Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Sample
2.2. Dietary Intake
2.3. Energy Expenditure
2.4. Micronutrients
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Energy Balance
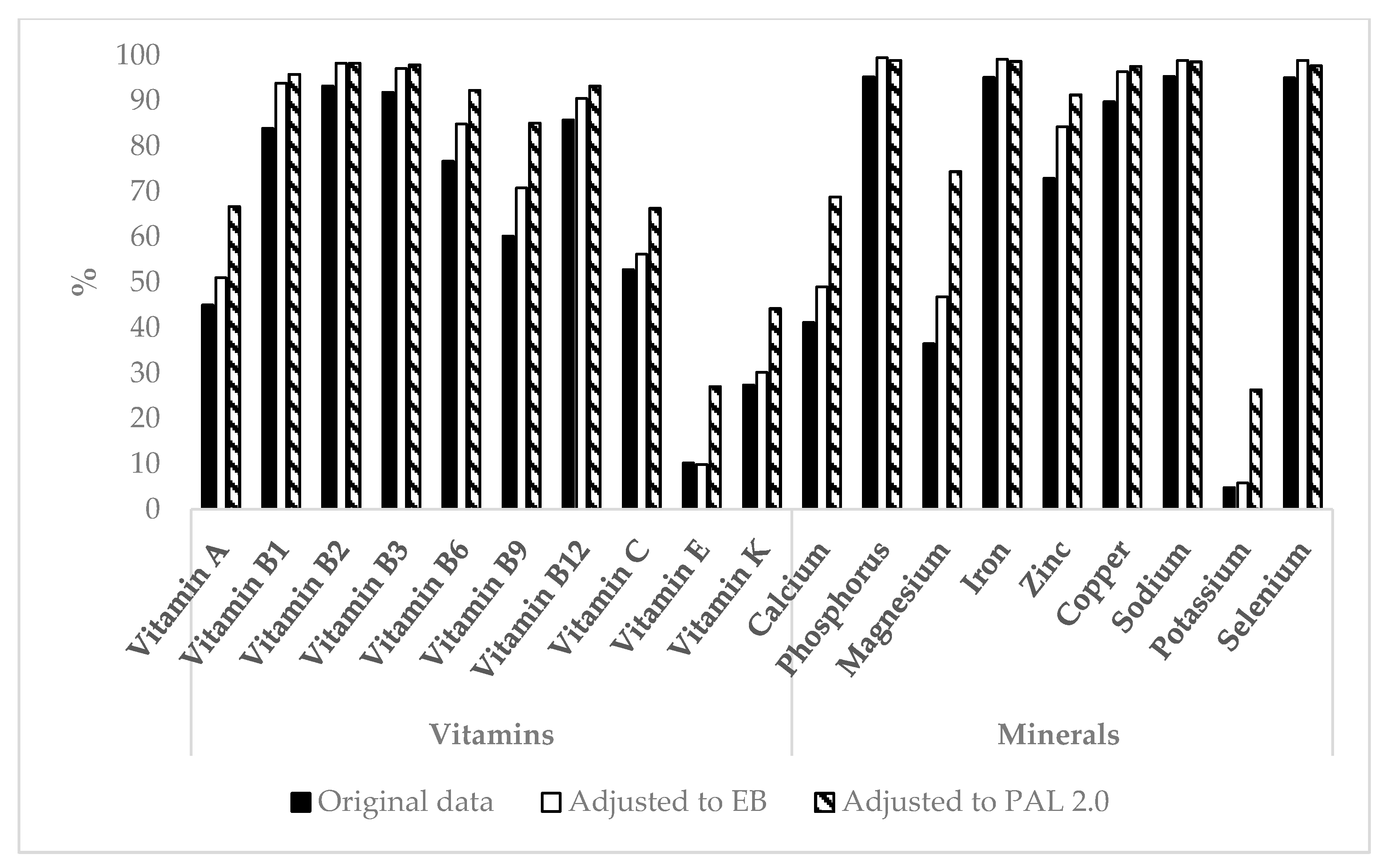
3.3. Micronutrient Intake
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fogelholm, M. Micronutrients: Interaction between physical activity, intakes and requirements. Public Health Nutr. 1999, 2, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Brummer, R.-J.; König, J.; O’Connell, M.A.; Ovesen, L.; Rechkemmer, G.; Stos, K.; Thurnham, D.I. Micronutrient deficiencies. Eur. J. Nutr. 2003, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef]
- Misner, B. Food alone may not provide sufficient micronutrients for preventing deficiency. J. Int. Soc. Sports Nutr. 2006, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed 2006, 8, 59. [Google Scholar] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid. Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the Scientific Committee on Food on the Revision of Reference Values for Nutrition Labelling. Available online: http://ec.europa.eu/food/fs/sc/scf/out171_en.pdf (accessed on 16 August 2016).
