4, pp. 27–44. [Google Scholar]
- Ross, C.; Santiago-Vázquez, L.; Paul, V. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2006, 78, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; MacLeod, S.L.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Ward, N.D.; Krusche, A.V.; Sawakuchi, H.O.; Brito, D.C.; Cunha, A.C.; Moura, J.M.S.; da Silva, R.; Yager, P.L.; Keil, R.G.; Richey, J.E. The compositional evolution of dissolved and particulate organic matter along the lower Amazon River-Óbidos to the ocean. Mar. Chem. 2015, 177, 244–256. [Google Scholar] [CrossRef]
- Moreira, C.; Ramos, V.; Azevedo, J.; Vasconcelos, V. Methods to detect cyanobacteria and their toxins in the environment. Appl. Microbiol. Biotechnol. 2014, 98, 8073–8082. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Holland, P.; Sprosen, J. Method for detecting classes of microcystins by combination of protein phosphatase inhibition assay and ELISA: Comparison with LC-MS. Toxicon 2005, 45, 199–206. [Google Scholar] [CrossRef]
- De Sá, L.L.C.; dos Vieira, J.M.S.; de Jesus, I.M.; de Mendes, R.A.; Pinheiro, S.C.C.; Vale, E.R.; dos Alves, F.A.S.; De Jesus, I.M.; de Santos, E.C.O.; Costa, V.B. da Ocorrência de uma floração de cianobactérias tóxicas na margem direita do rio Tapajós, no Município de Santarém (Pará, Brasil). Rev. Pan-Amazônica Saúde 2010, 1, 159–166. [Google Scholar]
- Vieira, J.M.D.S.; Azevedo, M.T.D.P.; De Oliveira Azevedo, S.M.F.; Honda, R.Y.; Corrêa, B. Microcystin production by Radiocystis fernandoi (Chroococcales, Cyanobacteria) isolated from a drinking water reservoir in the city of Belém, PA, Brazilian Amazonia region. Toxicon 2003, 42, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.D.S.; Azevedo, M.T.D.P.; de Azevedo, S.M.F.O.; Honda, R.Y.; Corrêa, B. Toxic cyanobacteria and microcystin concentrations in a public water supply reservoir in the Brazilian Amazonia region. Toxicon 2005, 45, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sioli, H. The Amazon and its main affluents: Hydrography, morphology of the river courses, and river types. In The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin; Sioli, H., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 127–165. [Google Scholar]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The fate of microcystins in the environment and challenges for monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Masui, H.; Uemura, H.; Mori, Y.; Harada, K. Analysis of microcystins in sediments using MMPB method. Toxicon 2001, 39, 687–692. [Google Scholar] [CrossRef]
- Cunha, A.C. Hidrodinâmica e Qualidade da Água como Subsídios ao Estudo de Emissário de Esgoto Subfluvial em Macapá-AP; Unifap: Macapá, Brazil, 2019; p. 50. [Google Scholar]
- Nimptsch, J.; Woelfl, S.; Osorio, S.; Valenzuela, J.; Moreira, C.; Ramos, V.; Castelo-Branco, R.; Leão, P.N.; Vasconcelos, V. First record of toxins associated with cyanobacterial blooms in oligotrophic North Patagonian lakes of Chile-a genomic approach. Int. Rev. Hydrobiol. 2016, 101, 57–68. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Neilan, B.A. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Iningainema pulvinus gen nov., sp nov. (Cyanobacteria, Scytonemataceae) a new nodularin producer from Edgbaston Reserve, north-eastern Australia. Harmful Algae 2017, 62, 10–19. [Google Scholar] [CrossRef]
- Welker, M.; Steinberg, C. Rates of humic substance photosensitized degradation of microcystin-lr in natural waters. Environ. Sci. Technol. 2000, 34, 3415–3419. [Google Scholar] [CrossRef]
