Vitamin A Status of Women and Children in Yaoundé and Douala, Cameroon, is Unchanged One Year after Initiation of a National Vitamin A Oil Fortification Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Sample Size
2.3. Participant Eligibility and Consent
2.4. Data Collection
2.4.1. Household Characteristics and Fortified/Fortifiable Food Consumption
2.4.2. Blood Samples
2.4.3. Breast Milk
2.4.4. Fortified Foods
2.4.5. Sample Storage and Shipping
2.4.6. Laboratory Analyses
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Micronutrient Content of Refined Oil Samples
3.3. Consumption of Fortified Foods and Predicted Impact on Dietary Adequacy
3.4. Change in Biomarkers of Vitamin A Status
3.5. Plausibility Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stevens, G.; Bennett, J.; Hennocq, Q.; Lu, Y.; De-Regil, L.; Rogers, L.; Danaei, G.; Li, G.; White, R.; Flaxman, S.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Horton, S.; Alderman, H.; Rivera, J.A. The challenge of hunger and malnutrition. In Copenhagen Consensus 2008 Challenge Paper; Copenhagen Consensus: Copenhagen, Denmark, 2008. [Google Scholar]
- Arroyave, G.; Mejía, L.A.; Aguilar, J.R. The effect of vitamin A fortification of sugar on the serum vitamin A levels of preschool Guatemalan children: A longitudinal evaluation. Am. J. Clin. Nutr. 1981, 34, 41–49. [Google Scholar] [PubMed]
- Murdiana, A.; Azis, I.; Saidin, S.; Jahari, A.B.; Karyadi, D. Vitamin A-fortified monosodium glutamate and vitamin A status: A controlled field trial. Am. J. Clin. Nutr. 1988, 48, 1265–1270. [Google Scholar]
- Davila, M.; Norris, L.; Cleary, M.; Ross, A. Vitamin A during lactation: Relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J. Nutr. 1985, 115, 1033–1041. [Google Scholar] [PubMed]
- Arroyave, G.; Aguilar, J.; Flores, M.; Guzman, M. Evaluation of Sugar Fortification with Vitamin A at the National Level; PAHO: Washington, DC, USA, 1979; pp. 1–82. [Google Scholar]
- Jus’at, I.; Jahari, A.B.; Ifrad; Htet, M.K.; Tilden, R.L.; Soekarjo, D.; Utomo, B.; Moench-Pfanner, R. Vitamin A-fortified cooking oil reduces vitamin A deficiency in infants, young children and women: Results from a programme evaluation in Indonesia. Public Health Nutr. 2522, 18, 2511–2522. [Google Scholar]
- Sablah, M.; Grant, F.K. E.; Fiedler, J.L. Food fortification in Africa: Progress to date and priorities moving forward. Sight Life Mag. 2013, 27, 18–24. [Google Scholar]
- Ministère de la Santé Publique. Cameroun Enquête Nationale sur la Carence en Vitamine A et l’Anémie Yaoundé; Ministère de la Santé Publique: Yaoundé, Cameroon, 2001.
- Helen Keller International Cameroun; Ministère de la Sante Publique du Cameroun; UNICEF. National Survey of Micronutrient Status and Consumption of Fortifiable Foods; Helen Keller International: Yaoundé, Cameroon, 2011. [Google Scholar]
- Engle-Stone, R.; Haskell, M.J.; Ndjebayi, A.O.; Nankap, M.; Erhardt, J.G.; Gimou, M.-M.; Brown, K.H. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J. Nutr. 2011, 141, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Ndjebayi, A.O.; Nankap, M.; Brown, K.H. Consumption of potentially fortifiable foods by women and young children varies by ecological zone and socio-economic status in Cameroon. J. Nutr. 2012, 142, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Haskell, M.; Nankap, M.; Ndjebayi, A.O.; Brown, K.H. Breast milk retinol and plasma retinol-binding protein concentrations provide similar estimates of vitamin A deficiency prevalence and identify similar risk groups among women in Cameroon but breast milk retinol underestimates the prevalence of deficiency among young children. J. Nutr. 2014, 144, 209–217. [Google Scholar] [PubMed]
- Micronutrient Initiative; PATH Canada Fortification Rapid Assessment Tool (FRAT). Available online: http://www.healthbridge.ca/FRATguidelines2003_Nov_20081PKE-1222008-1386.pdf (accessed on 20 August 2011).
