A Preliminary Survey of Cultured Fusaria from Symptomatic Legume Grains in North-Eastern Poland
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
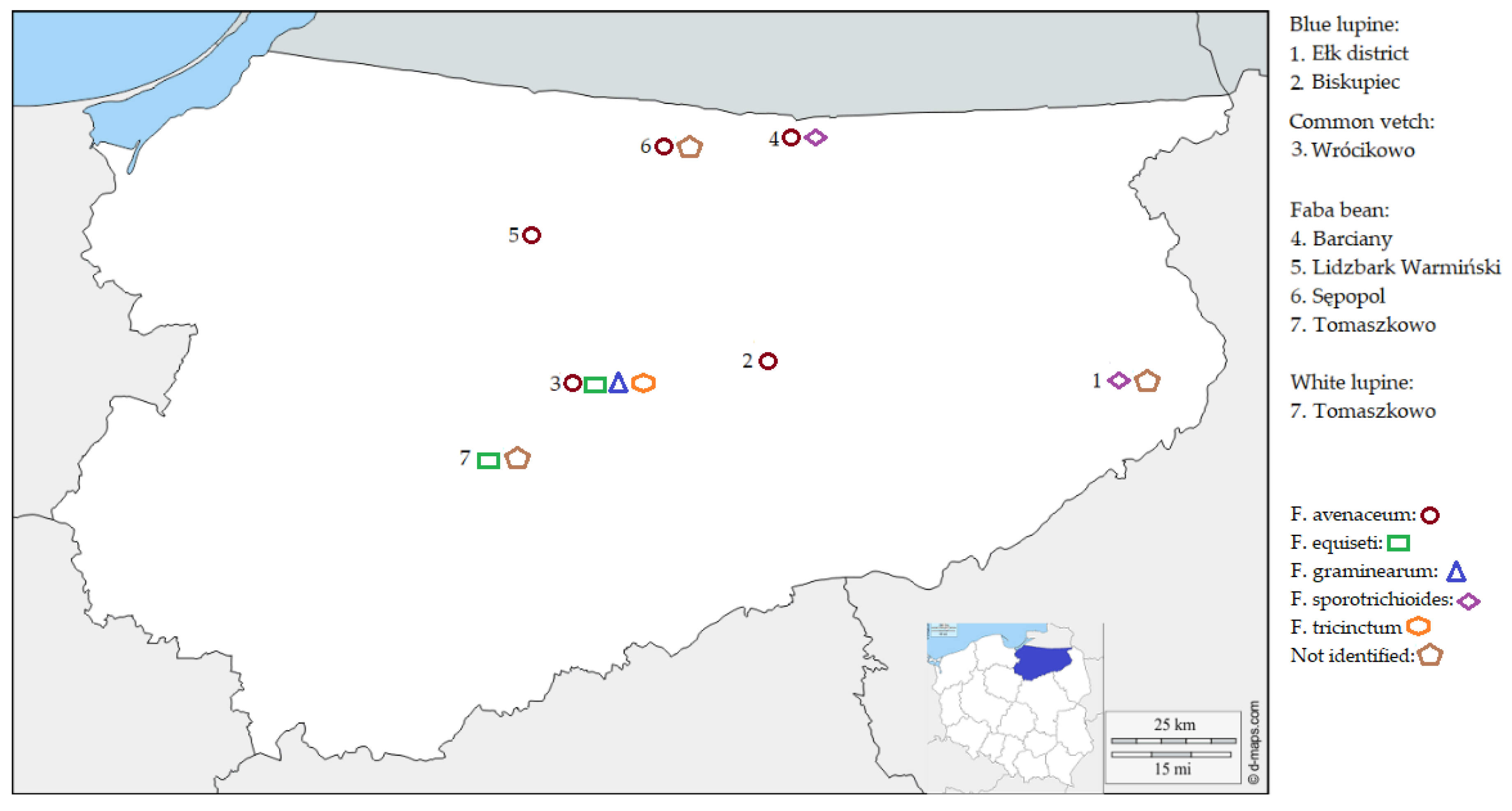
3.1. Legume Grain Samples
3.2. DNA Isolation and Species Identification
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Editorial: Protein Crops: Food and Feed for the Future. Front. Plant Sci. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food: Improve Health through Adequate Food, Edition 2017; Chavarri, M., Ed.; Intech Open: Rijeka, Croatia, 2017; Chapter 6; pp. 103–109. [Google Scholar]
- de Visser, C.; Schreuder, R.; Stoddard, F. The EU’s dependence on soya bean import for the animal feed industry and potential for EU produced alternatives. Oilseeds Fats Crop. Lipids 2014, 21, D407. [Google Scholar]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health benefits and culinary approaches to increase intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W. The occurrence of Fusarium species in Canada: VI. taxonomy and geographic distribution of Fusarium species on plants, insects, and fungi. Can. J. Bot. 1959, 37, 257–290. [Google Scholar] [CrossRef]
- Kurmut, A.; Nirenberg, H.; Bochow, H.; Buttner, C. Fusarium nygamai, causal agent of root rot of Vicia faba L. in the Sudan. Meded. -Fac. Landbouwkd. En Toegep. Biol. Wet. 2002, 67, 269–274. [Google Scholar]
- Pszczółkowska, A.; Okorski, A.; Fordoński, G.; Prusiński, J.; Faligowska, A.; Borowska, M. Fungal colonization of seeds of three lupine species in different regions of Poland. Acta Agrobot. 2017, 70. [Google Scholar] [CrossRef]
- Duke, J. Handbook of Legumes of World Economic Importance, 1st ed.; Plenum Press: New York, NY, USA; London, UK, 1981; pp. 281–283. [Google Scholar]
- Gorfu, D. Yield loss of faba bean caused by root rot (Fusarium avenaceum) [Vicia faba]. Faba Bean Inf. Serv. 1993, 33, 24–27. [Google Scholar]
- Sadowski, S. Występowanie chorób bobiku (Vicia faba L.) w rejonach olsztyńsko-elbląskim i bydgoskim [Occurrence of broad bean (Vicia faba L.) diseases in Olsztyn-Elbąg and Bydgoszcz Provinces]. Acta Agrobot. 1988, 41, 245–255. [Google Scholar] [CrossRef]
- Kulik, T.; Fordoński, G.; Pszczółkowska, A.; Płodzień, K.; Olszewski, J. Identyfikacja wybranych gatunków grzybów z rodzaju Fusarium z nasion niektórych gatunków roślin uprawnych metodą tradycyjnąa i BIO-PCR. Acta Agrobot. 2005, 58, 33–54. [Google Scholar] [CrossRef]
- Gleń, K.; Boligłowa, E.; Gospodarek, J. Grzyby zasiedlające nasiona bobu w zależności od sposobu ochrony roślin. Pol. J. Agron. 2013, 12, 9–16. [Google Scholar]