- Song, W.; Bardowell, S.O.; Shea, K.E. Mechanistic study and the influence of oxygen on the photosensitized transformations of microcystins (cyanotoxins). Environ. Sci. Technol. 2007, 41, 5336–5341. [Google Scholar] [CrossRef]
- Hitzfeld, B.C.; Hoger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar] [PubMed]
- Mayumi, T.; Kato, H.; Imanishi, S.; Kawasaki, Y.; Hasegawa, M.; Harada, K.I. Structural characterization of microcystins by LC/MS/MS under ion trap conditions. J. Antibiot. 2006, 59, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Kirkpatrick, B.; Backer, L.C. Characterization of aerosols containing microcystin. Mar. Drugs 2007, 5, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Koreivien, J.; Anne, O.; Kasperoviciene, J.; Burskyte, V. Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.L.F.F.; Calijuri, M.D.C.; Lorenzi, A.S.; Honda, R.Y.; Genuário, D.B.; Fiore, M.F. Morphological and molecular characterization of cyanobacteria from a Brazilian facultative wastewater stabilization pond and evaluation of microcystin production. Hydrobiologia 2009, 627, 195–209. [Google Scholar] [CrossRef]
- Bernard, C.; Froscio, S.; Campbell, R.; Monis, P.; Humpage, A.; Fabbro, L. Novel toxic effects associated with a tropical Limnothrix/Geitlerinema-like cyanobacterium. Environ. Toxicol. 2011, 26, 260–270. [Google Scholar] [CrossRef]
- Humpage, A.; Falconer, I.; Bernard, C.; Froscio, S.; Fabbro, L. Toxicity of the cyanobacterium Limnothrix AC0243 to male Balb/c mice. Water Res. 2012, 46, 1576–1583. [Google Scholar] [CrossRef]
- Whan, P.M. Investigation of a Novel Toxin Produced by a Limnothrix Cyanobacteria. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2015. [Google Scholar]
- Daniels, O. Autecology, Allelopathy and Toxicity of Limnothrix (Strain AC0243): Multiple-Organism Studies using Laboratory Cultures. Ph.D. Thesis, CQUniversity, Queensland, Australia, 2016. [Google Scholar]
- Genuário, D.B.; Lorenzi, S.; Fernanda, L.; Isaac, R.D.L.; Azevedo, T.D.P.; Neto, R.C.; Fiori, M.F. Cyanobacterial community and microcystin production in a recreational reservoir with constant Microcystis blooms. Hydrobiologia 2016, 779, 105–125. [Google Scholar] [CrossRef]
- Richardson, L.L.; Sekar, R.; Myers, J.L.; Gantar, M.; Voss, J.D.; Kaczmarsky, L.; Remily, E.R.; Boyer, G.L.; Zimba, P.V. The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS 2007, 272, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, L.R.; Costa-neves, A.; Geanne, A.A.; Brunetti, R.L.; Hentschke, G.S.; Malone, F.S.; Torres, L.M.B.; Anna, C.L.S. Biologically active compounds from cyanobacteria extracts: In vivo and in vitro aspects. Rev. Bras. Farmacogn. 2013, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Marsalek, B.; Blaha, L.; Babica, P. Analyses of microcystins in the biomass of Pseudanabaena limnetica collected in Znojmo reservoir. Czech Phycol. 2003, 3, 195–197. [Google Scholar]
- Oudra, B.; Loudiki, M.; Vasconcelos, V.; Sabour, B.; Sbiyyaa, B.; Oufdou, K.; Mezrioui, N. Detection and quantification of microcystins from cyanobacteria strains isolated from reservoirs and ponds in Morocco. Environ. Toxicol. 2002, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A. First report of toxic Cylindrospermopsis raciborskii and Raphidiopsis mediterranea (Cyanoprokaryota) in Egyptian fresh waters. FEMS Microbiol. Ecol. 2007, 59, 749–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Gómez, E.B.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Komárek, J. Heterocytous Genera, 1st ed.; Springer Spektrum: Heidelberg, Germany, 2013; p. 1131. [Google Scholar]