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Brown, K.H. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J. Nutr. 2014, 144, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.; Hess, S.Y.; Ouédraogo, Z.; Rouamba, N.; Ouédraogo, J.; Brown, K.H. Asymptomatic malaria infection affects the interpretation of biomarkers of iron and vitamin A status, even after adjusting for systemic inflammation, but does not affect plasma zinc concentrations among young children in Burkina Faso. J. Nutr. 2014, 144, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.; Desakorn, V.; Pongtavornpinyo, W.; Sahassananda, D.; Silamut, K.; Chotivanuch, K.; Newton, P.; Pitisuttithum, P.; Smithyman, S.; White, N.; et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005, 2, e204. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.; Underwood, B. Breast-milk vitamin A as an indicator of vitamin A status of women and infants. Bull. World Health Organ. 1995, 73, 703–711. [Google Scholar] [PubMed]
- Lucas, A.; Gibbs, J.; Lyster, R.; Baum, J. Creamatocrit: A simple clinical technique for estimating fat concentration and energy value of human milk. Br. Med. J. 1978, 1, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Rohner, F.; Frey, S.K.; Mothes, R.; Hurtienne, A.; Hartong, S.; Bosso, P.; Bui, M.; Schweigert, F.J.; Northrop-Clewes, C. Quantification of vitamin A in palm oil using a fast and simple portable device: Method validation and comparison to high-performance liquid chromatography. Int. J. Vitam. Nutr. Res. 2011, 81, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [PubMed]
- Handelman, G.; Shen, B.; Krinsky, N. High Resolution Analysis of Carotenoids in Human Plasma by High-Performance Liquid Chromatography. Methods Enzymol. 1992, 213, 336–346. [Google Scholar] [PubMed]
