Notes on the Cultivation of Two Mixotrophic Dinophysis Species and Their Ciliate Prey Mesodinium rubrum
Abstract
1. Introduction
2. Results
2.1. Optimizing Culture Medium for Phototrophic Growth of D. acuminata and D. acuta
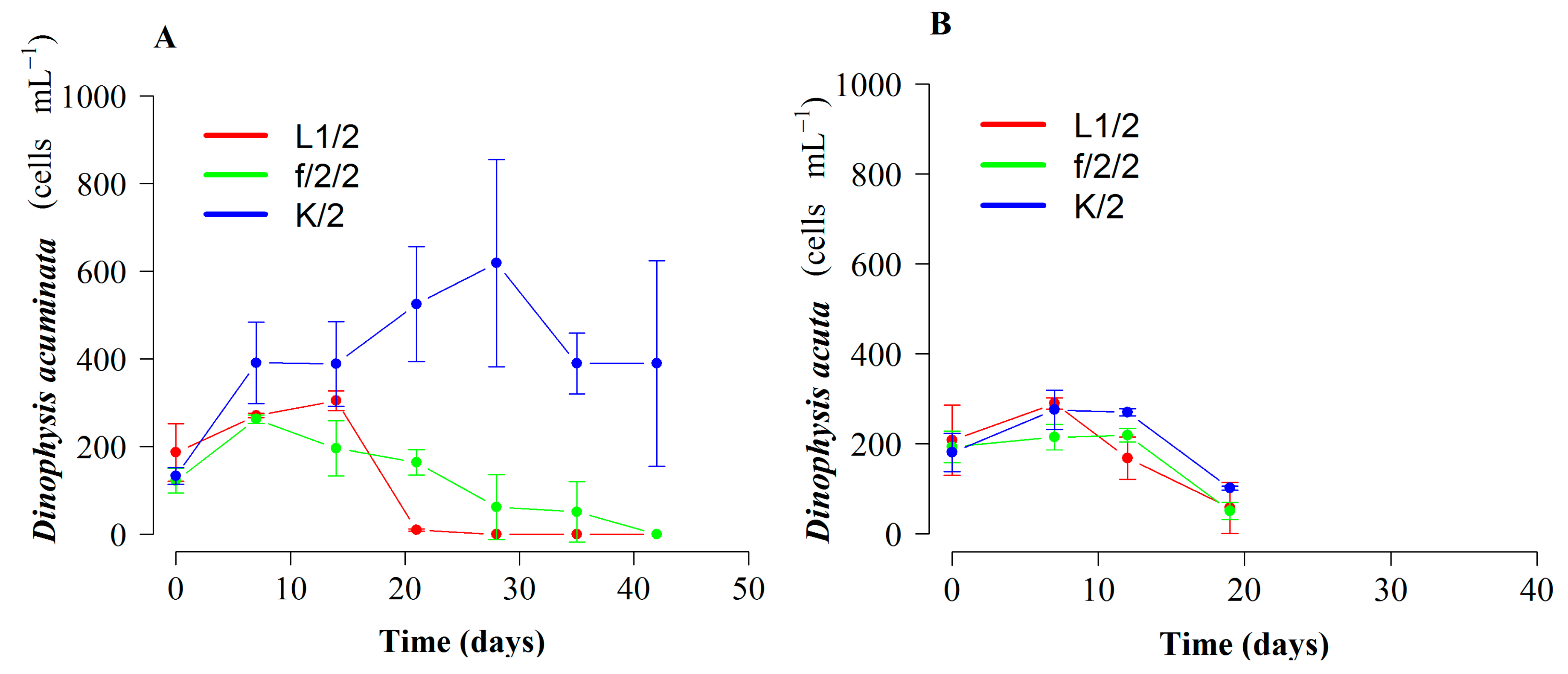
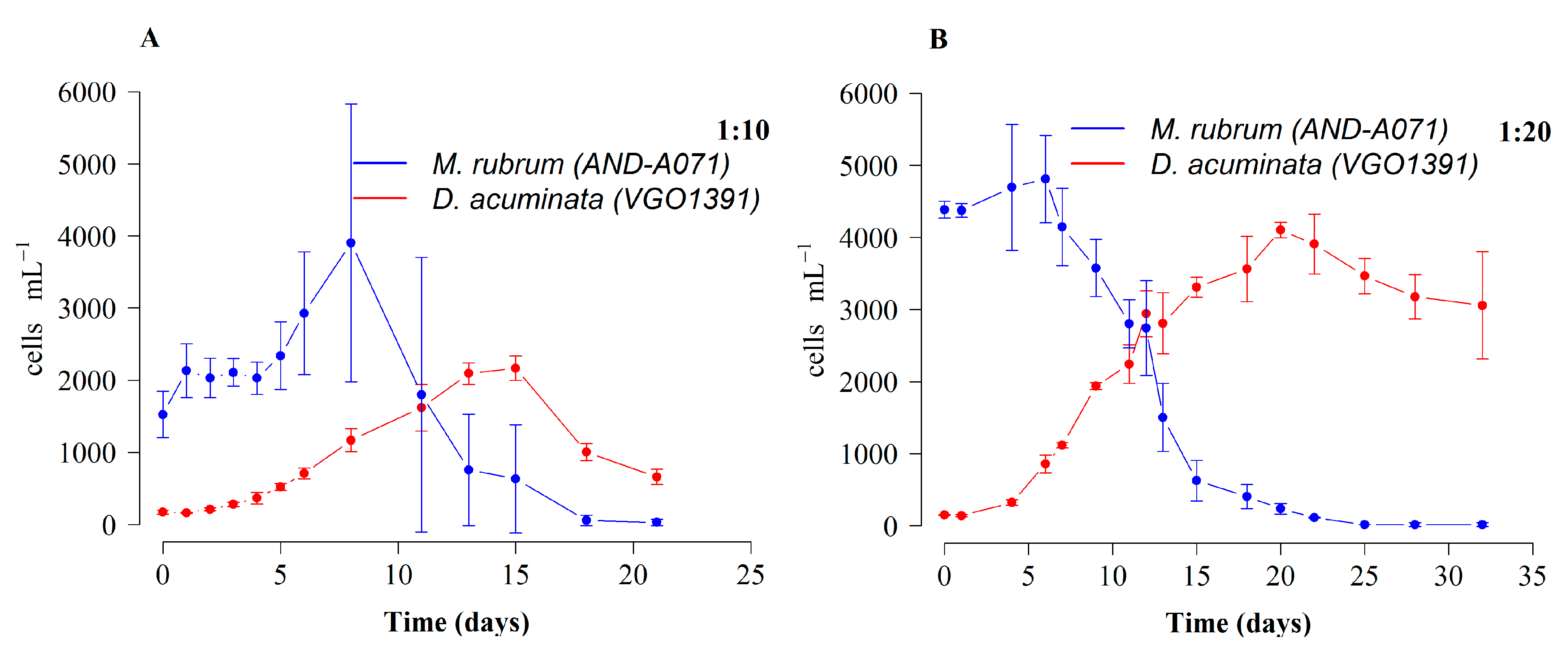
2.2. Growth and Cell Toxin Quota in 4 L Mixotrophic Cultures of D. acuminata and D. acuta
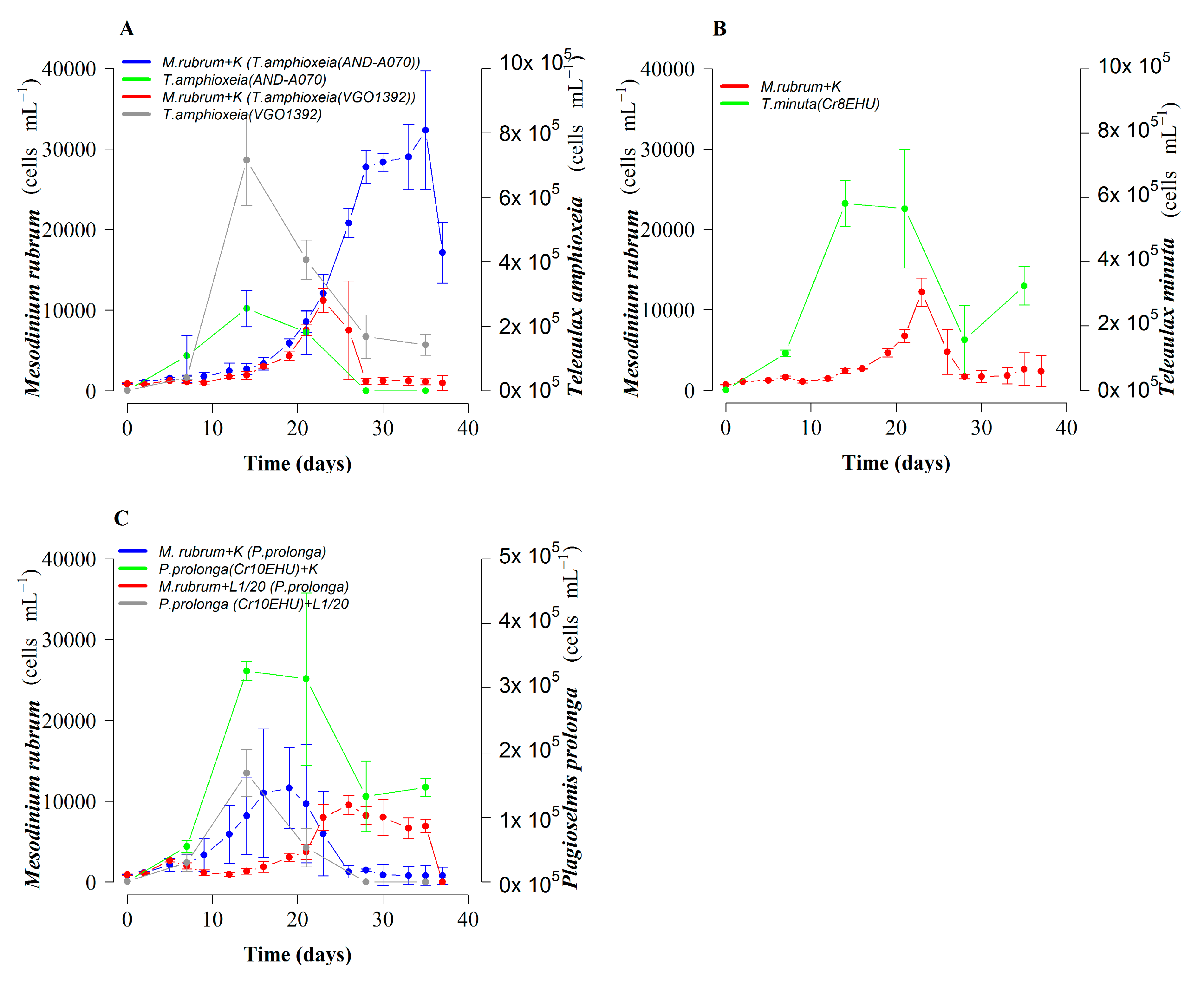
2.3. Optimization of M. rubrum Prey
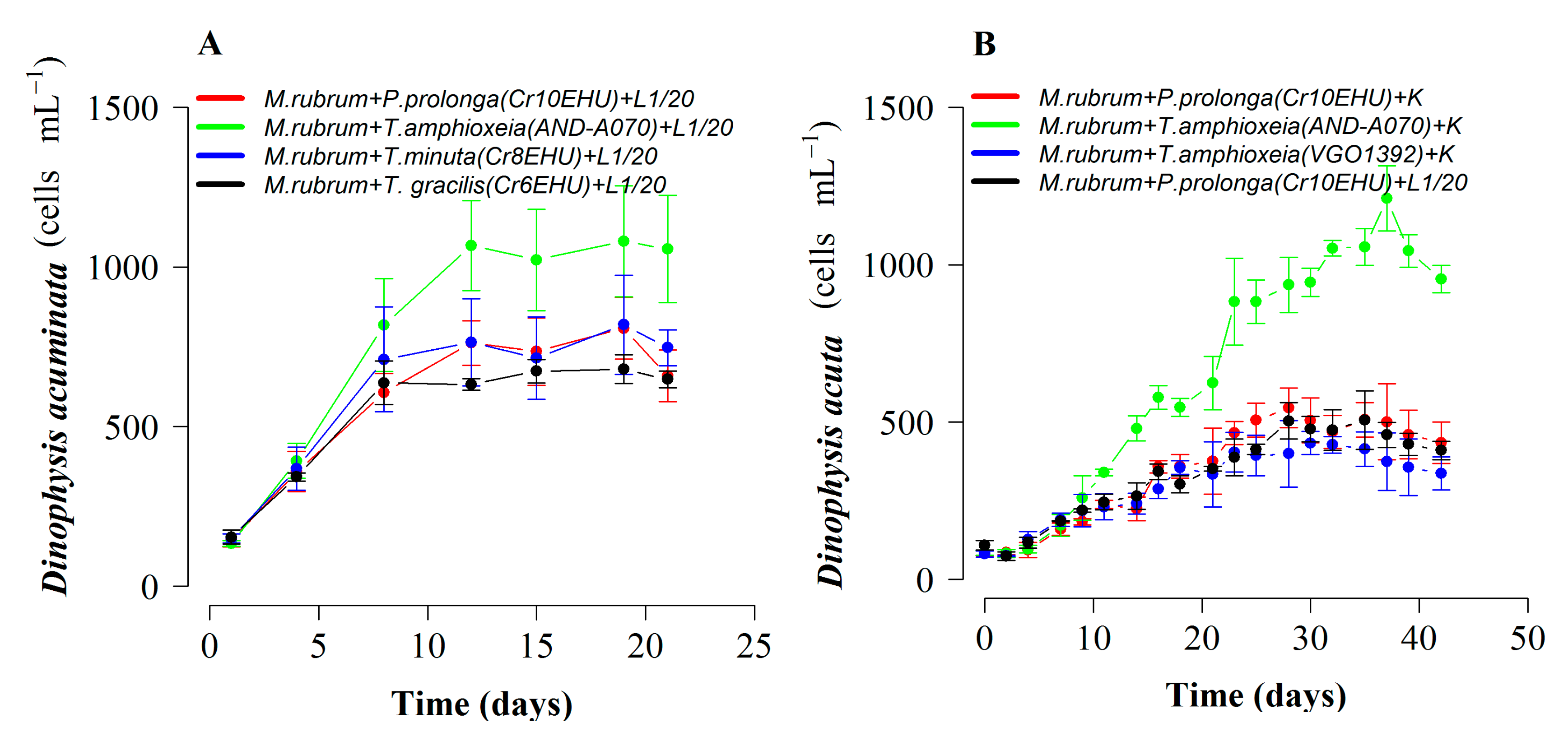
2.4. Optimal Cryptophyte Prey for M. rubrum and Dinophysis:Mesodinium (D:M) Ratio for Highest Dinophysis Growth and Survival
2.5. Mass Cultivation and Total Toxin Yield of Dinophysis in 30 L Photobioreactors
2.6. Dinophysis Vertical Distribution in the Culture Vessels
2.7. Nanoflagellate Contamination
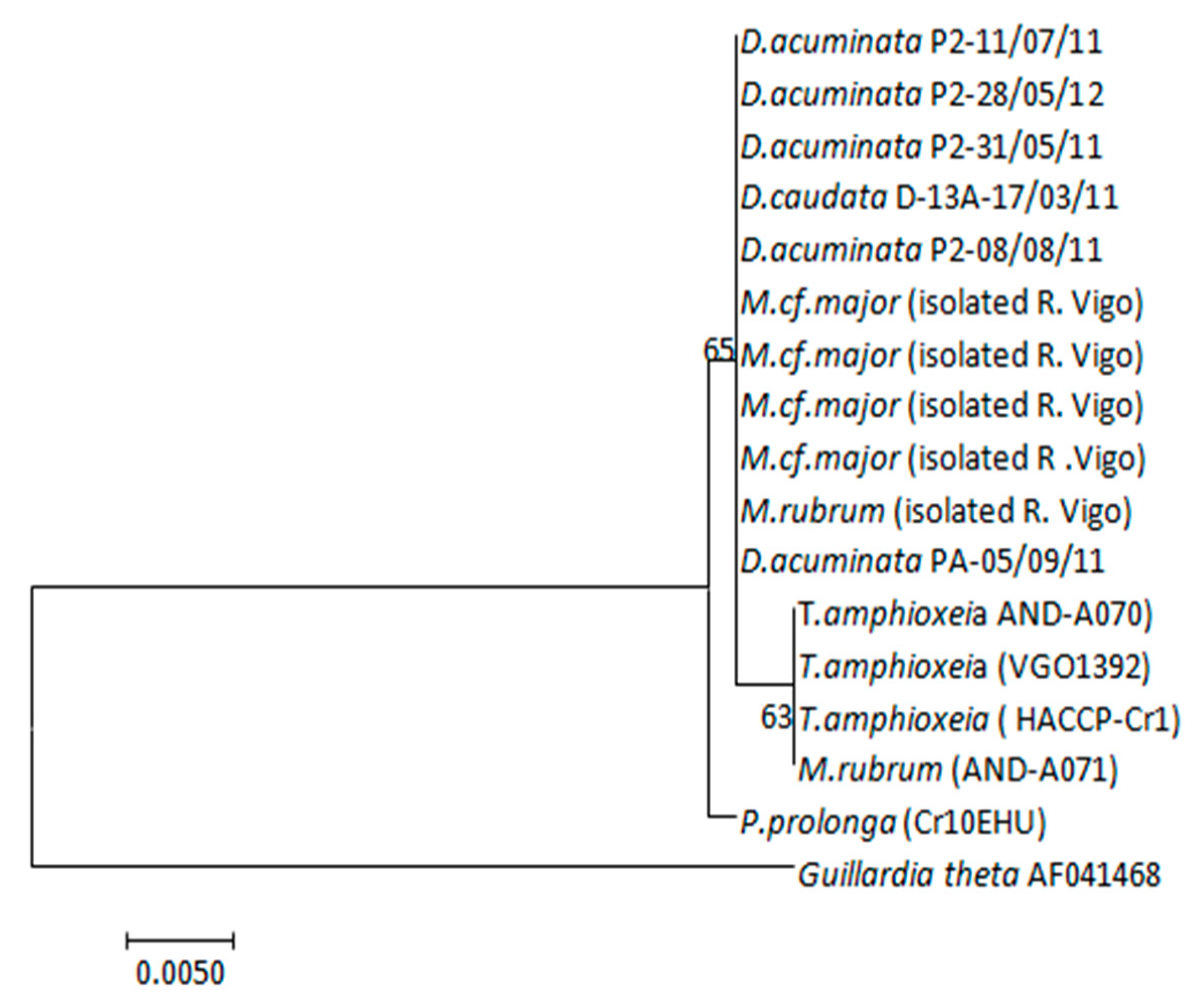
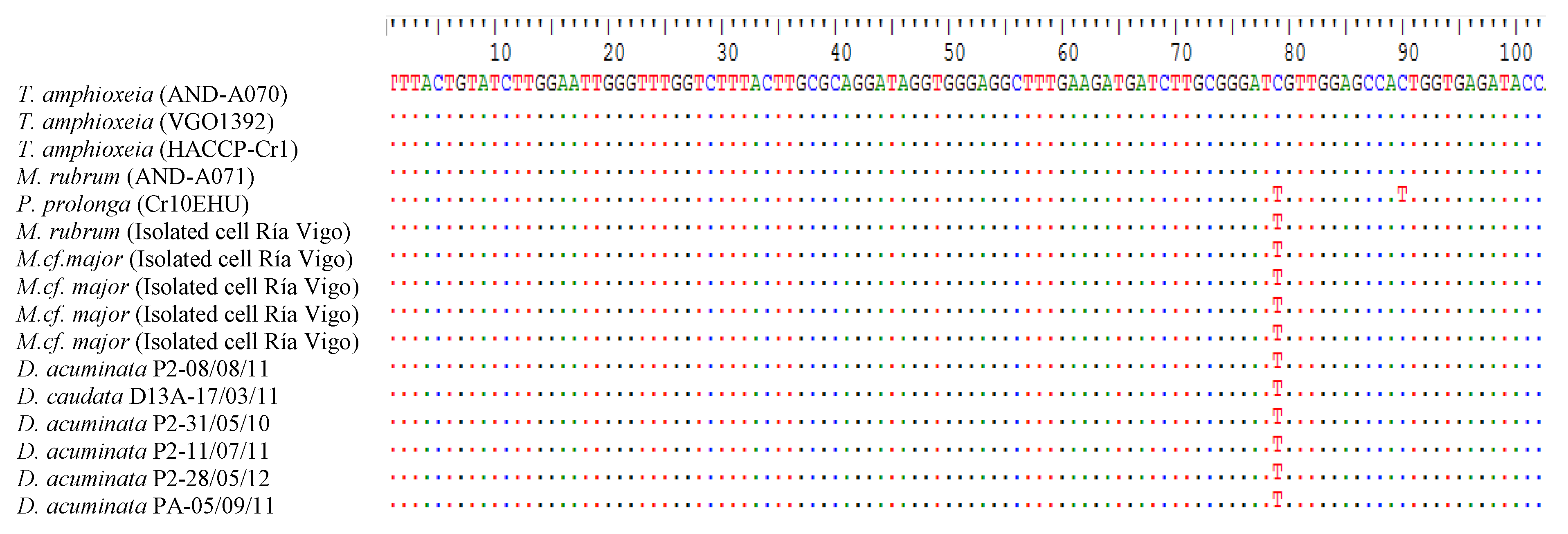
2.8. Sequencing and Phylogenetic Analysis
3. Discussion
3.1. K(-Si) Medium Best for Dinophysis Growth
3.2. Optimal Cryptophyte Prey for M. rubrum Growth
3.3. Best Results with Mass Production of Dinophysis and Other Considerations
3.4. Variability in Dinophysis Cell Toxin Quota and Culture Strategies
4. Conclusions
5. Materials and Methods
5.1. Cultures, Culturing Conditions, and Single-Cell Isolated Field Specimens
5.2. Cell Counts and Growth Rate Estimates
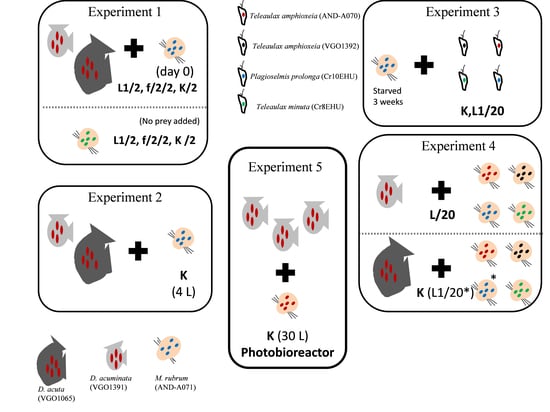
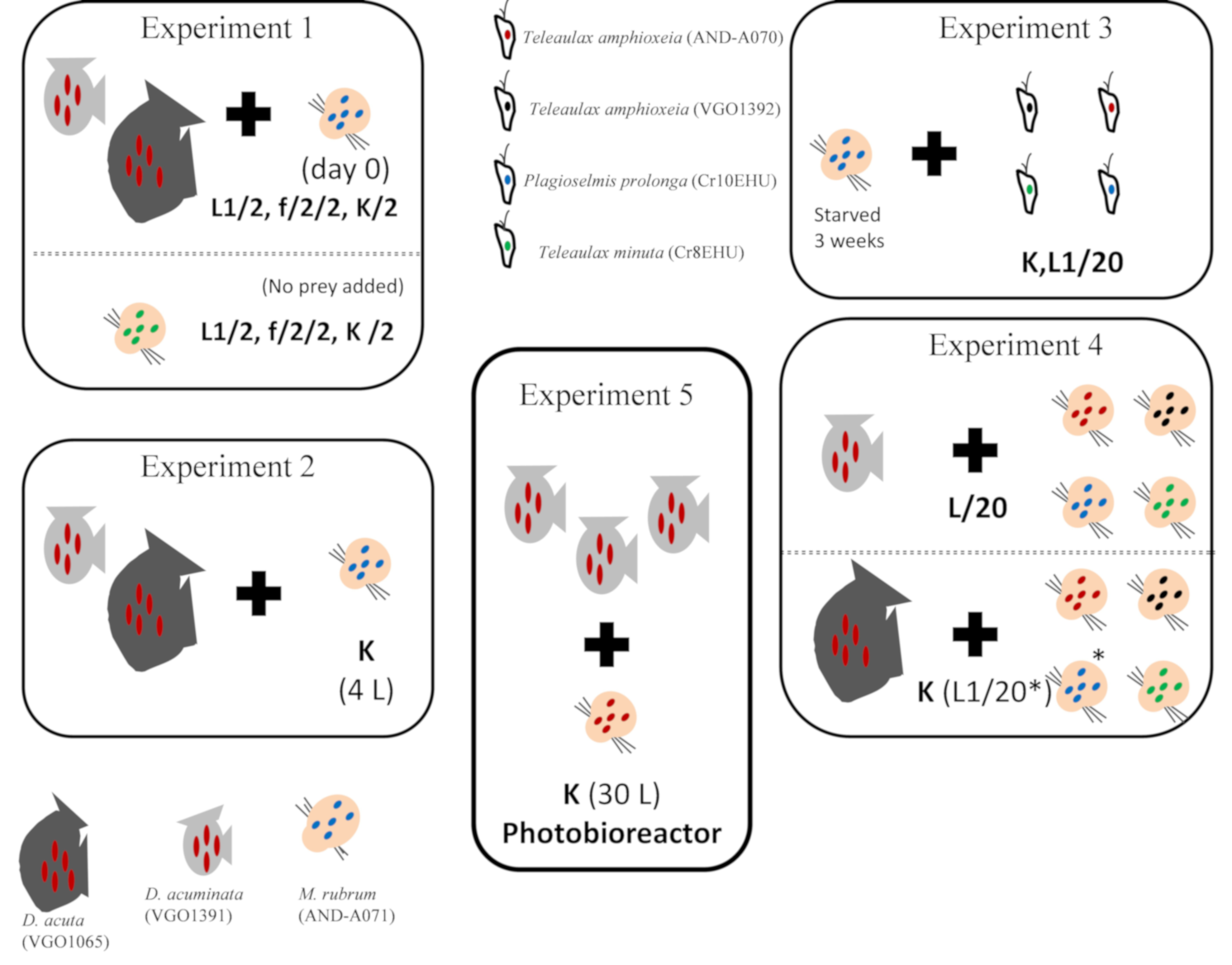
5.3. Experiment 1. Phototrophic Growth of D. acuminata, D. acuta, and M. rubrum with Different Culture Media
5.4. Experiment 2. Scaling up Mixotrophic Cultures of D. acuminata and D. acuta Cultures with K(-Si) Medium
5.5. Experiment 3. Mixotrophic Growth of M. rubrum with Different Cryptophytes
5.6. Experiment 4. Optimal Cryptophyte Prey for M. rubrum and Best M. rubrum Ratio to Feed Dinophysis
5.7. Experiment 5. Mass Production of D. acuminata in 30 L Photobioreactors
5.8. DNA Extraction, PCR Amplification and Sequencing
5.9. Phylogenetic Analysis
5.10. Harvesting and Total Toxin Extraction from Dinophysis Cultures