- Podleśny, J.; Podleśna, A.; Bieniaszewski, T. Occurrence of fungal diseases on blue lupine (Lupinus angustifolius L.) plants at different regions of PolandWystępowanie chorób grzybowych na roślinach łubinu wąskolistnego (Lupinus angustifolius L.) w różnych rejonach Polski. Prog. Plant Prot. 2016, 56, 25–33. [Google Scholar]
- Clarkson, J.D.S. Pathogenicity of Fusarium spp Associated with Foot-Rots of Peas and Beans. Plant Pathol. 1978, 27, 110–117. [Google Scholar] [CrossRef]
- Elwakil, M.; El-Refai, I.; Awadallah, O.; El-Metwally, M.; Mohammed, M. Seed-borne pathogens of faba bean in Egypt: Detection and pathogencity. Plant Pathol. J. 2009, 8, 90–97. [Google Scholar] [CrossRef]
- Belete, E.; Ayalew, A.; Ahmed, S. Associations of biophysical factors with faba bean root rot (Fusarium solani) epidemics in the northeastern highlands of Ethiopia. Crop Prot. 2013, 52, 39–46. [Google Scholar] [CrossRef]
- Neamat, A.K.; Abbas, M.S.; Sobhy, H.M.; Abou-Zeid, N.M.; Mahmoud, N.A. Induction of Systemic Resistance in Faba Bean Plants against Fusarium oxysporum the Causal of Wilt Disease. Egypt. J. Biol. Pest Control 2016, 26, 431–438. [Google Scholar]
- Miličević, T.; Kaliterna, J.; Ivić, D.; Stričak, A. Identification and occurrence of Fusarium species on seeds of common wetch, white lupine and some wild legumes. Poljoprivreda 2013, 19, 25–32. [Google Scholar]
- Liu, C.M.; Kachur, S.; Dwan, M.G.; Abraham, A.G.; Aziz, M.; Hsueh, P.R.; Huang, Y.T.; Busch, J.D.; Lamit, L.J.; Gehring, C.A.; et al. FungiQuant: A broad-coverage fungal quantitative real-time PCR assay. BMC Microbiol. 2012, 12, 255. [Google Scholar] [CrossRef]
- Waalwijk, C.; van der Heide, R.; de Vries, I.; van der Lee, T.; Schoen, C.; Costrel-de Corainville, G.; Hauser-Hahn, I.; Kastelein, P.; Kohl, J.; Lonnet, P.; et al. Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 2004, 110, 481–494. [Google Scholar] [CrossRef]
- Bilska, K.; Kulik, T.; Ostrowska-Kolodziejczak, A.; Busko, M.; Pasquali, M.; Beyer, M.; Baturo-Ciesniewska, A.; Juda, M.; Zaluski, D.; Treder, K.; et al. Development of a Highly Sensitive FcMito qPCR Assay for the Quantification of the Toxigenic Fungal Plant Pathogen Fusarium culmorum. Toxins 2018, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, M.; Supronien, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef]