- Van Kuijk, F.; Handelman, G.; Dratz, E. Rapid analysis of the major classes of retinoids by step gradient reversed-phase high-performance liquid chromatography using (O-ethyl) oxime derivatives. J. Chromatogr. 1985, 348, 241–251. [Google Scholar] [CrossRef]
- Tooze, J.A.; Kipnis, V.; Buckman, D.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Namaste, S.; Williams, A.M.; Engle-Stone, R.; Addo, O.Y.; Sullivan, K.M.; Suchdev, P.S.; Wirth, J.; Temple, V.; Serdula, M.K.; et al. Approaches to understand and adjust retinol binding protein concentration in the presence of inflammation in preschool-aged children and women of reproductive age: a multi-country analysis. Am. J. Clin. Nutr. In press.
- Shapiro, S.; Wilk, M. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zanutto, E. A comparison of propensity score and linear regression analysis of complex survey data. J. Data Sci. 2006, 4, 67–91. [Google Scholar]
- World Health Organization. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Palmer, A.C.; West, K.P.; Dalmiya, N.; Schultink, W. The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutr. 2012, 15, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, C.L.; Rowe, L.A.; Vossenaar, M.; Garrett, G.S. Regulatory monitoring of fortified foods: Identifying barriers and good practices. Glob. Helath Sci. Pract. 2015, 3, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Andarwalun, N.; Gitapratiwi, D.; Laillou, A.; Fitriani, D.; Hariyadi, P.; Moench-Pfanner, R.; Martianto, D. Quality of vegetable oil prior to fortification is an important criteria to achieve a health impact. Nutrients 2014. [Google Scholar] [CrossRef] [PubMed]
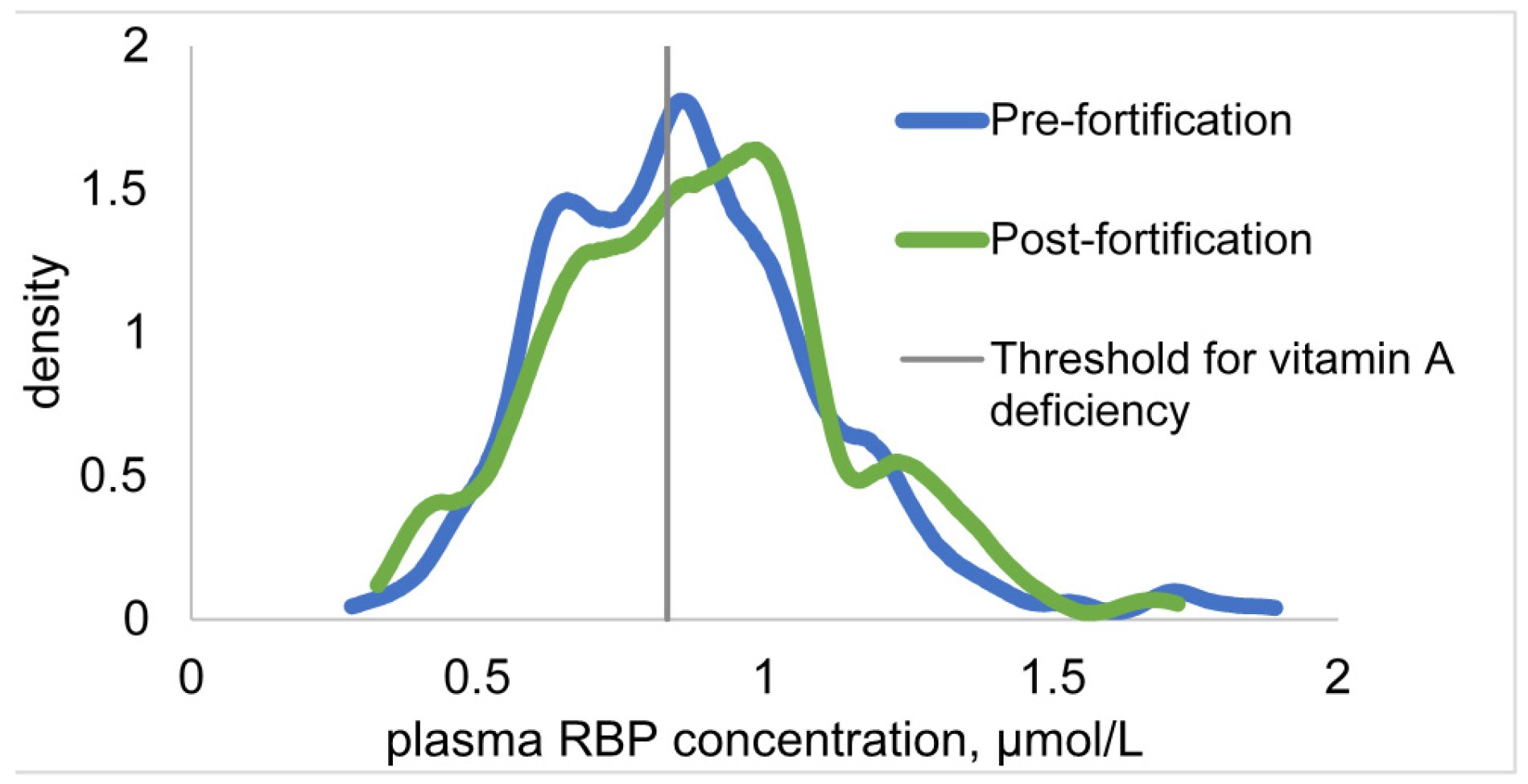
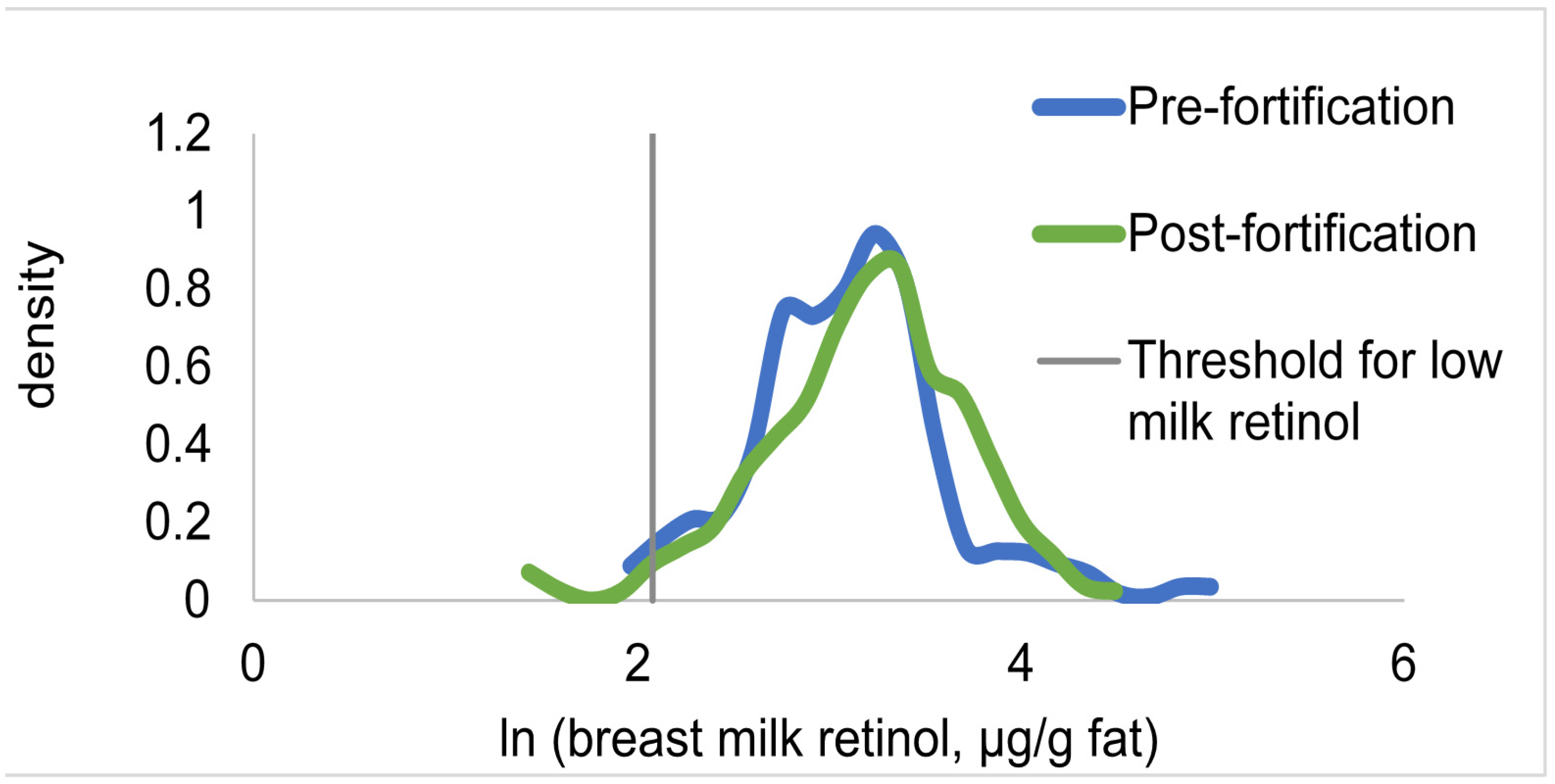
| Pre-Fortification (2009) | Post-Fortification (2012) | P Value | ||||
|---|---|---|---|---|---|---|