5.11. Toxin Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.; Glardy, J. Diarrhetic Shellfish Toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Reguera, B.; Pizarro, G. Planktonic dinoflagellates which produce polyether toxins of the old “DSP Complex”. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; Taylor & Francis: London, UK, 2008; pp. 257–284. [Google Scholar]
- Domínguez, H.P.; Paz, B.; Daranas, A.H.; Norte, M.; Franco, J.M.; Fernández, J.J. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implications. Toxicon 2010, 56, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Munday, R.; Dines, M.H.; Hawkes, A.D.; Briggs, L.R.; Sandvik, M.; Jensen, D.J.; Cooney, J.M.; Holland, P.T.; et al. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon 2004, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Regulation (EC)Nº853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. Eur. Commun 2004, 139, 55. [Google Scholar]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, H.P.; Aune, T.; Lassus, P.; Speijers, G.J.A.; Waldock, M. Paralytic and diarrhoeic shellfish poisons: Occurrence in Europe, toxicity, analysis and regulation. J. Nat. Toxins 2004, 2, 41–82. [Google Scholar]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Blanco, J.; Correa, J.; Muñiz, S.; Mariño, C.; Martín, H.; Arévalo, A. Evaluación del impacto de los métodos utilizados para el control de toxinas en el mejillón. Revista Galega dos Recursos Mariños (Art. Inf. Technol.) 2013, 3, 1–55. [Google Scholar]
- Kim, G.Y.; Kim, W.J.; Choi, Y.H. Pectenotoxin-2 from Marine Sponges: A Potential Anti-Cancer Agent—A Review. Mar. Drugs 2011, 9, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Kim, H.S.; Jeong, E.J.; Rho, J.R. Acuminolide A: Structure and bioactivity of a new polyether macrolide from dinoflagellate Dinophysis acuminata. Org. Lett. 2014, 16, 5362–5365. [Google Scholar] [CrossRef] [PubMed]
- Yih, W.; Rho, J.-R.; Kim, H.-S.; Kang, H.J. Methods for Massive Culture of Dinophysis acuminata and Isolation of Pectenotoxin-2. U.S. Patent No. US008247213B2, 21 August 2012. [Google Scholar]
- Schnepf, E.; Elbrächter, M. Cryptophycean-like double membrane-bound chloroplast in the dinoflagellate Dinophysis Ehrenb.: Evolutionary, phylogenetic and toxicological implications. Bot. Acta 1998, 101, 196–203. [Google Scholar] [CrossRef]