- Kulik, T.; Ostrowska, A.; Busko, M.; Pasquali, M.; Beyer, M.; Stenglein, S.; Zaluski, D.; Sawicki, J.; Treder, K.; Perkowski, J. Development of an FgMito assay: A highly sensitive mitochondrial based qPCR assay for quantification of Fusarium graminearum sensu stricto. Int. J. Food Microbiol. 2015, 210, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Jestoi, M.; Okorski, A. Development of TaqMan assays for the quantitative detection of Fusarium avenaceum/Fusarium tricinctum and Fusarium poae esyn1 genotypes from cereal grain. FEMS Microbiol. Lett. 2011, 314, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T. Development of TaqMan Assays for 3ADON, 15ADON and NIV Fusarium Genotypes Based on Tri12 Gene. Cereal Res. Commun. 2011, 39, 200–214. [Google Scholar] [CrossRef]
- Kulik, T.; Pszczolkowska, A.; Lojko, M. Multilocus Phylogenetics Show High Intraspecific Variability within Fusarium avenaceum. Int. J. Mol. Sci. 2011, 12, 5626–5640. [Google Scholar] [CrossRef] [PubMed]
- Haese, A.; Schubert, M.; Herrmann, M.; Zocher, R. Molecular Characterization of the Enniatin Synthetase Gene Encoding a Multifunctional Enzyme Catalyzing N-Methyldepsipeptide Formation in Fusarium-Scirpi. Mol. Microbiol. 1993, 7, 905–914. [Google Scholar] [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Zelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Villani, A.; Proctor, R.H.; Kim, H.-S.; Brown, D.W.; Logrieco, A.F.; Amatulli, M.T.; Moretti, A.; Susca, A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019, 20, 314. [Google Scholar] [CrossRef]
- Hartman, G.L.; McCormick, S.P.; O’Donnell, K. Trichothecene-Producing Fusarium Species Isolated from Soybean Roots in Ethiopia and Ghana and their Pathogenicity on Soybean. Plant Dis. 2019, 103, 2070–2075. [Google Scholar] [CrossRef]
- Jacobs, A.; Mojela, L.; Summerell, B.; Venter, E. Characterisation of members of the Fusarium incarnatum-equiseti species complex from undisturbed soils in South Africa. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018, 111, 1999–2008. [Google Scholar] [CrossRef]
- Gilbert, J.; Fernando, W.G.D. Epidemiology and biological control of Gibberella zeae Fusarium graminearum. Can. J. Plant Pathol. -Rev. Can. De Phytopathol. 2004, 26, 464–472. [Google Scholar] [CrossRef]
- Ruralis, E.; Chancellor, C.; Attila, S.B.M.; Duminicioiu, R. The Trouble with Soy: The Threats to Small-Scale Producers Across Europe; European Coordination Via Campesina (ECVC) and Eco Ruralis: Cluj Napoca, Romania, 2018. [Google Scholar]
| Fusarium Species | Host | Geographic Location | Sampling Season | References |
|---|---|---|---|---|
| F. acuminatum | Common vetch | Canada | - | [7] |
| F. acutatum | Faba bean | Sudan | - | [8] |
| F. avenaceum | Blue lupine | Poland | 2011–2013 | [9] |
| Common vetch | - | - | [10] | |
| Faba bean | Ethiopia | 1993 | [11] | |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| White lupine | Poland | 2011 | [9] | |
| F. culmorum | Faba bean | Poland | 1981–1984 | [12] |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| F. compactum | Faba bean | Sudan | - | [8] |
