The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro
Abstract
1. Introduction
2. Results
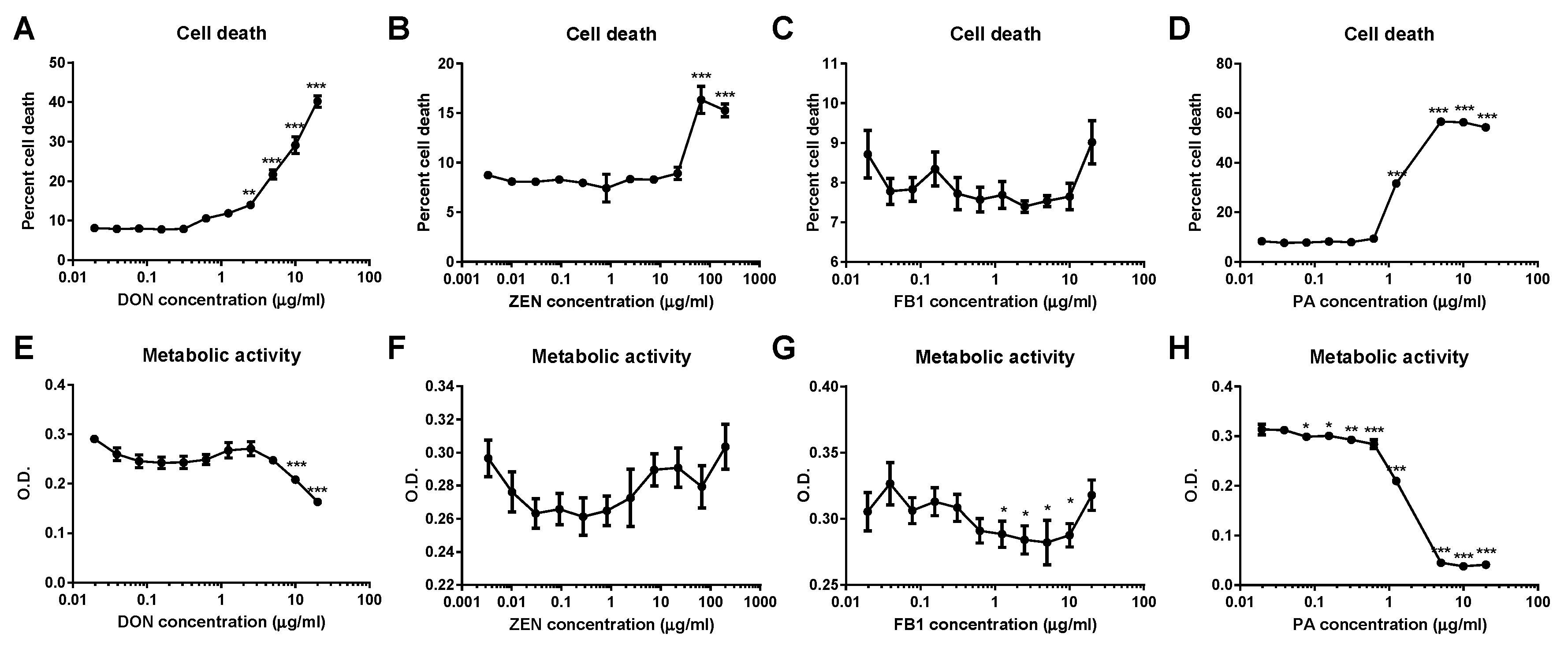
2.1. DON and ZEN Are Cytotoxic at High Doses but Do Not Reduce Cell Metabolism at Lower Doses
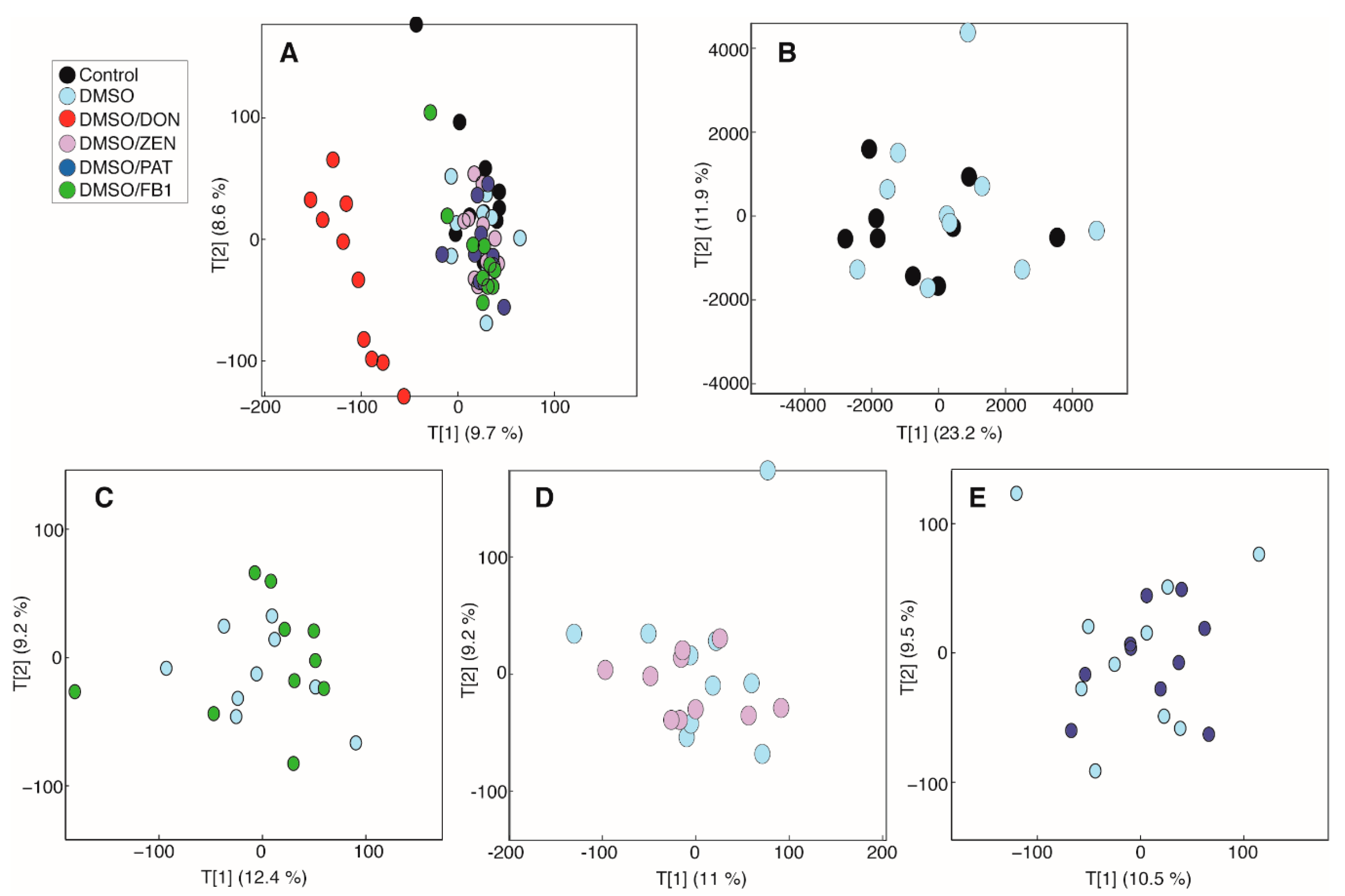
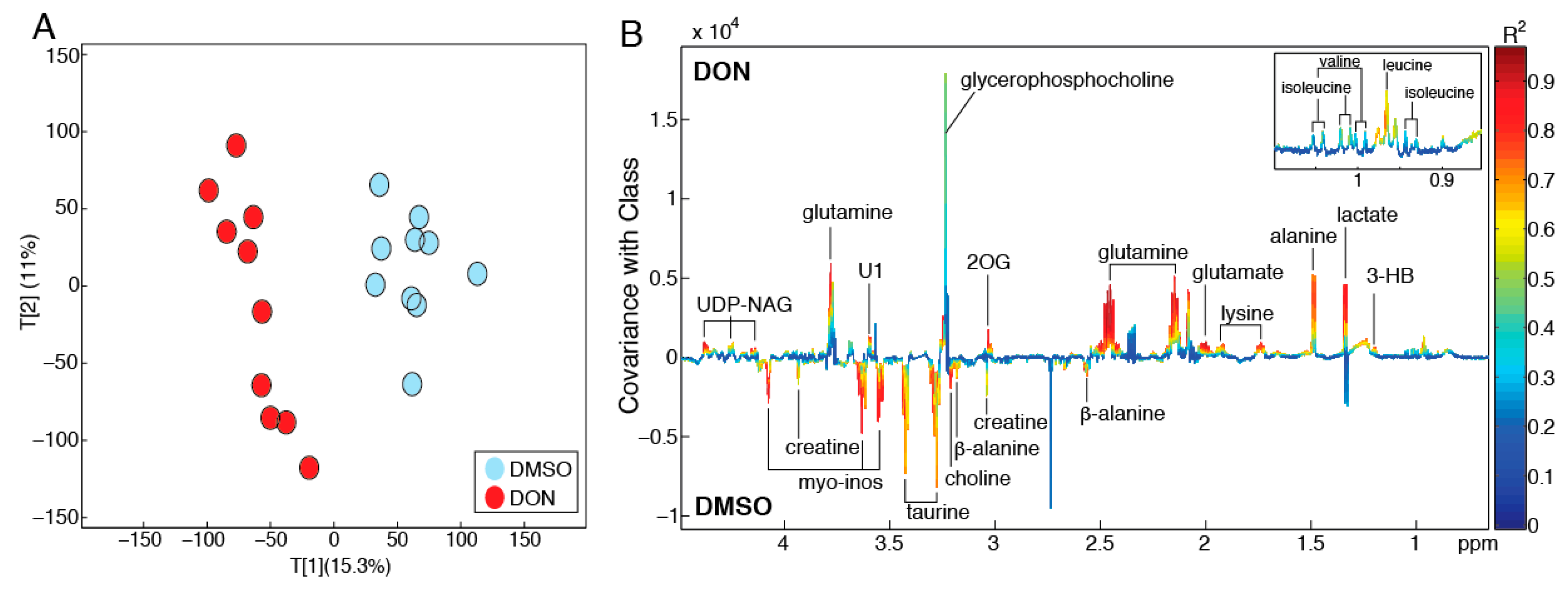
2.2. DON Induces Metabolic Perturbations in MDBK Cells
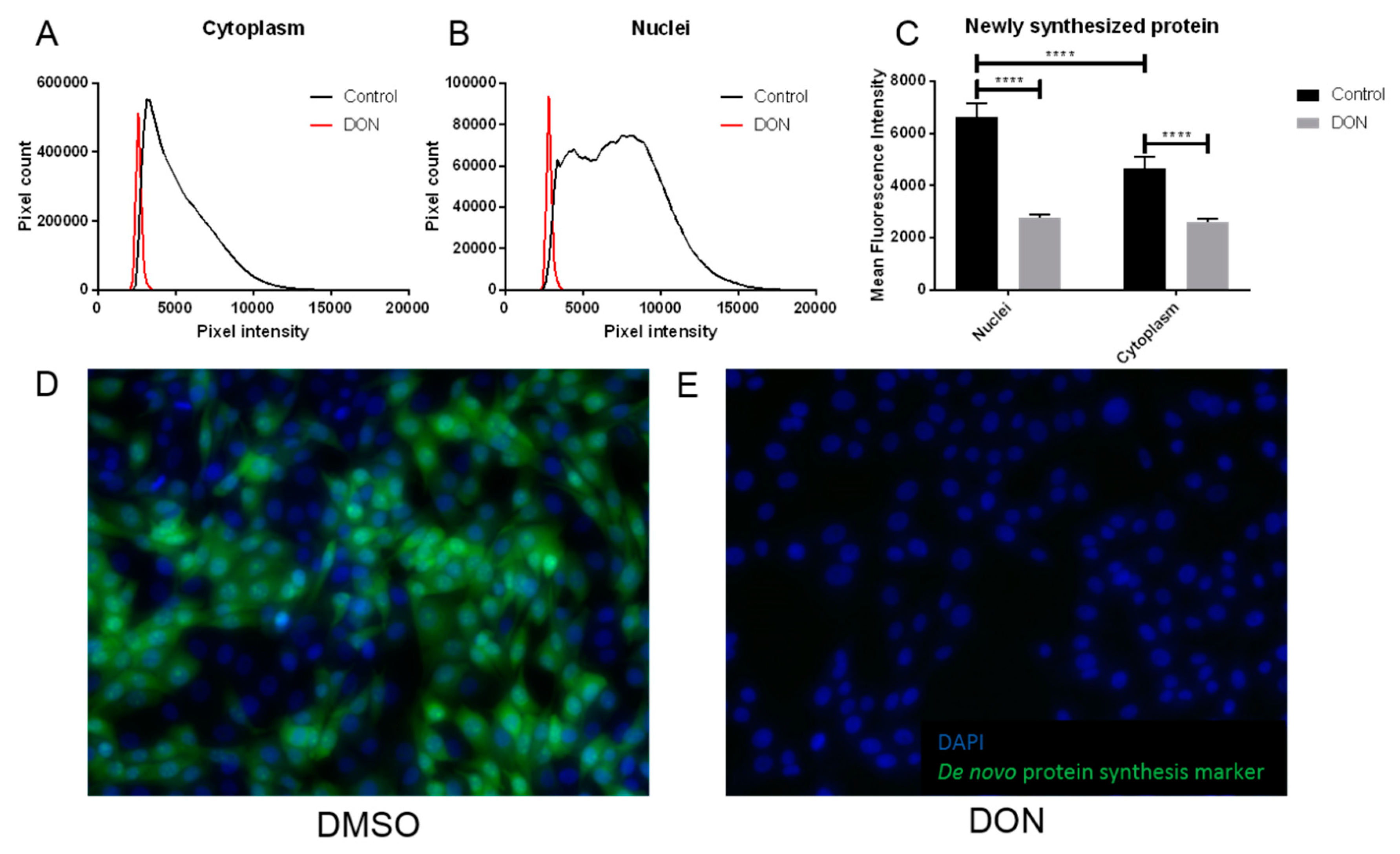
2.3. MDBK Cells Exposed to DON Showed Reduced Levels of Protein Synthesis
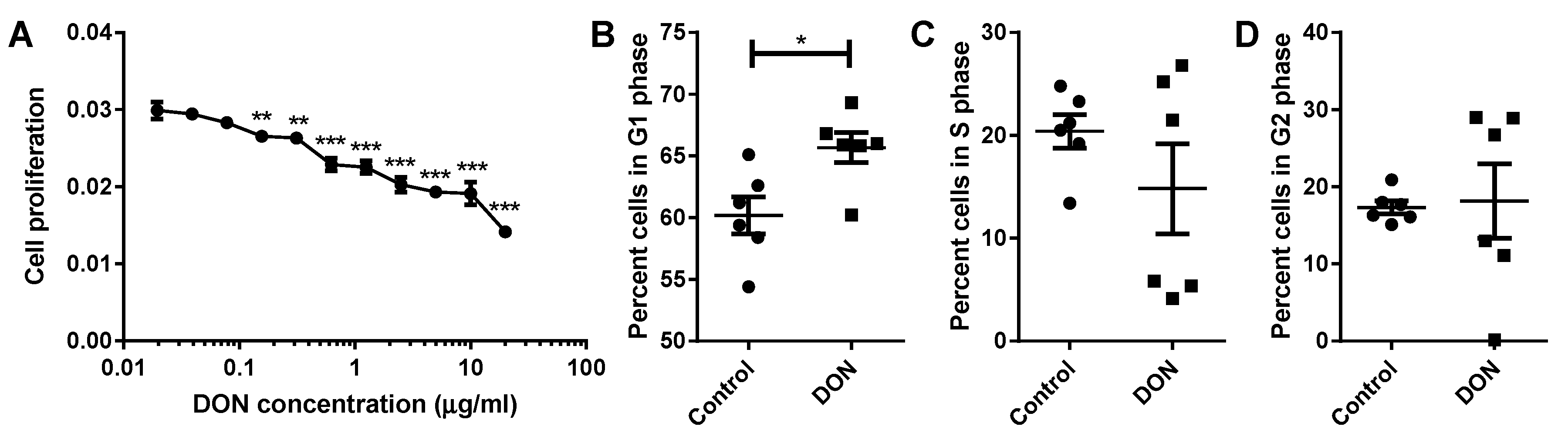
2.4. A Reduction in MDBK Proliferation Was Observed after DON Treatment
3. Discussion
3.1. DON Inhibits Protein Synthesis and Arrests the Cell Cycle in Bovine Renal Epithelial Cells
3.2. DON Disrupts Both Cellular and Mitochondrial Lipid Membrane Integrity, Likely Triggering Both Osmoregulatory Responses and the Hypoxia-Induced Factor (HIF) Pathway in Bovine Kidney Cells