- Maestrini, S.Y. Bloom dynamics and ecophysiology of Dinophysis spp. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; NATO ASI Series, Series G, Ecological Science; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; pp. 243–266. [Google Scholar]
- Jacobson, D.M.; Andersen, R.A. The discovery of mixotrophy in photosynthetic species of Dinophysis (Dinophyceae): Light and electron microscopical observations of food vacuoles in Dinophysis acuminata, D. norvegica and two heterotrophic dinophysoid dinoflagellates. Phycologia 1994, 33, 97–110. [Google Scholar] [CrossRef]
- Takishita, K.; Koike, K.; Maruyama, T.; Ogata, T. Molecular Evidence for Plastid Robbery (Kleptoplastidy) in Dinophysis, a Dinoflagellate causing Diarrhetic Shellfish Poisoning. Protist 2002, 153, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Takishita, K.; Koike, K.; Maruyama, T.; Nakayama, T.; Kobiyama, A.; Ogata, T. Development of molecular probes for Dinophysis (Dinophyceae) plastid: A tool to predict blooming and explore plastid origin. Mar. Biotechnol. 2005, 7, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, G.; Miyamura, K.; Imai, I. Trying to cultivation of Dinophysis caudata (Dinophyceae) and the appearence of small cells. Plankton Biol. Ecol. 2003, 50, 31–36. [Google Scholar]
- Janson, S. Molecular evidence that plastids in the toxin-producing dinoflagellate genus Dinophysis originate from the free-living cryptophyte Teleaulax amphioxeia. Environ. Microbiol. 2004, 6, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.D.E.; Stoecker, D.K.; Johnson, M.D.; Van Heukelem, W.F.; Sneider, K. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 2000, 405, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Yih, W.; Kim, H.S.; Jeong, H.J.; Myung, G.; Kim, Y.G. Ingestion of cryptophyte cells by the marine photosynthetic ciliate Mesodinium rubrum. Aquat. Microb. Ecol. 2004, 36, 165–170. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.G.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Jaen, D.; Mamán, L.; Domínguez, R.; Martín, E. First report of Dinophysis acuta in culture. Harmful Algae News 2009, 39, 1–2. [Google Scholar]