| F. equiseti | Blue lupine | Poland | 2012 | [9] |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| White lupine | Poland | 2011 | [9] | |
| F. graminearum | Faba bean | Poland | 2001 | [13] |
| F. nygamai | Faba bean | Sudan | - | [8] |
| F. oxysporum | Blue lupine | Poland | 2010–2012 | [15] |
| Common vetch | Canada | - | [7] | |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | United Kingdom | 1973 | [16] | |
| Faba bean | Sudan | - | [8] | |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Egypt | 2004–2005 | [17] | |
| Faba bean | Ethiopia | 2010–2011 | [18] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| Faba bean | Egypt | - | [19] | |
| F. poae | Blue lupine | Poland | 2012–2013 | [9] |
| White lupine | Poland | 2011–2012 | [9] | |
| F. proliferatum | Faba bean | Sudan | - | [8] |
| White lupine | Croatia | - | [20] | |
| F. semitectum | Faba bean | Sudan | - | [8] |
| Faba bean | Egypt | 2004–2005 | [17] | |
| F. solani | Faba bean | Poland | 1981–1984 | [12] |
| Faba bean | United Kingdom | 1973 | [16] | |
| Faba bean | Sudan | - | [8] | |
| Faba bean | Egypt | 2004–2005 | [17] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| Faba bean | Ethiopia | 2010–2011 | [18] | |
| F. sporotrichioides | Blue lupine | Poland | 2013 | [9] |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| F. tricinctum | Blue lupine | Poland | 2012–2013 | [9] |
| F. verticillioides | Common vetch | Croatia | - | [20] |
| Faba bean | Egypt | 2004–2005 | [17] | |
| White lupine | Croatia | - | [20] |
| Specificity of the qPCR Assay | Primer/Probe Sequence | Reaction Reagents | Reaction Conditions | References |
|---|---|---|---|---|
| Total fungal DNA | ||||
| FungiQuant | F: GGRAAACTCACCAGGTCCAG | A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [21] |
| R: GSWCTATCCCCAKCACGA | ||||
| Probe: (6FAM)-TGGTGCATGGCCGTT-(MGBNFQ) | ||||
| Species | ||||
| F. avenaceum | F: CCATCGCCGTGGCTTTC R: CAAGCCCACAGACACGTTGT Probe: FAM-ACGCAATTGACTATTGC-MGB | B | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 50 s) × 40 | [22] |
| F. culmorum | F: TCGTTGACGGTGAGGGTTGT R:GACTCGAACACGTCAACCAACT Probe: FAM-CGGTTATTATTTCGAAAAGT- MGB | A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [23] |
| F. equiseti | F: CACCGTCATTGGTATGTTGTCATC R: TGTTAGCATGAGAAGGTCATGAGTG | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. graminearum s.s. | F: TGGCCTGAATGAAGGATTTCTAG R: CATCGTTGTTAACTTATTGGAGATG Probe: FAM-TTAAACACTCAAACACTACA- MGB | A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [25] |