4. Conclusions
5. Materials and Methods
5.1. Determination of Cytotoxicity and Impact of Mycotoxins on Metabolic Activity of Bovine Kidney Epithelial Cells
5.2. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy-Based Metabolic Phenotyping
5.3. De Novo Synthesis of Protein by Bovine Kidney Epithelial Cells Following DON Exposure
5.4. Proliferation of Bovine Kidney Epithelial Cells Following Exposure to DON
5.5. Cell Cycle Analysis of Bovine Kidney Epithelial Cells Exposed to DON
Author Contributions
Funding
Conflicts of Interest
References
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef]
- Oswald, I.P.; Marin, D.E.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F.; Oswald, I. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Marczuk, J.; Obremski, K.; Lutnicki, K.; Gajęcka, M.; Gajęcki, M. Zearalenone and deoxynivalenol mycotoxicosis in dairy cattle herds. Pol. J. Vet. Sci. 2012, 15, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Vilar, M.; Maas, R.F.M.; De Bosschere, H.; Ducatelle, R.; Fink-Gremmels, J.; Bosschere, H. Patulin produced by an Aspergillus clavatus isolated from feed containing malting residues associated with a lethal neurotoxicosis in cattle. Mycopathology 2004, 158, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.F.; Girgin, G.; Baydar, T. Occurrence, prevention and limitation of mycotoxins in feeds. Anim. Nutr. Feed Technol. 2015, 1515, 471–490. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Responses of dairy cows to dietary aflatoxin: Feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 1982, 65, 1503–1508. [Google Scholar] [CrossRef]