- Nishitani, G.; Nagai, S.; Sakiyama, S.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis caudata (Dinophyceae). Plankton Benthos Res. 2008, 3, 78–85. [Google Scholar] [CrossRef]
- Nagai, S.; Nitshitani, G.; Tomaru, Y.; Sakiyama, S.; Kamiyama, T. Predation by the toxic dinoflagellate Dinophysis fortii on the ciliate Myrionecta rubra and observation of sequestration of ciliate chloroplasts. J. Phycol. 2008, 44, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, G.; Nagai, S.; Takano, Y.; Sakiyama, S.; Baba, K.; Kamiyama, T. Growth charareristics and phylogenetic analysis of the marine dinoflagellate Dinophysis infundibulus (Dinophyceae). Aquat. Microb. Ecol. 2008, 209–221. [Google Scholar] [CrossRef]
- Riobó, P.; Reguera, B.; Franco, J.M.; Rodríguez, F. First report of the toxin profile of Dinophysis sacculus Stein from LC–MS analysis of laboratory cultures. Toxicon 2013, 76, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Escalera, L.; Reguera, B.; Rial, P.; Riobó, P.; de Jesús da Silva, T. Morphological variability, toxinology and genetics of the dinoflagellate Dinophysis tripos (Dinophysiaceae, Dinophysiales). Harmful Algae 2012, 13, 26–33. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a Chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Hansen, P.J.; Nielsen, L.T.; Johnson, M.; Berge, T.; Flynn, K.J. Acquired phototrophy in Mesodinium and Dinophysis–A review of cellular organization, prey selectivity, nutrient uptake and bioenergetics. Harmful Algae 2013, 28, 126–139. [Google Scholar] [CrossRef]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R.; et al. Defining Planktonic Protist Functional Groups on Mechanism for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies. Protist 2016, 167, 106–120. [Google Scholar] [CrossRef] [PubMed]
- García-Portela, M.; Riobó, P.; Reguera, B.; Garrido, J.; Blanco, J.; Rodríguez, F. Comparative ecophysiology of Dinophysis acuminata and D. acuta: Effect of light intensity and quality on growth, cellular toxin content and photosynthesis. J. Phycol. [CrossRef]