- Vaz, M.G.M.V.; Genuário, D.B.; Andreote, A.P.D.; Malone, C.F.S.; Sant’Anna, C.L.; Barbiero, L.; Fiore, M.F. Pantanalinema gen. nov. and Alkalinema gen. nov.: Novel pseudanabaenacean genera (Cyanobacteria) isolated from saline–alkaline lakes. Int. J. Syst. Evol. Microbiol. 2015, 65, 298–308. [Google Scholar] [CrossRef]
- Da Malone, C.F.S.; Rigonato, J.; Laughinghouse, H.D.; Schmidt, É.C.; Bouzon, Z.L.; Wilmotte, A.; Fiore, M.F.; Sant’Anna, C.L. Cephalothrix gen. nov. (Cyanobacteria): Towards an intraspecific phylogenetic evaluation by multilocus analyses. Int. J. Syst. Evol. Microbiol. 2015, 65, 2993–3007. [Google Scholar] [CrossRef]
- Ward, N.D.; Sawakuchi, H.O.; Neu, V.; Less, D.F.S.; Valerio, A.M.; Cunha, A.C.; Kampel, M.; Bianchi, T.S.; Krusche, A.V.; Richey, J.E.; et al. Velocity-amplified microbial respiration rates in the lower Amazon River. Limnol. Oceanogr. Lett. 2018, 3, 265–274. [Google Scholar] [CrossRef]
- Dokulil, M.T. Environmental control of phytoplankton productivity in turbulent turbid systems. Hydrobiologia 1994, 289, 65–72. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Padisák, J. Are phytoplankton dynamics in rivers so different from those in shallow lakes? Hydrobiologia 1994, 289, 1–7. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton (Ecology, Biodiversity and Conservation); Cambridge University Press: Cambridge, UK, 2006; p. 535. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota, Part 2: Oscillatoriales, 1st ed.; Spektrum Akademischer Verlag: Berlin, Germany, 2005; p. 759. [Google Scholar]
- Padisák, J.; Soróczki-Pintér, É.; Rezner, Z. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton—An experimental study. Hydrobiologia 2003, 500, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Kruk, C.; Huszar, V.L.M.; Peeters, E.T.H.M.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Rücker, J.; Wiedner, C.; Zippel, P. Factors controlling the dominance of Planktothrix agardhii and Limnothrix redekei in eutrophic shallow lakes. Hydrobiologia 1997, 342, 107–115. [Google Scholar] [CrossRef]
- Fisher, T.R. Plâncton e produção primária em sistemas aquáticos da bacia da Amazônia Central. Acta Amazônica 1979, 8, 43–54. [Google Scholar] [CrossRef]
- Wood, E.J.F. A phytoplankton study of the Amazon Region. Bull. Mar. Sci. 1966, 102–123. [Google Scholar]
- Esteves, F.A. Fundamentos de Limnologia, 3rd ed.; Interciência: Rio de Janeiro, Brazil, 2011; p. 826. [Google Scholar]
- Viehman, T.S.; Richardson, L.L. Motility patterns of Beggiatoa and Phormidium corallyticum in black band disease. In Proceedings of the Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Volume 2, pp. 1251–1255. [Google Scholar]
- Myers, J.L.; Sekar, R.; Richardson, L.L. Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 2007, 73, 5173–5182. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.; Cunha, H.F.A.; Júnior, A.C.P.B.; Daniel, L.A.; Schulz, H.E. Qualidade microbiológica da água em rios de áreas urbanas e periurbanas no baixo Amazonas: O caso do Amapá. Eng. Sanit. e Ambient. 2004, 9, 322–328. [Google Scholar] [CrossRef]
- APHA-American Public Health Association. 10200 Plankton: 10200 B: Sample Collection. In Stand. Methods Exam. Wastewater; Rice, E.W., Ed.; APHA-American Public Health Association: Washington, DC, USA, 2005; pp. 2–31. [Google Scholar]
- INPE, CPTEC. Divisão de satélites e sistemas ambientais. Available online: http://satelite.cptec.inpe.br/radiacao/ (accessed on 29 April 2016).