- Calton, J.B. Prevalence of micronutrient deficiency in popular diet plans. J. Int. Soc. Sports Nutr. 2010, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Csizmadi, I.; Kelemen, L.E.; Speidel, T.; Yuan, Y.; Dale, L.C.; Friedenreich, C.M.; Robson, P.J. Are physical activity levels linked to nutrient adequacy? Implications for cancer risk. Nutr. Cancer 2014, 66, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Anastasiou, C.A.; Lentzas, Y.; Stefanadis, C. Physical activity, obesity status, and glycemic control: The ATTICA study. Med. Sci. Sports Exerc. 2007, 39, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Role of micronutrients in sport and physical activity. Br. Med. Bull. 1999, 55, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, I.; Rousseau, A.S. Does physical exercise modify antioxidant requirements? Nutr. Res. Rev. 2008, 21, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Micronutrient requirements for athletes. Clin. Sports Med. 2007, 26, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Perret, C.; Stoffel-Kurt, N. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J. Spinal Cord Med. 2011, 34, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Kayser, B.; Saris, W.H.; Pichard, C. Effects of physical activity on food intake. Clin. Nutr. 2005, 24, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [PubMed]
- Woodside, J.V.; McCall, D.; McGartland, C.; Young, I.S. Micronutrients: Dietary intake v. supplement use. Proc. Nutr. Soc. 2005, 64, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Camões, M.; Lopes, C. Dietary intake and different types of physical activity: Full-day energy expenditure, occupational and leisure-time. Public Health Nutr. 2008, 11, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Garcin, M.; Doussot, L.; Mille-Hamard, L.; Billat, V. Athletes’ dietary intake was closer to French RDA’s than those of young sedentary counterparts. Nutr. Res. 2009, 29, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. Vital Health Stat. 1 2013, 56, 1–37. [Google Scholar]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Vital Health Stat. 2 2013, 161, 1–24. [Google Scholar]
- Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 13 July 2017).
- Butte, N.F.; Wong, W.W.; Treuth, M.S.; Ellis, K.J.; O’Brian Smith, E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 2004, 79, 1078–1087. [Google Scholar] [PubMed]
- Löf, M.; Olausson, H.; Bostrom, K.; Janerot-Sjöberg, B.; Sohlstrom, A.; Forsum, E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am. J. Clin. Nutr. 2005, 81, 678–685. [Google Scholar] [PubMed]
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiother. 2013, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://riskfactor.cancer.gov/tools/nhanes_pam/ (accessed on 13 July 2017).
- Kcal Estimates from Activity Counts Using the Potential Energy Method. Available online: http://actigraphcorp.com/research-database/kcal-estimates-from-activity-counts-using-the-potential-energy-method/ (accessed on 16 August 2016).
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Manore, M.M.; Thompson, J.L. Energy requirements of the athlete: Assessment and evidence of energy efficiency. In Clinical Sports Nutrition, 4th ed.; Burke, L.M., Deakin, V., Eds.; McGraw-Hill Australia Pty Ltd.: North Ryde, Australia, 2010; pp. 96–115. [Google Scholar]
- Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Available online: http://www.fao.org/3/a-y5686e.pdf (accessed on 16 August 2016).