| n | Mean ± SE or % | n | Mean ± SE or % | |||
| Women | ||||||
| Age, year | 279 | 27.1 ± 0.4 | 302 | 29.1 ± 0.4 | 0.002 | |
| Pregnant, % | 214 | 11 | 293 | 16 | 0.07 | |
| Lactating, % | 228 | 26 | 302 | 28 | 0.71 | |
| Received postpartum VAS 1 (among lactating women) 2, % | 106 | 43 | 131 | 47 | 0.54 | |
| Milk fat content 2, g/L | 130 | 47.1 ± 2.4 | 151 | 51.7 ± 3.5 | 0.075 | |
| CRP, mg/L | 273 | 2.67 ± 0.22 | 305 | 3.52 ± 0.37 | 0.0002 | |
| AGP, g/L | 273 | 0.73 ± 0.01 | 305 | 0.72 ± 0.01 | 0.070 | |
| Inflammation, % | 273 | 18 | 305 | 22 | 0.19 | |
| Malaria, % | 261 | 7 | 299 | 5 | 0.39 | |
| Children | ||||||
| Age, year | 272 | 30.3 ± 1.0 | 303 | 32.9 ± 0.8 | 0.036 | |
| Male, % | 288 | 49 | 308 | 50 | 0.86 | |
| Stunted (HAZ < −2), % | 255 | 13.0 | 300 | 15.6 | 0.29 | |
| Breastfeeding, % | 239 | 5 | 281 | 4 | 0.69 | |
| Received VAS in past 6 month, % | 233 | 76 | 289 | 51 | 0.002 | |
| CRP, mg/L | 254 | 4.20 ± 0.33 | 297 | 4.49 ± 0.45 | 0.58 | |
| AGP, g/L | 254 | 0.90 ± 0.02 | 297 | 0.97 ± 0.02 | 0.12 | |
| Inflammation, % | 254 | 38 | 297 | 46 | 0.10 | |
| Malaria, % | 234 | 13 | 294 | 8 | 0.088 | |
| Yaoundé | Douala | Total | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | ||
| Detectable VA, % | |||||||
| “Brand name” oil | 28 | 81.4 (65.7–97.1) | 39 | 71.8 (56.4–87.2) | 67 | 75.6 (64.8–86.3) | |
| “Bulk” oil | 62 | 40.6 (28.4–52.8) | 53 | 10.1 (0.6–19.6) | 115 | 26.1 (18.1–34.1) | |
| Overall2 | 96 | 54.0 (44.0–63.9) | 96 | 34.9 (21.5–48.4) | 192 | 44.1 (36.1–52.2) | |
| Average VA concentration, among samples with VA, µg RE/g | |||||||
| “Brand name” oil | 23 | 13.8 (11.7–16.0) | 26 | 12.1 (10.7–13.6) | 49 | 12.9 (11.6–14.1) | |
| “Bulk” oil | 25 | 9.9 (8.4–11.3) | 5 | 8.3 (3.2–13.4) | 30 | 9.6 (8.1–11.0) 3 | |
| Overall 2 | 52 | 11.5 (9.9–13.0) | 31 | 11.5 (10.1–12.9) | 83 | 11.5 (10.5–12.5) | |