- INMET. Precipitation and Air Temperature data for Macapá 2015 and 2016; INMET: Macapá, Brazil, 2016.
- ANA Séries Históricas de Estações. Available online: http://www.snirh.gov.br/hidroweb/publico/medicoes_historicas_abas.jsf (accessed on 29 April 2016).
- Nubel, U.; Muyzer, G.; Garcia-pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria PCR Primers To Amplify 16S rRNA Genes from Cyanobacteria. Microbiology 1997, 63, 3327–3332. [Google Scholar]
- Neilan, B.A.; Jacobs, D.; Therese, D.D.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA Sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Lopes, V.R.; Ramos, V.; Martins, A.; Sousa, M.; Welker, M.; Antunes, A.; Vasconcelos, V.M. Phylogenetic, chemical and morphological diversity of cyanobacteria from Portuguese temperate estuaries. Mar. Environ. Res. 2012, 73, 7–16. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schembri, M.A.; Neilan, B.A.; Saint, C.P. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 2001, 16, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales, 1st ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1999; p. 548. [Google Scholar]
- Ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team, 2017. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 15 November 2019).
- Oksanen, J. Constrained ordination: Tutorial with R and vegan. R—Packace Vegan. 2012, pp. 1–10. Available online: http://cc.oulu.fi/~jarioksa/opetus/metodi/sessio2.pdf (accessed on 15 November 2019).


| Sampling | Raw Water | Treated Water | ||
|---|---|---|---|---|
| mcyE | ELISA | mcyE | ELISA | |
| Apr15 | - | - | - | - |
| May | - | - | - | - |
| Jun | * 1 | + | + | + |
| Jul | + | + | + | + |
| Aug | - | + | - | - |
| Sep | - | - | - | - |
| Oct | - | - | - | - |
| Nov | - | - | - | - |
| Dec | - | - | - | - |
| Jan | - | - | - | - |
| Feb | - | - | - | - |
| Mar | - | - | - | - |
| Apr16 | - | - | - | - |
| Parameter | Raw Water | Treated Water | Brazilian Guideline Values for Drinking Water [20] | ||
|---|---|---|---|---|---|
| Average ± SD | Min–Max | Average ± SD | Min–Max | ||
| pH | 6.6 ± 0.28 | 6.0–7.2 | 6.0 ± 0.30 | 5.4–6.7 | In accordance |
| Dissolved Oxygen (mg L−1) | 6.0 ± 0.71 | 4.8–6.8 | 4.2 ± 0.20 | 3.8–4.5 | - 1 |
| Microcystin– LR (µg L−1) | 0.4 ± 0.69 | 0.0–2.1 | 0.01 ± 0.03 | 0.0–0.1 | In accordance |
| Nitrate (mg L−1) | 0.4 ± 0.83 | 0.0–2.5 | 0.9 ± 1.30 | 0.02–4.5 | In accordance |
| Ammonia (mg L−1) | 0.7 ± 0.54 | 0.05–1.6 | 0.1 ± 0.05 | 0.0–0.2 | In accordance |
| Orthophosphate (mg L−1) | 0.3 ± 0.44 | 0.02–1.6 | 0.13 ± 0.09 | 0.05–0.4 | - 1 |
| Chloride (mg L−1) | 8.7 ± 9.17 | 1.6–25.8 | 3.8 ± 1.70 | 1.8–6.8 | In accordance |