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Food and Nutrition Board; Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Archer, E.; Hand, G.A.; Blair, S.N. Validity of U.S. Nutritional Surveillance: National Health and Nutrition Examination Survey Caloric Energy Intake Data, 1971–2010. PLoS ONE 2013, 8, e76632. [Google Scholar] [CrossRef]
- Didier, J.P.; Guilloux, D.; Rouhier-Marcer, I. Coût énergétique de la marche à vitesse confortable et adaptation respiratoire dans deux groupes de personnes jeunes et âgées. Ann. Réadapt. 1995, 38, 475–480. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; WHO Technical Report Series 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef] [PubMed]
- Moor de Burgos, A.; Wartanowicz, M.; Ziemlanski, S. Blood vitamin and lipid levels in overweight and obese women. Eur. J. Clin. Nutr. 1992, 46, 803–808. [Google Scholar] [PubMed]
- Reitman, A.; Friedrich, I.; Ben-Amotz, A.; Levy, Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr. Med. Assoc. J. 2002, 4, 590–593. [Google Scholar] [PubMed]
- Wallstrom, P.; Wirfalt, E.; Lahmann, P.H.; Gullberg, B.; Janzon, L.; Berglund, G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am. J. Clin. Nutr. 2001, 73, 777–785. [Google Scholar] [PubMed]
- Canoy, D.; Wareham, N.; Welch, A.; Bingham, S.; Luben, R.; Day, N.; Khaw, K.T. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am. J. Clin. Nutr. 2005, 82, 1203–1209. [Google Scholar] [PubMed]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum selenium determinants in French adults: The SU.VI.M.AX study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.P.; Bingham, S.; Johansson, I.; Wallstrom, P.; Overvad, K.; Tjonneland, A.; et al. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Rock, C.L.; Eldridge, A.L.; Kristal, A.R.; Patterson, R.E.; Cooper, D.A.; Neumark-Sztainer, D.; Cheskin, L.J.; Thornquist, M.D. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J. Nutr. 2001, 131, 2184–2191. [Google Scholar] [PubMed]
- Parikh, S.J.; Edelman, M.; Uwaifo, G.I.; Freedman, R.J.; Semega-Janneh, M.; Reynolds, J.; Yanovski, J.A. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J. Clin. Endocrinol. Metab. 2004, 89, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J. Pediatr. 1999, 134, 160–165. [Google Scholar] [PubMed]
- Hill, R.J.; Davies, P.S.W. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br. J. Nutr. 2001, 85, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Briefel, R.R.; Sempos, C.T.; McDowell, M.A.; Chien, S.; Alaimo, K. Dietary methods research in the third National Health and Nutrition Examination Survey: Underreporting of energy intake. Am. J. Clin. Nutr. 1997, 65, 1203S–1209S. [Google Scholar] [PubMed]
- Melzer, K.; Renaud, A.; Zurbuchen, S.; Tschopp, C.; Lehmann, J.; Malatesta, D.; Ruch, N.; Schutz, Y.; Kayser, B.; Mäder, U. Alterations in energy balance from an exercise intervention with ad libitum food intake. J. Nutr. Sci. 2016, 5, e7. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.; Blair, S.N.; van Baak, M.A.; Eaton, S.B.; Davies, P.S.; Di Pietro, L.; Fogelholm, M.; Rissanen, A.; Schoeller, D.; Swinburn, B.; et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes. Rev. 2003, 4, 101–114. [Google Scholar] [PubMed]
- Raichlen, D.A.; Pontzer, H.; Harris, J.A.; Mabulla, A.Z.; Marlowe, F.W.; Josh Snodgrass, J.; Eick, G.; Colette Berbesque, J.; Sancilio, A.; Wood, B.M. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. 2017, 29, e22919. [Google Scholar] [CrossRef] [PubMed]