- Kiyothong, K.; Rowlinson, P.; Wanapat, M.; Khampa, S. Effect of mycotoxin deactivator product supplementation on dairy cows. Anim. Prod. Sci. 2012, 52, 832–841. [Google Scholar] [CrossRef]
- Morgavi, D.; Riley, R.; Morgavi, D. An historical overview of field disease outbreaks known or suspected to be caused by consumption of feeds contaminated with Fusarium toxins. Anim. Feed Sci. Technol. 2007, 137, 201–212. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Vasanthi, S.; Bhat, R.V. Mycotoxins in foods—Occurrence, health and economic significance and food control measures. Indian J. Med. Res. 1998, 108, 212–224. [Google Scholar] [PubMed]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 6868, 1–19. [Google Scholar] [CrossRef]
- D’Mello, J.; Placinta, C.; Macdonald, A. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Dänicke, S.; Seeling, K. Relevance of the Fusarium toxins deoxynivalenol and zearalenone in ruminant nutrition. A review. J. Anim. Feed Sci. 2005, 14, 3–40. [Google Scholar]
- Cogan, T.; Hawkey, R.; Higgie, E.; Lee, M.R.F.; Mee, E.; Parfitt, D.; Raj, J.; Roderick, S.; Walker, N.; Ward, P.; et al. Silage and total mixed ration hygienic quality on commercial farms: Implications for animal production. Grass Forage Sci. 2017, 72, 601–613. [Google Scholar] [CrossRef]
- HY-SIL Collaborators, HY-SIL Project Farm Study on Silage and Animal Health. 2015. Available online: http://www.bovmycotox.co.uk/wp-content/uploads/2015/08/MycotoxinFarmSurvey2015.pdf. (accessed on 5 October 2018).