| Sulfate (mg L−1) | 1.8 ± 1.48 | 0.0–4.0 | 14.0 ± 3.60 | 9.0–21.0 | In accordance |
| Aluminum (mg L−1) | 0.1 ± 0.07 | 0.03–0.3 | 0.11 ± 0.06 | 0.006–0.2 | In accordance |
| Iron (mg L−1) | 2.2 ± 1.26 | 0.89–5.4 | 0.7 ± 1.10 | 0.07–3.9 | Above the guideline value 0.3 |
| Transparency (cm) | 25.3 ± 9.60 | 12.0–36.5 | - | - | - 1 |
| Euphotic Zone (cm) | 75.9 ± 28.90 | 36.0–109.5 | - | - | - 1 |
| Turbidity (NTU) | 55.5 ± 41.0 | 19.8–122.0 | 9.8 ± 13.50 | 1.4–48.9 | Above the guideline value 5.0 |
| Total Dissolved Solids (ppm) | 24.3 ± 4.41 | 20.0–30.0 | 31.2 ± 8.20 | 20.0–40.0 | In accordance |
| Electrical Conductivity (µS cm−1) | 50.4 ± 7.81 | 40.0–60.0 | 68.2 ± 10.70 | 50.0–80.0 | - 1 |
| Water Temperature (°C) | 28.8 ± 1.70 | 25.0–30.3 | 28.9 ± 1.70 | 24.4–30.7 | - 1 |
| Minimum Air Temperature (°C) | 24.3 ± 0.80 | 22.7–25.7 | 24.3 ± 0.80 | 22.7–25.7 | - 1 |
| Maximum Air Temperature (°C) | 33.2 ± 2.00 | 28.9–35.3 | 33.2 ± 2.00 | 28.9–35.3 | - 1 |
| Insolation (h) | 7.7 ± 3.40 | 1.3–10.6 | 7.7 ± 3.40 | 1.3–10.6 | - 1 |
| Irradiation (W m−2) | 241.9 ± 75.60 | 86.6–312.3 | 241.9 ± 75.60 | 86.6–312.3 | - 1 |
| Daily Rain Precipitation (mm) | 5.3 ± 7.60 | 0.0–22.4 | 5.3 ± 7.60 | 0.0–22.4 | - 1 |
| Monthly Rain Precipitation (mm) | 206.0 ± 209.00 | 0.0–528.2 | 206.0 ± 209.00 | 0.0–528.2 | - 1 |
| Water Level (m) | 3.1 ± 0.20 | 2.9–3.4 | 3.1 ± 0.20 | 2.9–3.4 | - 1 |
| Flow (m3 s−1) | 177.376 ± 72.82 | 89.3–279.9 | 177.376 ± 72.82 | 89.3–279.9 | - 1 |
| Total Coliforms (TC/100 mL) | 12838.7 ± 7239.23 | 2658.0–22494.0 | 0.0 | 0.0 | In accordance |
| E. coli (E. coli/100 mL) | 1082.7 ± 1832.57 | 68.0–6780.0 | 0.0 | 0.0 | In accordance |
| Cyanobacteria (cells mL−1) | 214.7 ± 277.81 | 89.4–1090.0 | - | - | In accordance |
| Type | Parameter | Unity | Method/Equipment |
|---|---|---|---|
| Chemical | Ph | pH | pH-meter OrionStar A121 Thermoscientific |
| Dissolved Oxygen | mg L−1 | YSI 550 A DO probe | |
| Microcystin- LR | µg L−1 | ELISA, molecular biology, LC-ESI-MS/MS | |
| Nitrate | mg L−1 | Reduction Cadmium/Spectrophotometer | |
| Ammonia | mg L−1 | Nessler/Spectrophotometer | |
| Orthophosphate | mg L−1 | Phosver3/Spectrophotometer | |
| Chloride | mg L−1 | Mercuric Thiocyanate/Spectrophotometer | |
| Sulfate | mg L−1 | Method Sulfaver/Spectrophotometer | |
| Aluminum | mg L−1 | Aluver/Spectrophotometer | |
| Iron | mg L−1 | Ferrover/Spectrophotometer | |
| Physical | Transparency | Cm | Secchi Disk |
| Euphotic Zone | Cm | Secchi Disk x 3.0 | |
| Turbidity | NTU | Turbidimeter AP2000 Policontrol | |
| Total Dissolved Solids | Ppm | Portable EC, TDS and Temperature meter HI8730 Hanna | |