- Lanningham-Foster, L.; Nysse, L.J.; Levine, J.A. Labor saved, calories lost: The energetic impact of domestic labor-saving devices. Obes. Res. 2003, 11, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Foster, R.C.; Eide, D.S.; Levine, J.A. Feasibility of a walking workstation to increase daily walking. Br. J. Sports Med. 2008, 42, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Miller, J.M. The energy expenditure of using a “walk-and-work” desk for office workers with obesity. Br. J. Sports Med. 2007, 41, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Penner, S.B.; Campbell, N.R.C.; Chockalingam, A.; Zarnke, K.; Van Vliet, B. Dietary sodium and cardiovascular outcomes: A rational approach. Can. J. Cardiol. 2007, 23, 567–572. [Google Scholar] [CrossRef]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Pavela, G.; Lavie, C.J. A Discussion of the Refutation of Memory-Based Dietary Assessment Methods (M-BMs): The Rhetorical Defense of Pseudoscientific and Inadmissible Evidence. Mayo Clin. Proc. 2015, 90, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Blair, S.N. Implausible data, false memories, and the status quo in dietary assessment. Adv. Nutr. 2015, 6, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [PubMed]

| Participants | n | Age (Years) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|---|
| Males | 2070 | 52.5 ± 17.9 | 86.5 ± 18.7 a | 175 ± 8 a | 28.3 ± 5.3 |
| Females | 1945 | 52.8 ± 17.5 | 74.8 ± 19.0 | 161 ± 7 | 28.9 ± 6.9 |
| Total | 4015 | 52.7 ± 17.7 | 80.8 ± 19.7 | 168 ± 10 | 28.6 ± 6.1 |
| PAL | n | Age (Years) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|---|
| <1.4 | 2440 | 57.2 ± 18.3 a | 79.4 ± 20.0 b | 167 ± 10 a | 28.4 ± 6.2 b |
| 1.4- <1.7 | 1469 | 45.9 ± 14.2 | 83.1 ± 19.0 | 169 ± 10 | 28.4 ± 5.1 |
| ≥1.7 | 106 | 42.5 ± 13.9 | 82.1 ± 19.1 | 170 ± 9 | 28.9 ± 6.1 |
| PAL | n | Energy Intake (kcal/Day) | Energy Balance | EI/BMR | |
|---|---|---|---|---|---|
| kcal/Day | % of TEE | ||||
| <1.4 | 2440 | 1942 ± 731 a | −78 ± 690 a | −2.5 ± 33.8 a | 1.26 ± 0.44 a |
| 1.4- <1.7 | 1469 | 2286 ± 904 | −216 ± 847 | −8.0 ± 33.2 | 1.37 ± 0.50 |
| ≥1.7 | 106 | 2589 ± 1003 b | −574 ± 1041 b | −16.9 ± 31.6 b | 1.52 ± 0.58 b |
| BMI (kg/m2) | n | Energy Balance | EI/BMR | |
|---|---|---|---|---|
| kcal/Day | % of TEE | |||
| <18.5 | 55 | 529 ± 816 a | 31.9 ± 46.9 a | 1.74 ± 0.63 a |
| 18.5- <25 | 1144 | 175 ± 712 b | 9.4 ± 35.6 b | 1.49 ± 0.49 b |
| 25- <30 | 1462 | −136 ± 693 c | −6.0 ± 30.1 c | 1.30 ± 0.43 |
| ≥ 30 | 1354 | −443 ± 762 | −17.2 ± 29.2 | 1.14 ± 0.41 |
| Males (n = 2070) | Females (n = 1945) | |||||
|---|---|---|---|---|---|---|
| Intake | DRI * | Intake | DRI * | |||
| Vitamins | Vitamin A | [µg/day] | 677 ± 660 a | 625 | 584 ± 454 | 500 |
| [µg/MJ] | 71.6 ± 78.4 a | 83.2 ± 66.8 | ||||
| Vitamin B1 | [mg/day] | 1.9 ± 0.9 a | 1.0 | 1.9 ± 0.8 | 0.9 | |
| [mg/MJ] | 0.19 ± 0.07 a | 0.20 ± 0.07 | ||||
| Vitamin B2 | [mg/day] | 2.5 ± 1.2 a | 1.1 | 1.9 ± 0.8 | 0.9 | |
| [mg/MJ] | 0.26 ± 0.10 a | 0.26 ± 0.10 | ||||
| Vitamin B3 | [mg/day] | 28.2 ± 12.9 a | 12 | 20.1 ± 8.4 | 11 | |
| [mg/MJ] | 2.9 ± 1.0 | 2.8 ± 1.0 | ||||
| Vitamin B6 | [mg/day] | 2.2 ± 1.1 a | 1.1 | 1.7 ± 0.8 | 1.1 | |
| [mg/MJ] | 0.23 ± 0.10 | 0.24 ± 0.11 | ||||
| Vitamin B9 | [µg/day] | 446 ± 222 a | 320 | 354 ± 173 | 320 | |
| [µg/MJ] | 45.8 ± 18.5 a | 49.8 ± 22.0 | ||||
| Vitamin B12 | [µg/day] | 6.4 ± 7.5 a | 2.0 | 4.5 ± 4.2 | 2.0 | |