- García-Portela, M.; Reguera, B.; Sibat, M.; Altenburger, A.; Rodríguez, F.; Hess, P. Metabolomic profiles of Dinophysis acuminata and Dinophysis acuta using non-targeted high-resolution mass espectrometry: Effect of nutritional status and prey. Mar. Drugs 2018, 16, 143–168. [Google Scholar] [CrossRef] [PubMed]
- García-Portela, M.; Reguera, B.; Ribera d’Alcalà, M.; Rodríguez, F.; Montresor, M. The turbulent life of Dinophysis. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; Book of Abstracts O-051. p. 64. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Tong, M.; Smith, J.L.; Kulis, D.M.; Anderson, D.M. Role of dissolved nitrate and phosphate in isolates of Mesodinium rubrum and toxin-producing Dinophysis acuminata. Aquat. Microb. Ecol. 2015, 75, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Seeyave, S.; Probyn, T.A.; Pitcher, G.C.; Lucas, M.I.; Purdie, D.A. Nitrogen nutrition in assemblages dominated by Pseudo-nitzschia spp., Alexandrium catenella and Dinophysis acuminata off the west coast of South Africa. Mar. Ecol. Prog. Ser. 2009, 379, 91–107. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.; Gobler, C.J. The contribution of inorganic and organic nutrients to the growth of a North American isolate of the mixotrophic dinoflagellate, Dinophysis acuminata. Limnol. Oceanogr. 2015, 60, 1588–1603. [Google Scholar] [CrossRef]
- García-Portela, M. Comparative Ecophysiology of Two Mixotrophic Species of Dinophysis Producers of Lipophilic Toxins. Ph.D Thesis, Univerdidad de Vigo, Vigo, Spain, 2018. [Google Scholar]
- Smith, M.; Hansen, P.J. Interaction between Mesodinium rubrum and its prey: Importance of prey concentration, irradiance and pH. Mar. Ecol. Prog. Ser. 2007, 338, 61–70. [Google Scholar] [CrossRef]
- Park, J.S.; Myung, G.; Kim, H.S.; Cho, B.C.; Yih, W. Growth responses of the marine photosynthetic ciliate Myrionecta rubra to different cryptomonad strains. Aquat. Microb. Ecol. 2007, 48, 83–90. [Google Scholar] [CrossRef]
- Myung, G.; Kim, H.S.; Park, J.S.; Park, M.G.; Yih, W. Population growth and plastid type of Myrionecta rubra depend on the kinds of available cryptomonad prey. Harmful Algae 2011, 10, 536–541. [Google Scholar] [CrossRef]