| Women | Children | |||||
|---|---|---|---|---|---|---|
| Pre-fortification (2009) | Post-fortification (2012) | P value | Pre-fortification (2009) | Post-fortification (2012) | P value | |
| N (FFQ) | 290 | 309 | 290 | 309 | ||
| Refined oil consumption in past week, % | 82.4 ± 2.8 | 93.6 ± 1.4 | <0.0001 | 81.2 ± 3.0 | 91.7 ± 1.5 | 0.0002 |
| Frequency of oil consumption in past week, among consumers, times/wk | 10.3 ± 0.5 | 9.2 ± 0.3 | 0.14 | 10.7 ± 0.6 | 9.1 ± 0.5 | 0.009 |
| Frequency of refined oil consumption, all participants, times/wk | 8.5 ± 0.5 | 8.6 ± 0.4 | 0.13 | 8.7 ± 0.6 | 8.3 ± 0.5 | 0.59 |
| N (24-h dietary recall) | 297 | - | - | 229 | - | - |
| Mean oil intake in the past day, among consumers, g/day | 32 ± 2 | - | - | 18 ± 2 | - | - |
| Mean usual oil intake (total population) 2, g/day | 17.2 ± 0.6 | - | - | 10.1 ± 0.4 | - | - |
| Total usual VA intake 2, µg RAE/d | 449 ± 20 | 630 ± 20 | - | 194 ± 8 | 285 ± 9 | - |
| Vitamin A intake < EAR 2, % | 59 ± 15 | 16 ± 2 | - | 64 ± 2 | 38 ± 5 | - |
| Pre-Fortification (2009) | Post-Fortification (2012) | P 2 | P 3 | ||
|---|---|---|---|---|---|
| Women | |||||
| N | 273 | 305 | |||
| RBP, µmol/L | 1.41 ± 0.02 | 1.40 ± 0.02 | 0.70 | 0.35 | |
| Adjusted4 RBP, µmol/L | 1.65 ± 3.0 | 1.66 ± 0.02 | 0.34 | - | |
| RBP < 0.78 µmol/L, % | 2.4 ± 0.9 | 3.4 ± 1.1 | 0.54 | 0.58 | |
| RBP < 1.17 µmol/L, % | 22.5 ± 3.1 | 25.9 ± 2.9 | 0.42 | 0.28 | |
| Adjusted 4 RBP < 0.78 µmol/L, % | 1.5 ± 0.7 | 1.7 ± 0.7 | 0.83 | - | |
| Adjusted 4 RBP < 1.17 µmol/L, % | 10.3 ± 1.9 | 14.8 ± 1.7 | 0.80 | - | |
| N | 134 | 154 | |||
| Breast milk vitamin A, µmol/L | 3.67 ± 0.21 | 4.38 ± 0.25 | 0.011 | 0.15 | |
| Breast milk vitamin A, µg/g fat | 26.1 ± 1.8 | 27.1 ± 1.2 | 0.41 | - | |
| Breast milk vitamin A < 1.05 µmol/L, % | 7.6 ± 1.8 | 2.5 ± 1.2 | 0.050 | 0.85 | |