| [µg/MJ] | 0.66 ± 0.84 | 0.63 ± 0.59 | ||||
| Vitamin C | [mg/day] | 96.5 ± 83.5 a | 75 | 86.9 ± 72.6 | 60 | |
| [mg/MJ] | 10.1 ± 8.7 a | 12.5 ± 11.1 | ||||
| Vitamin E | [mg/day] | 7.6 ± 4.4 a | 12 | 6.3 ± 3.9 | 12 | |
| [mg/MJ] | 0.77 ± 0.35 a | 0.87 ± 0.49 | ||||
| Vitamin K | [µg/day] | 105 ± 141 | 120 | 99 ± 121 | 90 | |
| [µg/MJ] | 11.2 ± 16.8 a | 14.5 ± 21.7 | ||||
| Minerals | Calcium | [mg/day] | 948 ± 509 a | 800 | 789 ± 398 | 800 |
| [mg/MJ] | 96.2 ± 40.1 a | 110 ± 48 | ||||
| Phosphorus | [mg/day] | 1473 ± 586 a | 580 | 1112 ± 419 | 580 | |
| [mg/MJ] | 149 ± 34 a | 154 ± 37 | ||||
| Magnesium | [mg/day] | 321 ± 131 a | 350 | 254 ± 105 | 265 | |
| [mg/MJ] | 33.0 ± 9.9 a | 35.7 ± 11.7 | ||||
| Iron | [mg/day] | 18.0 ± 8.5 a | 6.0 | 13.7 ± 6.3 | 8.1 | |
| [mg/MJ] | 1.9 ± 0.7 a | 1.9 ± 0.8 | ||||
| Zinc | [mg/day] | 13.9 ± 9.0 a | 9.4 | 10.0 ± 5.3 | 6.8 | |
| [mg/MJ] | 1.4 ± 0.9 | 1.4 ±0.7 | ||||
| Copper | [mg/day] | 1.5 ± 1.1 a | 0.7 | 1.2± 0.7 | 0.7 | |
| [mg/MJ] | 0.15 ± 0.13 a | 0.16 ± 0.09 | ||||
| Potassium | [mg/day] | 2990 ± 1152 a | 4700 | 2365 ± 878 | 4700 | |
| [mg/MJ] | 309 ± 92 a | 334 ± 105 | ||||
| Selenium | [µg/day] | 124 ± 55 a | 45 | 92 ± 40 | 45 | |
| [µg/MJ] | 12.6 ± 3.8 | 12.8 ±4.1 | ||||
| Sodium | [mg/day] | 3781 ± 1644 a | 1500 | 2825 ± 1101 | 1500 | |
| [mg/MJ] | 382 ± 113 a | 392 ± 104 | ||||
| Micronutrient Intake | PAL | |||
|---|---|---|---|---|
| <1.4 (n = 2440) | 1.4 ≤ 1.7 (n = 1469) | ≥1.7 (n = 106) | ||
| Vitamins | Vitamin A [µg/day] | 625 ± 516 | 644 ± 658 | 636 ± 503 |
| Vitamin B1 [mg/day] | 1.6 ± 0.7 a | 1.8 ± 0.9 | 1.9 ± 0.8 b | |
| Vitamin B2 [mg/day] | 2.1 ± 1.0 a | 2.3 ± 1.1 | 2.4 ± 1.1 | |
| Vitamin B3 [mg/day] | 22.9 ± 11.1 a | 26.5 ± 12.2 | 27.6 ± 11.4 | |
| Vitamin B6 [mg/day] | 1.9 ± 1.0 a | 2.1 ± 1.1 | 2.2 ± 1.1 | |
| Vitamin B9 [µg/day] | 385 ± 197 a | 424 ± 215 | 459 ± 219 | |
| Vitamin B12 [µg/day] | 5.3 ± 5.9 b | 5.8 ± 6.8 | 5.7 ± 3.9 | |
| Vitamin C [mg/day] | 88 ± 74 b | 97 ± 84 | 107 ± 97 | |
| Vitamin E [mg/day] | 6.6 ± 4.0 a | 7.5 ± 4.5 | 7.8 ± 4.7 | |
| Vitamin K [µg/day] | 100 ± 124 | 104 ± 135 | 121 ± 220 | |
| Minerals | Calcium [mg/day] | 824 ± 431 a | 942 ± 502 | 968 ± 555 |
| Phosphorus [mg/day] | 1219 ± 496 a | 1412 ± 578 | 1543 ± 703 | |
| Magnesium [mg/day] | 273 ± 115 a | 310 ± 129 | 344 ± 163 | |
| Iron [mg/day] | 15.3 ± 7.3 a | 16.9 ± 8.4 | 17.3 ± 8.5 | |
| Zinc [mg/day] | 11.5 ± 8.3 a | 12.8 ± 6.6 | 13.4 ± 6.5 | |
| Copper [mg/day] | 1.3 ± 0.8 a | 1.5 ± 1.2 | 1.5 ± 0.7 | |
| Potassium [mg/day] | 2580 ± 994 a | 2831 ± 1146 | 3167 ± 1433 | |
| Selenium [µg/day] | 103 ± 47 a | 117 ± 54 | 133 ± 63 b | |
| Sodium [mg/day] | 3141 ± 1377 a | 3578 ± 1598 | 3766 ± 1670 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydenreich, J.; Melzer, K.; Flury, C.; Kayser, B. Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES. Nutrients 2017, 9, 754. https://doi.org/10.3390/nu9070754
Heydenreich J, Melzer K, Flury C, Kayser B. Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES. Nutrients. 2017; 9(7):754. https://doi.org/10.3390/nu9070754
Chicago/Turabian StyleHeydenreich, Juliane, Katarina Melzer, Céline Flury, and Bengt Kayser. 2017. "Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES" Nutrients 9, no. 7: 754. https://doi.org/10.3390/nu9070754
APA StyleHeydenreich, J., Melzer, K., Flury, C., & Kayser, B. (2017). Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES. Nutrients, 9(7), 754. https://doi.org/10.3390/nu9070754