- Peltomaa, E.; Johnson, M.D. Mesodinium rubrum exhibits genus-level but not species-level cryptophyte prey selection. Aquat. Microb. Ecol. 2017, 78, 147–159. [Google Scholar] [CrossRef]
- Rial, P.; Laza-Martínez, A.; Reguera, B.; Raho, N.; Rodríguez, F. Origin of cryptophyte plastids in Dinophysis from Galician waters: Results from field and culture experiments. Aquat. Microb. Ecol. 2015, 76, 163–174. [Google Scholar] [CrossRef]
- Gao, H.; Hua, C.; Tong, M. Impact of Dinophysis acuminata feeding Mesodinium rubrum on nutrient dynamics and bacterial composition in a microcosm. Toxins 2018, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Effects of light and food availability on toxin production, growth and photosynthesis in Dinophysis acuminata. Mar. Ecol. Prog. Ser. 2012, 471, 37–50. [Google Scholar] [CrossRef]
- Lindahl, O.; Lundve, B.; Johansen, M. Toxicity of Dinophysis spp. in relation to population abundance and environmental condition on the Swedish west coast. Harmful Algae 2007, 6, 218–231. [Google Scholar] [CrossRef]
- Pizarro, G.; Paz, B.; González-Gil, S.; Franco, J.M.; Reguera, B. Seasonal variability of lipophilic toxins during a Dinophysis acuta bloom in Western Iberia: Differences between picked cells and plankton concentrates. Harmful Algae 2009, 8, 926–937. [Google Scholar] [CrossRef]
- Pizarro, G.; Moroño, A.; Paz, B.; Franco, J.M.; Reguera, B. Evaluation of passive samples as a monitoring tool for early warning of Dinophysis toxins in shelfish. Mar. Drugs 2013, 11, 3823–3845. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.M.; Kulis, D.M.; Fux, E.; Smith, J.L.; Hess, P.; Zhou, Q.X.; Anderson, D.M. The effects of growth phase and light intensity on toxin production by Dinophysis acuminata from the northeastern United States. Harmful Algae 2011, 10, 254–264. [Google Scholar] [CrossRef]
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta—Effects of light, food availability and growth phase. Harmful Algae 2013, 23, 34–45. [Google Scholar] [CrossRef]