| F. langsethiae | F: CAAGTCGACCACTGTGAGTACCTCT R: TGTCAAAGCATGTCAGTAAAGATGAC | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. poae | F: AAATCGGCGTATAGGGTTGAGATA R: GCTCACACAGAGTAACCGAAACCT Probe: FAM-CAAAATCACCCAACCGACCCTTTC-TAMRA | B | 50 °C for 2 min, 95 °C for 10 min, (95 °C for 15 s, 60 °C for 60 s) × 40 | [22] |
| F. proliferatum | F: CTTCGATCGCGCGTCCT R: CACGTTTCGAATCGCAAGTG | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. sporotrichioides | F: GCAAGTCGACCACTGTGAGTACA R: CTGTCAAAGCATGTCAGTAAAAATGAT | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. subglutinans | F: TCATTGGTATGTTGTCGCTCATG R: GTGATATGTTAGTACGAATAAAGGGAGAAC | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. verticillioides | F: CGTTTCTGCCCTCTCCCA R: TGCTTGACACGTGACGATGA | C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| Enniatin genotype | ||||
| esyn1 | F: AGCAGTCGAGTTCGTCAACAGA R: GGCYTTTCCTGCGAACTTG Probe: FAM-CCGTCGAGTCCTCT-MGB | B | 95 °C for 20 s, (95 °C for 3 s, 60 °C for 30 s) × 40 | [26] |
| Tri genotypes | ||||
| 3ADON | F: CATGCGGGACTTTGATCGAT | B | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 50 s) × 40 | [27] |
| R: TTTGTCCGCTTTCTTTCTATCATAAA | ||||
| Probe: FAM-CTCACCGATCATGTTC-MGB | ||||
| 15ADON | F: TCCAATCATTGCCAGCCTCTA | |||
| R: TGATGCGGAACATGGTCTGT | ||||
| Probe: FAM-ATGAGGGACTTTGACCAAT-MGB | ||||
| NIV | F: TCGCCAGTCTCTGCATGAAG | |||
| R: CCTTATCCGCTTTCTTTCTATCATAAA | ||||
| Probe: FAM-CTGATCATGTCCCGCATC-MGB |
| Plant Host | F. avenaceum | F. equiseti | F. graminearum s.s. | F. sporotrichioides | F. tricinctum | Not Identified |
|---|---|---|---|---|---|---|
| Blue lupine | 7 | - | - | 3 | - | 1 |
| Common vetch | 7 | 9 | 1 | - | 1 | - |
| Faba bean | 7 | - | - | 1 | - | 4 |
| White lupine | - | 1 | - | - | - | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żelechowski, M.; Olszewski, J.; Kulik, T. A Preliminary Survey of Cultured Fusaria from Symptomatic Legume Grains in North-Eastern Poland. Toxins 2019, 11, 569. https://doi.org/10.3390/toxins11100569
Żelechowski M, Olszewski J, Kulik T. A Preliminary Survey of Cultured Fusaria from Symptomatic Legume Grains in North-Eastern Poland. Toxins. 2019; 11(10):569. https://doi.org/10.3390/toxins11100569
Chicago/Turabian StyleŻelechowski, Maciej, Jacek Olszewski, and Tomasz Kulik. 2019. "A Preliminary Survey of Cultured Fusaria from Symptomatic Legume Grains in North-Eastern Poland" Toxins 11, no. 10: 569. https://doi.org/10.3390/toxins11100569
APA StyleŻelechowski, M., Olszewski, J., & Kulik, T. (2019). A Preliminary Survey of Cultured Fusaria from Symptomatic Legume Grains in North-Eastern Poland. Toxins, 11(10), 569. https://doi.org/10.3390/toxins11100569