| Electrical Conductivity | µS cm−1 | Portable EC, TDS and Temperature meter HI8730 Hanna | |
| Water Temperature | °C | pH-meter OrionStar A121 Thermoscientific | |
| Air Temperature Min | °C | INPE | |
| Air Temperature Max | °C | INPE | |
| Insolation | H | INPE | |
| Irradiation | W m−2 | INPE | |
| Daily Rain | Mm | Weather station/INMET | |
| Monthly Rain | Mm | Weather station/INMET | |
| Water Level | M | Tidal tables | |
| Flow | m3 s−1 | HidroWeb/ANA | |
| Microbiol. | Total Coliforms | TC 100 mL−1 | Chromogenic Substrate |
| E. coli | E. coli 100 mL−1 | Chromogenic Substrate | |
| Cyanobacteria | Cells mL−1 | Utermöhl |
| Gene | Primer | Primer Sequence 5’-3’ | Size (bp) | Reference | Positive Control |
|---|---|---|---|---|---|
| 16S | CYA106F/CYA781R | CGGACGGGTGAGTAACGCGTGA GACTACTGGGGTATCTAATCCCATT | 675 | [81] | - |
| 16S | CYA359F/1494R | GGGGAATYTTCCGCAATGGG TACGGCTACCTTGTTACGAC | 1135 | [81,82] | - |
| mcyA | CD1F/CD1R | AAAATTAAAAGCCGTATCAAA AAAAGTGTTTTATTAGCGGCTCAT | 297 | [83] | LEGE91339-Microcystis aeruginosa |
| mcyE | HEPF/HEPR | TTTGGGGTTAACTTTTTTGGGCATAGTC AATTCTTGAGGCTGTAAATCGGGTTT | 472 | [40] | LEGE91339-Microcystis aeruginosa |
| sxtI | SXTI 682F/SXTI 877R | GGATCTCAAAGAAGATGGCA GCCAAACGCAGTACCACTT | 100 | [84] | LMECYA 040-Aphanizomenon gracile |
| anaC | anaCF/anaCR | TCTGGTATTCAGTCCCCTCTAT CCCAATAGCCTGTCATCAA | 366 | [85] | LEGE X-002-Anabaena sp. |
| pks | M4/M5 | GAAGCTCTGGAATCCGGTAA AATCCTTACGGGATCCGGTGC | 535/ 540 | [86] | LEGE 97,047-Cylindrospermopsis raciborskii |
| ps | M13/ M14 | GGCAAATTGTGATAGCCACGAGC GATGGAACATCGCTCACTGGTG | 511/ 534 | [86] | LEGE 97,047-Cylindrospermopsis raciborskii |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D.C. Oliveira, E.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil. Toxins 2019, 11, 669. https://doi.org/10.3390/toxins11110669
D.C. Oliveira E, Castelo-Branco R, Silva L, Silva N, Azevedo J, Vasconcelos V, Faustino S, Cunha A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil. Toxins. 2019; 11(11):669. https://doi.org/10.3390/toxins11110669
Chicago/Turabian StyleD.C. Oliveira, Elane, Raquel Castelo-Branco, Luis Silva, Natalina Silva, Joana Azevedo, Vitor Vasconcelos, Silvia Faustino, and Alan Cunha. 2019. "First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil" Toxins 11, no. 11: 669. https://doi.org/10.3390/toxins11110669
APA StyleD.C. Oliveira, E., Castelo-Branco, R., Silva, L., Silva, N., Azevedo, J., Vasconcelos, V., Faustino, S., & Cunha, A. (2019). First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil. Toxins, 11(11), 669. https://doi.org/10.3390/toxins11110669