- Scudamore, K.; Livesey, C.T. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 1998, 77, 1–17. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 33, 94–99. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- May, H.D.; Wu, Q.; Blake, C.K. Effects of the Fusarium spp. mycotoxins fusaric acid and deoxynivalenol on the growth of Ruminococcus albus and Methanobrevibacter ruminantium. Can. J. Microbiol. 2000, 46, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Vatzia, E.; Springler, A.; Pierron, A.; Gerner, W.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G.; Mayer, E. Bovine peripheral blood mononuclear cells are more sensitive to deoxynivalenol than those derived from poultry and swine. Toxins 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Chorfi, Y.; Guerrero-Netro, H.M.; Price, C. The mycotoxin metabolite deepoxy-deoxynivalenol increases apoptosis and decreases steroidogenesis in bovine ovarian theca cells. Biol. Reprod. 2017, 97, 746–757. [Google Scholar]
- Pizzo, F.; Caloni, F.; Schreiber, N.B.; Cortinovis, C.; Spicer, L.J. In vitro effects of deoxynivalenol and zearalenone major metabolites alone and combined, on cell proliferation, steroid production and gene expression in bovine small-follicle granulosa cells. Toxicon 2016, 109, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, V.I.; Anderson, P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999, 274, 13985–13992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-R.; Lau, A.S.; Pestka, J.J. Role of double-stranded RNA-Activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol. Sci. 2003, 74, 335–344. [Google Scholar] [CrossRef]
- Li, M.; Pestka, J.J. Comparative induction of 28S ribosomal RNA cleavage by ricin and the trichothecenes deoxynivalenol and T-2 toxin in the macrophage. Toxicol. Sci. 2008, 105, 67–78. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. MAP kinase pathways. In Molecular Cell Biology, 6th ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2000. [Google Scholar]
- Yang, H.; Chung, D.H.; Kim, Y.B.; Choi, Y.H.; Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology 2008, 243, 145–154. [Google Scholar] [CrossRef]
- Li, D.; Ye, Y.; Lin, S.; Deng, L.; Fan, X.; Zhang, Y.; Deng, X.; Li, Y.; Yan, H.; Ma, Y. Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: Cell-Cycle arrest, oxidative stress, and apoptosis. Environ. Toxicol. Pharmacol. 2014, 37, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Diesing, A.-K.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H.-J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE 2011, 6, e17472. [Google Scholar] [CrossRef] [PubMed]
- Moonen, L.; D’Haese, P.C.; Vervaet, B.A. Epithelial cell cycle behaviour in the injured kidney. Int. J. Mol. Sci. 2018, 19, 2038. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V.; Krishnaswamy, D.R. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-κB nuclear localization and down regulation of NF-κB and Cyclo-Oxygenase—2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Buffington, D.A.; Humes, H.D. Kidney epithelial cells. In Methods in Enzymology; Klimanskaya, I., Lanza, R., Eds.; Academic Press: Cambridge, MA, USA, 2006; pp. 194–207. [Google Scholar]
- Gallazzini, M.; Burg, M.B. What’s new about osmotic regulation of glycerophosphocholine. Physiology 2009, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Horio, M.; Yamauchi, A.; Moriyama, T.; Imai, E.; Orita, Y. Osmotic regulation of amino acids and system A transport in Madin-Darby canine kidney cells. Am. J. Physiol. Cell Physiol. 1997, 272, C804–C809. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W.; Han, X.; Patters, A.B. Taurine and the renal system. J. Biomed. Sci. 2010, 17 (Suppl. 1), S4. [Google Scholar] [CrossRef]
- Jones, D.P.; Miller, L.A.; Chesney, R.W. Polarity of taurine transport in cultured renal epithelial cell lines: LLC-PK1 and MDCK. Am. J. Physiol. Renal Physiol. 1993, 265, F137–F145. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, P.; Rajashree, K.; Priya, L.B.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Vaz, F.M.; Wanders, R.J.A. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Mechanisms of hypoxia responses in renal tissue. J. Am. Soc. Nephrol. 2013, 24, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Usman, I.M.; Sun, L.; Kanwar, Y.S. Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1304–1321. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.J.; Amiri, M.; Dick, F.A. Analysis of cell cycle position in mammalian cells. J. Vis. Exp. JoVE 2012, 59, e3491. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, J.R.; Breton, J.; Panic, G.; Cogan, T.A.; Bailey, M.; Swann, J.R.; Lee, M.R.F. The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins 2019, 11, 554. https://doi.org/10.3390/toxins11100554
Bailey JR, Breton J, Panic G, Cogan TA, Bailey M, Swann JR, Lee MRF. The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins. 2019; 11(10):554. https://doi.org/10.3390/toxins11100554
Chicago/Turabian StyleBailey, Jennifer R., Jonathan Breton, Gordana Panic, Tristan A. Cogan, Michael Bailey, Jonathan R. Swann, and Michael R. F. Lee. 2019. "The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro" Toxins 11, no. 10: 554. https://doi.org/10.3390/toxins11100554
APA StyleBailey, J. R., Breton, J., Panic, G., Cogan, T. A., Bailey, M., Swann, J. R., & Lee, M. R. F. (2019). The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins, 11(10), 554. https://doi.org/10.3390/toxins11100554