- Smith, J.L.; Tong, M.; Kulis, D.; Anderson, D.M. Effect of ciliate strain, size, and nutritional content on the growth and toxicity of mixotrophic Dinophysis acuminata. Harmful Algae 2018, 78, 95–105. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, L.; Beuzenberg, V.; Holland, P.; McNabb, P.; Selwood, A. Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon 2004, 44, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Rundberget, T.; Sandvik, M.; Larsen, K.; Pizarro, G.M.; Reguera, B.; Castberg, T.; Gustad, E.; Loader, J.I.; Rise, F.; Wilkins, A.L.; et al. Extraction of microalgal toxins by large-scale pumping of seawater in Spain and Norway, and isolation of okadaic acid and dinophysistoxin-2. Toxicon 2007, 50, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Richlen, M.L.; Barber, P.H. A technique for the rapid extraction of microalgal DNA from single live and preserved cells. Mol. Ecol. Notes 2005, 5, 688–691. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in Eukaryotic algae and Cyanobacteria. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W:Improving the sensivity of progresive multiple sequence alignement though sequence weighting, position specific gap penalties and matrix choice. Nucleic Aids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]

| Species | Ref. | Experimental Conditions | V (mL) | T (°C) | L:D Cycle (h) | Medium | OA (pg cell−1) | DTX2 (pg cell−1) | PTX2 (pg cell−1) |
|---|---|---|---|---|---|---|---|---|---|
| D. acuta | [34] | Well-fed (ES) | 250 | 15 | 12:12 | L1-Si/20 | 12.2 ± 2.3 | 4.4 ± 0.9 | 22.2 ± 9.4 |
| [34] | Well-fed (ES) | 150 | 17 | 14:10 | L1-Si/20 | 41.0 ± 4.9 | 17.4 ± 4.1 | 38.0 ± 8.2 | |
| Prey-limited (ES) | 150 | 17 | 14:10 | 74.1 ± 8.2 | 32.4 ± 3.8 | 59.3 ± 11.8 | |||
| [34] | Well-fed (DK) | 150 | 17 | 14:10 | L1-Si/20 | 35.9 ± 7.07 | 16.5 ± 0.8 | 70.0 ± 0.8 | |
| Prey-limited (DK) | 150 | 17 | 14:10 | 38.6 ± 4.5 | 19.0 ± 2.3 | 43.6 ± 6.2 | |||
| [33] | Low light | 250 | 15 | 12:12 | L1-Si/40 | 3.3 ± 1.6 | 2.2 ± 0.1 | 71.5 ± 14.2 | |
| High light | 250 | 15 | 12:12 | 50.2 ± 20.1 | 35.4 ± 17.4 | 187.8 ± 104.1 | |||
| This work | Mass culture-sta | 1450 | 15 | 12:12 | L1-Si/20 | 30.2 * | 7.3 * | 48.2 * | |
| Mass culture-sta | 3500 | 15 | 12:12 | K-Si | 15.5 * | 5.2 * | 50.5 * | ||
| Mass culture-exp | 4000 | 19 | 16:8 | K-Si | 7.7 | 2.9 | 8.2 | ||