| Breast milk vitamin A < 8 µg/g fat | 2.5 ± 1.9 | 2.6 ± 1.5 | 0.96 | - | |
| Children | |||||
| N | 254 | 297 | |||
| RBP, µmol/L | 0.87 ± 0.02 | 0.88 ± 0.02 | 0.68 | 0.18 5 | |
| Adjusted 4 RBP, µmol/L | 0.98 ± 0.02 | 1.00 ± 0.02 | 0.49 | - | |
| RBP < 0.83 µmol/L, % | 44.2 ± 3.6 | 41.2 ± 3.2 | 0.50 | 0.18 6 | |
| Adjusted 4 RBP < 0.83 µmol/L, % | 30.6 ± 3.9 | 26.6 ± 2.2 | 0.28 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engle-Stone, R.; Nankap, M.; Ndjebayi, A.; Gimou, M.-M.; Friedman, A.; Haskell, M.J.; Tarini, A.; Brown, K.H. Vitamin A Status of Women and Children in Yaoundé and Douala, Cameroon, is Unchanged One Year after Initiation of a National Vitamin A Oil Fortification Program. Nutrients 2017, 9, 522. https://doi.org/10.3390/nu9050522
Engle-Stone R, Nankap M, Ndjebayi A, Gimou M-M, Friedman A, Haskell MJ, Tarini A, Brown KH. Vitamin A Status of Women and Children in Yaoundé and Douala, Cameroon, is Unchanged One Year after Initiation of a National Vitamin A Oil Fortification Program. Nutrients. 2017; 9(5):522. https://doi.org/10.3390/nu9050522
Chicago/Turabian StyleEngle-Stone, Reina, Martin Nankap, Alex Ndjebayi, Marie-Madeleine Gimou, Avital Friedman, Marjorie J. Haskell, Ann Tarini, and Kenneth H. Brown. 2017. "Vitamin A Status of Women and Children in Yaoundé and Douala, Cameroon, is Unchanged One Year after Initiation of a National Vitamin A Oil Fortification Program" Nutrients 9, no. 5: 522. https://doi.org/10.3390/nu9050522
APA StyleEngle-Stone, R., Nankap, M., Ndjebayi, A., Gimou, M.-M., Friedman, A., Haskell, M. J., Tarini, A., & Brown, K. H. (2017). Vitamin A Status of Women and Children in Yaoundé and Douala, Cameroon, is Unchanged One Year after Initiation of a National Vitamin A Oil Fortification Program. Nutrients, 9(5), 522. https://doi.org/10.3390/nu9050522