| Mass culture-sta | 5000 | 15 | 12:12 | L1-Si/20 | 61.5 * | 20.3 * | 3 * | ||
| D. acuminata | [34] | Well-fed (ES) | 250 | 15 | 12:12 | L1-Si/20 | 35.2 ± 6.8 | ||
| [34] | Well-fed (ES) | 150 | 17 | 14:10 | L1-Si/20 | 6.0 ± 2.8 | |||
| Prey-limited (ES) | 150 | 17 | 14:10 | 21.5 ± 0.7 | |||||
| [34] | Well-fed (DK) | 150 | 17 | 14:10 | L1-Si/20 | 9.8 ± 1.7 | |||
| Prey-limited (DK) | 150 | 17 | 14:10 | 32.3 ±4.7 | |||||
| [33] | Low light | 250 | 15 | 12:12 | L1-Si/40 | 14.7 ± 12.1 | |||
| High light | 250 | 15 | 12:12 | 41.4 ± 4 | |||||
| This work | Mass culture-sta | 2200 | 15 | 12:12 | L1-Si/20 | 33.3 * | |||
| Mass culture-sta | 2700 | 15 | 12:12 | L1-Si/20 | 122.2 * | ||||
| Mass culture-exp | 4000 | 19 | 16:8 | K-Si | 9.9 | ||||
| Mass culture-sta | 4500 | 15 | 12:12 | K-Si | 20.3 * | ||||
| Mass culture-sta | 17,900 | 15 | 12:12 | K-Si | 28.9 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Urcera, J.; Rial, P.; García-Portela, M.; Lourés, P.; Kilcoyne, J.; Rodríguez, F.; Fernández-Villamarín, A.; Reguera, B. Notes on the Cultivation of Two Mixotrophic Dinophysis Species and Their Ciliate Prey Mesodinium rubrum. Toxins 2018, 10, 505. https://doi.org/10.3390/toxins10120505
Hernández-Urcera J, Rial P, García-Portela M, Lourés P, Kilcoyne J, Rodríguez F, Fernández-Villamarín A, Reguera B. Notes on the Cultivation of Two Mixotrophic Dinophysis Species and Their Ciliate Prey Mesodinium rubrum. Toxins. 2018; 10(12):505. https://doi.org/10.3390/toxins10120505
Chicago/Turabian StyleHernández-Urcera, Jorge, Pilar Rial, María García-Portela, Patricia Lourés, Jane Kilcoyne, Francisco Rodríguez, Amelia Fernández-Villamarín, and Beatriz Reguera. 2018. "Notes on the Cultivation of Two Mixotrophic Dinophysis Species and Their Ciliate Prey Mesodinium rubrum" Toxins 10, no. 12: 505. https://doi.org/10.3390/toxins10120505
APA StyleHernández-Urcera, J., Rial, P., García-Portela, M., Lourés, P., Kilcoyne, J., Rodríguez, F., Fernández-Villamarín, A., & Reguera, B. (2018). Notes on the Cultivation of Two Mixotrophic Dinophysis Species and Their Ciliate Prey Mesodinium rubrum. Toxins, 10(12), 505. https://doi.org/10.3390/toxins10120505